Abstract
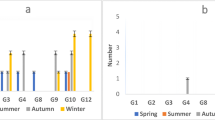
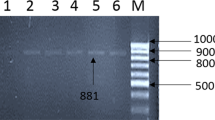
Enteric viruses, especially human rotaviruses present in aquatic environments, are microbial criteria in quality assessment of water resources. The present research aimed to investigate molecular monitoring of human rotavirus and efficacy evaluation of Isfahan water treatment plant (WTP) in the elimination of viruses. In total, 60 water samples were collected from different units of WTP. Zeta plus electropositive Virosorb cartridge filter and elution buffer was used for concentrating water samples. Enzyme-linked immunosorbent assay (ELISA) was used for detecting rotavirus antigen. Quantitative real-time reverse transcription PCR (qRT-PCR) with SYBR Green I fluorescent dye was performed for molecular detection of rotavirus. Multiplex nested reverse transcription-polymerase chain reaction (RT-PCR) was used for rotavirus G genotyping. Total coliform count varies from 102–103 CFU/mL in the raw water resources. Rotavirus antigen was detected in 17 samples (28.33%) by ELISA, and 13 samples (21.67%) were found positive by RT-PCR. These included 41.18% (7 cases) of raw water influent, 29.41% (5 cases) after sedimentation, 23.52% (4 cases) after ozonation, and 5.88% (1 case) after filtration in ELISA method. The highest number of rotaviruses was detected by qRT-PCR in autumn (46.15% (6 cases)). The commonest circulating G type in the sampling points was the mixed types, which was identified in 6 samples (46.15%), followed by non-typeable (23.07%), G3 (15.38%), G1 (7.69%), and G8 (7.69%), respectively. Despite the presence of rotavirus in raw water, after clarification and ozonation, filtration and treated water did not show the presence of rotavirus. The results of this study showed that multi-stage treatment has a positive effect on virus removal in WTP.





Similar content being viewed by others
Abbreviations
- IWTP:
-
Isfahan water treatment plant
- ELISA:
-
Enzyme-linked immunosorbent assay
- rpm:
-
Revolutions per minute
- MPN:
-
Most probable number
- NT:
-
Non-typeable samples
- CFU:
-
Colony-forming unit
- OD:
-
Optical density
- NSP:
-
Nonstructural proteins
- PMMoV:
-
Pepper mild mottle virus
- Nested RT-PCR:
-
Nested reverse transcription (RT) PCR
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- WHO/CDC:
-
World Health Organization and Centers for Disease Control and Prevention.
- EC:
-
Electrical conductivity
- RVA:
-
Rotavirus A
- PAC:
-
Poly aluminum chloride
- NTU:
-
Nephelometric turbidity unit
- Ct:
-
Cycle threshold
- WTP:
-
Water treatment plant
- DWTP:
-
Drinking water treatment plant
- VP:
-
Viral protein
- PBS:
-
Phosphate-buffered saline
- FC:
-
Fecal coliform
References
Adefisoye, M. A., Nwodo, U. U., Green, E., & Okoh, A. I. (2016). Quantitative PCR detection and characterization of human adenovirus, rotavirus and hepatitis A virus in discharged effluents of two wastewater treatment facilities in the Eastern Cape, South Africa. Food Environmental Virology, 8, 262–274.
Asami, T., Katayama, H., Torrey, J. R., Visvanathan, C., & Furumai, H. (2016). Evaluation of virus removal efficiency of coagulation-sedimentation and rapid sand filtration processes in a drinking water treatment plant in Bangkok, Thailand. Water Research, 101, 84–94. https://doi.org/10.1016/j.watres.2016.05.012.
Assis, A. S. F., Cruz, L. T., Ferreira, A. S., Bessa, M. E., de Oliveira, P. M. A., Vieira, C. B., et al. (2015). Relationship between viral detection and turbidity in a watershed contaminated with group A rotavirus. Environmental Science and Pollution Research, 22, 6886–6897. https://doi.org/10.1007/s11356-014-3874-8.
Atabakhsh, P., Kargar, M., & Doosti, A. (2019). Molecular detection and genotyping of group A rotavirus in two wastewater treatment plants, Iran. Brazilian of microbiology, 1–7. https://doi.org/10.1007/s42770-019-00131-0.
Banerjee, I., Ramani, S., Primrose, B., Iturriza-Gomara, M., Gray, J. J., Brown, D. W., & Kang, G. (2008). Modification of rotavirus multiplex RT-PCR for the detection of G12 strains based on characterization of emerging G12 rotavirus strains from South India. Medical Virology, 79(9), 1413–1421.
Barril, P. A., Prez, V. E., & Nates, S. V. (2016). Rotavirus monitoring in drinking and surface waters of Cordoba, Argentina. Gastrointestinal Diseases, 1–11. https://smjournals.com/ebooks/gastrointestinal-diseases/chapters/GD-16-01.pdf
Bosch, A., Guix, S., Sano, D., & Pinto, M. R. (2008). New tools for the study and direct surveillance of viral pathogens in water. Current Opinion in Biotechnology, 19, 295–301. https://doi.org/10.1016/j.copbio.2008.04.006.
Centers for Disease, C, Prevention. (2014). Rotavirus surveillance-worldwide. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt13-otavirus.html.
Delgado-Gardea, M. C. E., Tamez-Guerra, P., Gomez-Flores, R., Mendieta-Mendoza, A., Zavala-Diaz de la Serna, F. J., et al. (2017). Prevalence of rotavirus genogroup A and norovirus genogroup II in Bassaseachic falls national park surface waters in Chihuahua, Mexico. International Journal of Environmental Research and Public Health, 14, 482.
Fongaro, G., Nascimento, M., Vianccelli, A., Tonetta, D., Petrucio, M., & Barardi, C. (2012). Surveillance of human viral contamination and physicochemical profiles in a surface water lagoon. Water Science and Technology, 66, 2682–2687. https://doi.org/10.2166/wst.2012.504.
Fout, G. S., Schaefer III, F. W., Messer, J. W., Dahling, D. R., & Stetler, R. E. (1996). Information collection rule (ICR) microbial laboratory manual. EPA/600/R-95/178. Washington, DC: U.S. Environmental Protection Agency.
Gibson, K. E., Opryszko, C. M., Schissler, J. T., Guo, Y., & Schwab, K. J. (2011). Evaluation of human enteric viruses in surface water and drinking water resources in Southern Ghana. American Society of Tropical Medicine and Hygiene, 84(1), 20–29.
Grassi, T., Bagordo, F., Idolo, A., Lugoli, F., Gabutti, G., & Donno, A. D. (2010). Rotavirus detection in environmental water samples by tangential flow ultrafiltration and RT-nested PCR. Environmental Monitoring and Assessment, 164, 199–205.
Grøndahl-Rosado, R. C., Yarovitsyna, E., Trettenes, E., Myrmel, M., & Robertson, L. J. (2014). A one year study on the concentrations of norovirus and enteric adenoviruses in wastewater and a surface drinking water source in Norway. Food Environmental Virology, 6, 232–245.
Hamza, I. A., Jurzik, L., Uberla, K., & Wilhelm, M. (2011). Methods to detect infectious human enteric viruses in environmental water samples. International Journal of Hygiene and Environmental Health, 214, 424–436.
Ibfelt, T., Frandsen, T., Permin, A., Andersen, L. P., & Schultz, A. C. (2016). Test and validation of methods to sample and detect human virus from environmental surfaces using norovirus as a model virus. Hospital Infection, 92(4), 378–384. https://doi.org/10.1016/j.jhin.2016.01.003
Ibrahim, C., Hassen, A., Pothier, P., Mejri, S., & Hammami, S. (2018). Molecular detection and genotypic characterization of enteric adenoviruses in a hospital wastewater. Environmental Science and Pollution Research, 25, 10977–10987.
Inker, L. A., Gerba, C. P., & Bright, K. R. (2012). Concentration and recovery of viruses from water: a comprehensive review. Food environmental virology, 4, 41–67.
Kargar, M., Javdani, N., Najafi, A., & Tahamtan, Y. (2013). First molecular detection of group A rotavirus in urban and hospital sewage systems by nested-RT PCR in Shiraz, Iran. Environmental Health Science Engineering, 11, 4. https://doi.org/10.1186/2052-336X-11-4.
Karim, M. R., Rhodes, E. R., Brinkman, N., Wymer, L., & Fout, G. S. (2009). New electropositive filter for concentrating enteroviruses and noroviruses from large volumes of water. Applied and Environmental Microbiology, 75, 2393–2399. https://doi.org/10.1128/AEM.00922-08.
Kiulia, N.M., Hofstra, N., Vermeulen, L.C., Obara, M.A., Medema, G., & Rose, J.B. (2015). Global Occurrence and Emission of Rotaviruses to Surface Waters. Pathogens, 4, 229-255. https://doi.org/10.3390/pathogens4020229.
Kluge, M., Fleck, J. D., Soliman, M. C., Luz, R. B., Fabres, R. B., Comerlato, J., et al. (2014). Human adenovirus (HAdV), human enterovirus (HEV), and genogroup A rotavirus (GARV) in tap water in southern Brazil. Water Health, 12, 26–532. https://doi.org/10.2166/wh.2014.202.
Lee, D. Y., Leung, K. T., Lee, H., & Habas, M. B. (2016). Simultaneous detection of selected enteric viruses in water samples by multiplex quantitative PCR. Water, Air, and Soil Pollution, 227, 107–111. https://doi.org/10.1007/s11270-016-2811-5.
Liu, J., Lurain, K. U., Sobuz, S., Begum, S., & Kumburu, H. (2015). Molecular genotyping and quantitation assay for rotavirus surveillance. Virological Methods, 213, 157–163.
Maite, M. (2016). Human norovirus in artificial and environmental marine water: development of antibody based rapid methods. a dissertation submitted to the graduate faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The School of Nutrition and Food Science.
Najafi, A., Kargar, M., & Jafarpour, T. (2012). Burden and typing of rotavirus group A in children with acute gastroenteritis in Shiraz, Southern Iran. Iranian Red Crescent Medical, 14, 531.
Rames, E., Roiko, A., Stratton, H., & Macdonald, J. (2016). Technical aspects of using human adenovirus as a viral water quality indicator. Water Research, 96, 308–326.
Rice, E. W., Baird, R. B., Eaton, A. D., & Clesceri, L. S. (2012). Standard methods for the examination of water and wastewater Washington. 22nd Edition, American Public Health Association, American Water Works, Water Environment Federation, Washington DC., USA.
Rizk, N. M., & Allayeh, A. K. (2018). Multiplex semi-nested RT-PCR for genotyping of rotaviruses group A in Giza Tap Water, Egypt. Asian Journal of Water, Environment and Pollution, 15, 217–221. https://doi.org/10.3233/AJW-180034.
Salvo, M., Lizasoain, A., Castells, M., Bortagaray, V., Castro, S., Colina, R., Tort, F. L., & Victoria, M. (2018). Human bocavirus: detection, quantification and molecular characterization in sewage and surface waters in Uruguay. Food environmental virology, 10, 193–200. https://doi.org/10.1007/s12560-017-9334-0.
Silva, M., Miagostovich, M., & Victoria, M. (2016). Rotavirus and astrovirus. global water pathogen project.
Soltan, M. A., Tsai, Y-L., Lee, P-YA., Tsai, C-F., Chang, H-FG., Wang, H-TT., et al. (2016). Comparison of electron microscopy, ELISA, real time RT-PCR and insulated isothermal RT-PCR for the detection of Rotavirus group A (RVA) in feces of different animal species. Virological methods, 235, 99–104.
Verheyen, J., Timmen-Wego, M., Laudien, R., Boussaad, I., Sen, S., Koc, A., et al. (2009). Detection of adenoviruses and rotaviruses in drinking water sources used in rural areas of Benin, West Africa. Applied and Environmental Microbiology, 75(9), 2798–2801.
WHO Geneva. (2009). Department of Immunization, Vaccines and Biological manual of rotavirus detection and characterization methods. CH-1211, Switzerland This publication is available on the Internet at: http://whqlibdoc.who.int/hq/2008/WHO_IVB_08.17_eng.pdf.
WHO. (2014). Progress on drinking water and sanitation: update. Geneva: Switzerland.
Ye, Y. X., Ming, X., Zhang, L. Y., Xiao, W. Q., Huang, X. N., Cao, Y. G., & Gu, K. D. (2012). Real-time PCR detection of enteric viruses in source water and treated drinking water in Wuhan, China. Current Microbiology, 65, 244–253. https://doi.org/10.1007/s00284-012-0152-1.
Yousuf, F. A., Siddiqui, R., & Ahmed Khan, N. (2017). Presence of rotavirus and free-living amoebae in the water supplies of Karachi, Pakistan. Sao Paulo Institute of Tropical Medicine, 59, 1–7. https://doi.org/10.1590/s1678-9946201759032.
Acknowledgments
The authors are grateful to the Islamic Azad University of Jahrom and Isfahan water treatment plant for their executive support of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atabakhsh, P., Kargar, M. & Doosti, A. Molecular surveillance of human rotaviruses in drinking water and investigation of the efficiency of their removal in Isfahan water treatment plant. Environ Monit Assess 191, 759 (2019). https://doi.org/10.1007/s10661-019-7834-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7834-0