Abstract
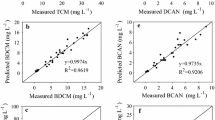
Despite there being numerous models of trihalomethane (THM) formation, they are limited by high estimation errors, which can be close to the regulatory limits for THMs, due to the fluorescence quenching effect. In this research, the estimation error for THM formation was reduced by correcting the quenching effect. The trihalomethane formation potential (THMFP) test was conducted in the presence of chlorine and bromine, individually and in mixtures. The THM precursors used in this study were protein (bovine serum albumin; BSA), amino acids (tryptophan and tyrosine), chlorine, bromine, and Suwannee River natural organic matter (SWNOM). BSA tended to form bromodichloromethane (BDCM) rather than trichloromethane (TCM) during chlorination in the presence of bromide (Br−). In contrast, SWNOM tended to form chlorinated THMs (TCM) rather than brominated THMs (BDCM and dibromochloromethane; DBCM), and no TBMs were formed in these processes. BSA with SWNOM decreased the formation of TCM due to the decrease in the amount of TCM precursor in SWNOM through binding with BSA. The concentration of each THM species was predicted from the fluorescence intensity of peak C, corrected fluorescence intensity of peak T, and Br− concentration. The use of humic-like and corrected protein-like fluorescence in the excitation–emission matrix model for predicting THM species reduced the prediction error. In this research, correction of the fluorescence quenching decreased the mean percentage estimation error for TCM, BDCM, and DBCM from 47%, 35%, and > 100% in classical approaches to 6.6%, 26.9%, and 2.0%, respectively. This study is expected to make contributions in reporting the relationship between the concentration of natural organic matter compositions and the formation of THM species.
Graphic Abstract






Similar content being viewed by others
Data availability
All data in this manuscript are available on reasonable request. All the analyses were performed in triplicate, and the data reported are average with standard deviation.
References
Boyer, T. H. (2015). Meta-analysis of trihalomethane formation models and application to bromide intrusion, in: Recent Advances in Disinfection By-Products (pp. 97–116), ACS Publications.
Chaukura, N., Marais, S. S., Moyo, W., Mbali, N., Thakalekoala, L. C., Ingwani, T., Mamba, B. B., Jarvis, P., & Nkambule, T. T. I. (2020). Contemporary issues on the occurrence and removal of disinfection byproducts in drinking water-A review. Journal of Environmental Chemical Engineering, 8, 103659.
Chen, B., & Westerhoff, P. (2010). Predicting disinfection by-product formation potential in water. Water Research, 44, 3755–3762. https://doi.org/10.1016/j.watres.2010.04.009
Chu, W.-H., Gao, N.-Y., Deng, Y., & Krasner, S. W. (2010). Precursors of dichloroacetamide, an emerging nitrogenous DBP formed during chlorination or chloramination. Environmental Science and Technology, 44, 3908–3912.
Ged, E. C., Chadik, P. A., & Boyer, T. H. (2015). Predictive capability of chlorination disinfection byproducts models. Journal of Environmental Management, 149, 253–262. https://doi.org/10.1016/j.jenvman.2014.10.014
Gopal, K., Tripathy, S. S., Bersillon, J. L., & Dubey, S. P. (2007). Chlorination byproducts, their toxicodynamics and removal from drinking water. Journal of Hazardous Materials, 140, 1–6.
Hao, R., Ren, H., Li, J., Ma, Z., Wan, H., Zheng, X., & Cheng, S. (2012). Use of three-dimensional excitation and emission matrix fluorescence spectroscopy for predicting the disinfection by-product formation potential of reclaimed water. Water Research, 46(17), 5765–5776.
Hua, B., Veum, K., Yang, J., Jones, J., & Deng, B. (2010). Parallel factor analysis of fluorescence EEM spectra to identify THM precursors in lake waters. Environmental Monitoring and Assessment, 161(1), 71–81.
Jutaporn, P., Laolertworakul, W., Armstrong, M. D., & Coronell, O. (2019). Fluorescence spectroscopy for assessing trihalomethane precursors removal by MIEX resin. Water Science and Technology, 79(5), 820–832.
Jutaporn, P., Laolertworakul, W., Tungsudjawong, K., Khongnakorn, W., & Leungprasert, S. (2021). Parallel factor analysis of fluorescence excitation emissions to identify seasonal and watershed differences in trihalomethane precursors. Chemosphere 131061.
Landis, M. S., Kamal, A. S., Kovalcik, K. D., Croghan, C., Norris, G. A., & Bergdale, A. (2016). The impact of commercially treated oil and gas produced water discharges on bromide concentrations and modeled brominated trihalomethane disinfection byproducts at two downstream municipal drinking water plants in the upper Allegheny River, Pennsylvania, USA. Science of the Total Environment, 542, 505–520.
Langsa, M., Heitz, A., Joll, C. A., Von Gunten, U., & Allard, S. (2017). Mechanistic aspects of the formation of adsorbable organic bromine during chlorination of bromide-containing synthetic waters. Environmental Science and Technology, 51, 5146–5155. https://doi.org/10.1021/acs.est.7b00691
Liang, L. I. N., Singer, P. C., Hill, C., & Carolina, N. (2003). Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environmental Science and Technology, 37, 2920–2928.
Malcolm, P. (1993). Bay-Delta water quality modeling. Rep. No. 15–041, Los Angeles.
McTigue, N. E., Cornwell, D. A., Graf, K., & Brown, R. (2014). Occurrence and consequences of increased bromide in drinking water sources. Journal-American Water Work. Assoc., 106, E492–E508.
MHLW. (2010). http://www.mhlw.go.jp/english/policy/health/water_sup- ply/4.html. Ministry of Health, Labour and Welfare, Japan.. Water quality standard item and standard value (51 items), Water quality management target setting item and target value (26 items) in tap water quality standard of Ministry of Health, Labour and Welfare, Japan (www.mhlw.go.jp)
Montgomery, W. (1993). Mathematical modeling of the formation of THMs and HAAs in chlorinated natural waters, American Water Works Association, Denver, Colorado.
Murphy, K. R., Stedmon, C. A., Graeber, D., & Bro, R. (2013). Fluorescence spectroscopy and multi-way techniques. PARAFAC. Analytical Methods, 5, 6557. https://doi.org/10.1039/c3ay41160e
Pifer, A. D., & Fairey, J. L. (2014). Suitability of organic matter surrogates to predict trihalomethane formation in drinking water sources. Environmental Engineering Science, 31(3), 117–126.
Raeke, J., Lechtenfeld, O. J., Tittel, J., Oosterwoud, M. R., Bornmann, K., & Reemtsma, T. (2017). Linking the mobilization of dissolved organic matter in catchments and its removal in drinking water treatment to its molecular characteristics. Water Research, 113, 149–159. https://doi.org/10.1016/j.watres.2017.01.066
Rathbun, R. E. (1996). Speciation of trihalomethane mixtures for the Mississippi, Missouri, and Ohio Rivers. Science of the Total Environment, 180, 125–135. https://doi.org/10.1016/0048-9697(95)04938-X
Richardson, S. D., & Postigo, C. (2011). Drinking water disinfection by-products, in: Emerging Organic Contaminants and Human Health. Springer, pp. 93–137.
Saipetch, K., Khanal, R., Yamazaki, M., Fu, Q. -L., Yoshimura, C., & Jin, X. (2021a). Exploring the fluorescence quenching interaction of amino acids and protein with natural organic matter by multi-spectroscopic method. Water Supply, ws2021103.
Saipetch, K., & Yoshimura, C. (2019). Importance of correcting for fluorescence quenching in fluorescence-based prediction of trihalomethane formation potential. Water Supply, 19(6), 1677–1685. https://doi.org/10.2166/ws.2019.039
Saipetch, K., Khanal, R., & Yoshimura, C. (2021b). Empirical equation for the correction of fluorescence quenching of bovine serum albumin by Suwannee river natural organic matter. Water Practice and Technology, wpt2021001. https://doi.org/10.2166/wpt.2021.001
Shan, J., Hu, J., Kaplan-Bekaroglu, S. S., Song, H., & Karanfil, T. (2012). The effects of pH, bromide and nitrite on halonitromethane and trihalomethane formation from amino acids and amino sugars. Chemosphere, 86(4), 323–328.
Tian, C., Liu, R., Guo, T., Liu, H., Luo, Q., & Qu, J. (2013). Chlorination and chloramination of high-bromide natural water: DBPs species transformation. Separation and Purification Technology, 102, 86–93.
Tungsudjawong, K., Leungprasert, S., & Peansawang, P. (2018). Investigation of humic acids concentration in different seasons in a raw water canal, Bangkok, Thailand. Water Science and Technology: Water Supply, 18(5), 1727–1738. https://doi.org/10.2166/ws.2017.235
US EPA. (2009). National primary drinking water regulations: Current drinking water regulations [WWW Document]. www.epa.gov/safewater/mcl.html mcls.
Watson, K., Farré, M. J., Leusch, F. D. L., & Knight, N. (2018). Using fluorescence-parallel factor analysis for assessing disinfection by-product formation and natural organic matter removal efficiency in secondary treated synthetic drinking waters. Science of the Total Environment, 640, 31–40.
Wenk, J., Aeschbacher, M., Salhi, E., Canonica, S., Von Gunten, U., & Sander, M. (2013). Chemical oxidation of dissolved organic matter by chlorine dioxide, chlorine, and ozone: Effects on its optical and antioxidant properties. Environmental Science and Technology, 47, 11147–11156.
Williams, C. J., Frost, P. C., Morales-Williams, A. M., Larson, J. H., Richardson, W. B., Chiandet, A. S., & Xenopoulos, M. A. (2016). Human activities cause distinct dissolved organic matter composition across freshwater ecosystems. Global Change Biology, 22, 613–626. https://doi.org/10.1111/gcb.13094
Acknowledgements
We thank the Open Research Facilities for Life Science and Technology of the Tokyo Institute of Technology for technical support in measuring THMs. Thanks also to Jimmy in discussing the comments.
Funding
This study was financially supported by the Ministry of the Environment of Japan [S-13–2-3] and JSPS KAKENHI [grant number 18H01566].
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial or competing interests to disclose. The expression expressed in this paper is that of the authors alone, and categorically does not reflect the opinion of their organization of their affiliation or the funder. The inclusion of commercial name of the products is for research purpose only, and also does not reflect endorsement by the authors or their affiliation or the funder.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•BSA tends to form BDCM rather than TCM during chlorination in the presence of Br−.
•SWNOM tends to form chlorinated THMs (TCM) rather than brominated THMs (BDCM and DBCM).
•BSA and SWNOM decrease the formation of TCM, possibly by decreasing the amount of TCM precursor in SWNOM by binding to BSA.
•Using humic-like and corrected protein-like fluorescence in the EEM model for predicting THM species reduces the prediction error.
Appendix 1
Appendix 1
-
A1 GC-BID running program.
-
Column: SH-Rtx-5 (Crossbond 5% diphenyl/95% dimethyl polysiloxane.
-
Auto sampler settings (AOC-20i) as follows:
Injection volume | 1.5 μL |
|---|---|
Washing with solvent before | 3 times |
Washing with solvent after injection | 1 time |
Wash with sample | 2 times |
-
Injection temperature of 200 °C in split mode.
-
BID detector temperature of 300 °C.
-
Oven temperatures as follows:
Rate | Temperature (°C) | Hold (min) |
|---|---|---|
30 | 5.5 | |
10 | 35 | 4 |
10 | 40 | 3 |
15 | 150 | 0 |
Rights and permissions
About this article
Cite this article
Saipetch, K., Khanal, R. & Yoshimura, C. Integration of fluorescence quenching correction into trihalomethane formation prediction models. Environ Monit Assess 193, 845 (2021). https://doi.org/10.1007/s10661-021-09649-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09649-z