Abstract
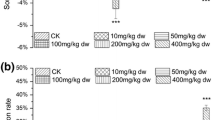
Both the evaluation and the determination of toxicity of chemical substances present in the environment have implications in human health. In this present study, the natural phenomenon named autotomy, a self-defense mechanism employed by several animals against the toxic chemical contaminants, was considered to assess the toxicity of different chemical substances. We investigated the effects of glucose, sodium chloride, kanamycin, mercuric chloride, arsenic trioxide, and lead oxide on the phenotypes of earthworm Eudrilus eugeniae. Depending on the concentration of different chemicals, worms exhibit unique phenotypes. These phenotypes can be used to identify the toxicity as well as the toxic concentration of the chemicals. Upon exposure to toxic chemicals, worms use different mechanical forces at the site of cleavage furrow to detach its segments. During the detachment, there is no apparent blood loss at both the ends of the worm. Our results show that the mercuric chloride is toxic at the concentration above 5 μg when compared to other chemicals. Based on our findings, the toxic effects of a chemical and the toxic concentration of a chemical can be evaluated in both cost and time-efficient manner; in addition, these chemicals can be classified into the following categories: (1) mercuric chloride is extreme-toxic, (2) arsenic trioxide and lead oxide is toxic, (3) kanamycin and sodium chloride is low-toxic, and (4) glucose is non-toxic.









Similar content being viewed by others
References
Allen, J. W., Mutkus, L. A., & Aschner, M. (2001). Mercuric chloride, but not methylmercury, inhibits glutamine synthetase activity in primary cultures of cortical astrocytes. Brain Research, 891(1), 148–157. https://doi.org/10.1016/S0006-8993(00)03185-1
Ames, B. N., Lee, F. D., & Durston, W. E. (1973). An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proceedings of the National Academy of Sciences, 70(3), 782–786. https://doi.org/10.1073/pnas.70.3.782
Aschner, M., Mullaney, K., Wagoner, D., Lash, L., & Kimelberg, H. (1994). Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)-and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocytes cultures. Brain Research, 664(1), 133–140. https://doi.org/10.1016/0006-8993(94)91963-1
ATSDR (Agency for Toxic Substances and Disease Registry) (1999). Toxicological profile for mercury. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA:ATSDR: https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf. Accessed 15 Dec 2017.
ATSDR (Agency for Toxic Substances and Disease Registry). 2011. Case studies in environmental medicine: arsenic toxicity. Atlanta, GA: ATSDR. http://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=0. Accessed 15 Dec 2017.
Boyd, W. A., & Williams, P. L. (2003). Comparison of the sensitivity of three nematode species to copper and their utility in aquatic and soil toxicity tests. Environmental Toxicology andChemistry, 22(11), 2768–2774. https://doi.org/10.1897/02-573
Bressler, J. P., & Goldstein, G. W. (1991). Mechanisms of lead neurotoxicity. Biochemical Pharmacology, 41(4), 479–484. https://doi.org/10.1016/0006-2952(91)90617-E
Bressler, J., Kim, K.-A., Chakraborti, T., & Goldstein, G. (1999). Molecular mechanisms of lead neurotoxicity. Neurochemical Research, 24(4), 595–600. https://doi.org/10.1023/A:1022596115897
Brown, C. A., & Formanowicz, D. R. (2012). The effect of leg autotomy on terrestrial and aquatic locomotion in the wolf spider Pardosavalens (Araneae: Lycosidae). The Journal of Arachnology, 40(2), 234–239. https://doi.org/10.1636/Hill-59.1
Burg, R. V. (1995). Inorganic mercury. Journal of Applied Toxicology, 15(6), 483–493. https://doi.org/10.1002/jat.2550150610
Burns, C. G., Milan, D. J., Grande, E. J., Rottbauer, W., MacRae, C. A., & Fishman, M. C. (2005). High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nature Chemical Biology, 1(5), 263–264. https://doi.org/10.1038/nchembio732
Cao, X., Song, Y., Kai, J., Yang, X., & Ji, P. (2012). Evaluation of EROD and CYP3A4 activities in earthworm Eiseniafetida as biomarkers for soil heavy metal contamination. Journal of Hazardous Materials, 243, 146–151. https://doi.org/10.1016/j.jhazmat.2012.10.012
Chen, C., Wang, Y., Qian, Y., Zhao, X., & Wang, Q. (2015). The synergistic toxicity of the multiple chemical mixtures: implications for risk assessment in the terrestrial environment. Environment International, 77, 95–105. https://doi.org/10.1016/j.envint.2015.01.014
Chick, H. (1908). An investigation of the laws of disinfection. Journal of Hygiene, 8(01), 92–158. https://doi.org/10.1017/S0022172400006987
Clarkson, T. W., & Magos, L. (2006). The toxicology of mercury and its chemical compounds. Critical Reviews in Toxicology, 36(8), 609–662. https://doi.org/10.1080/10408440600845619
Craig, W. A. (2010). Kanamycin. In M. L. Grayson (Ed.), Kucers’ the use of antibiotics. A clinical review of antibacterial, antifungal and antiviral drugs (Sixth ed., pp. 668–669). Boca Raton: CRC press, Taylor & Francis.
Dibas, A., Prasanna, G., & Yorio, T. (1999). Phenylarsine oxide inhibits phosphate uptake in human ciliary non-pigmented epithelial cells. Journal of Ocular Pharmacology and Therapeutics, 15(3), 241–250. https://doi.org/10.1089/jop.1999.15.241
EC (2004). Biological test method: tests for toxicity of contaminated soil to earthworms (Eiseniafoetida, Eiseniaandrei, or Lumbricusterrestris).Report EPS 1/RM/43, Environment Canada, Environmental Technology Centre, Ottawa.
Edwards, C. A., & Bater, J. E. (1992). The use of earthworms in environmental management. Soil Biology and Biochemistry, 24(12), 1683–1689. https://doi.org/10.1016/0038-0717(92)90170-3
Elaiya Raja, S., Kumar, S. D., Johnson Retnaraj Samuel, S. C., Kalidas, R., Nino, G. D., Jackson Durairaj, S. C., Kasimani, R., Sundar, K., & Sudhakar, S. (2017). Studies on organogenesis during regeneration in the earthworm, Eudriluseugeniae, in support of symbiotic association with bacillus endophyticus. Turkish Journal of Biology, 41, 113–126.
Engelhardt, H. (1922). Untersuchungeniuber das Mechanismus der Sublimatwirkung auf Bakterien. Desinfektion, 7, 81–83.
Flora, S. J. S., Saxena, G., & Mehta, A. (2007). Reversal of lead-induced neuronal apoptosis by chelation treatment in rats: role of reactive oxygen species and intracellular Ca2+. The Journal of Pharmacology and Experimental Therapeutics, 322(1), 108–116. https://doi.org/10.1124/jpet.107.121996
Ganry, O., Boudet, J., Wargon, C., Hornych, A., & Meyer, P. (1993). Effect of sodium bicarbonate and sodium chloride on arterial blood pressure, plasma renin activity and urinary prostaglandins in healthy volunteers. Journal of Hypertension, 11(5), S202–S203.
Gerald, G. W., Thompson, M. M., Levine, T. D., & Wrinn, K. M. (2017). Interactive effects of leg autotomy and incline on locomotor performance and kinematics of the cellar spider.Pholcusmanueli. Ecology and Evolution, 7(7), 6729–6735. https://doi.org/10.1002/ece3.3231
Gourevitch, A., Rossomano, V., Puglisi, T., Tynda, J., & Lein, J. (1958). Microbiological studies with kanamycin. Annals of the New York Academy of Sciences, 76(1), 31–41.
Hayon, I. L. (2014). Signal, transduction, and the hematopoietic stem cell. Rambam Maimonides Med J, 5(4), e0033. https://doi.org/10.5041/RMMJ.10167
Hisamoto, N., & Matsumoto, K. (2017). Signal transduction cascades in axon regeneration: insights from C. elegans. Current Opinion in Genetics & Development, 44, 54–60. https://doi.org/10.1016/j.gde.2017.01.010
Hodgson, E. (2004). A textbook of modern toxicology (Third ed.). Publication, United Kingdom: A JOHN WILEY & SONS, INC.. https://doi.org/10.1002/0471646776
Hughes, M. F. (2002). Arsenic toxicity and potential mechanisms of action. Toxicology Letters, 133(1), 1–16. https://doi.org/10.1016/S0378-4274(02)00084-X
Jager, T., Wal, L. V., Fleuren, R. H. L. J., Barendregt, A., & Hermens, J. L. M. (2005). Bioaccumulation of organic chemicals in contaminated soils: evaluation of bioassays with earthworms. Environmental Science & Technology, 39(1), 293–298. https://doi.org/10.1021/es035317o
Jiang, Y., Chen, J., Wu, Y., Wang, Q., & Li, H. (2016). Sublethal toxicity endpoints of heavy metals to the nematode Caenorhabditiselegans. PLoS One, 11(1), e0148014. https://doi.org/10.1371/journal.pone.0148014
Johnson Retnaraj Samuel, S. C., Elaiya Raja, S., Beryl Vedha, Y., Edith Arul Jane, A., Amutha, K., Dinesh, S. M., et al. (2011). Autofluorescence in BrdU-positive cells and augmentation of regeneration kinetics by riboflavin. Stem Cells and Development, 21(11), 2071–2083.
Ju, J., Saul, N., Kochan, C., Putschew, A., Pu, Y., Yin, L., & Steinberg, C. E. W. (2014). Cyanobacterial xenobiotics as evaluated by a Caenorhabditiselegans neurotoxicity screening test. International Journal of Environmental Research and Public Health, 11(5), 4589–4606. https://doi.org/10.3390/ijerph110504589
Kalidas, R. M., Raja, S. E., Mydeen, S. A. K. N. M., Samuel, S. C. J. R., Durairaj, S. C. J., Nino, G. D., Palanichelvam, K., Vaithi, A., & Sudhakar, S. (2015). Conserved lamin A protein expression in differentiated cells in the earthworm Eudrilus eugeniae. Cell Biology International, 39(9), 1036–1043. https://doi.org/10.1002/cbin.10479
Khanna, N., Cressman, C. P., Tatara, C. P., & Williams, P. L. (1997). Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media. Archives of Environmental Contamination and Toxicology, 32(1), 110–114. https://doi.org/10.1007/s002449900162
Lesiuk, N. M., &Drewes, C. D. (1999). Autotomy reflex in a freshwater oligochaete, Lumbriculusvariegatus (Clitellata: Lumbriculidae) Aquatic Oligochaetes (pp. 253–261): Springer.
Lim, P. N., Wu, T. Y., Sim, E. Y. S., & Lim, S. L. (2011). The potential reuse of soybean husk as feedstock of Eudrilus eugeniae in vermicomposting. Journal of the Science of Food and Agriculture, 91(14), 2637–2642. https://doi.org/10.1002/jsfa.4504
Lim, P. N., Wu, T. Y., Clarke, C., & Nik Daud, N. N. (2015). A potential bioconversion of empty fruit bunches into organic fertilizer using Eudrilus eugeniae. International journal of Environmental Science and Technology, 12(8), 2533–2544. https://doi.org/10.1007/s13762-014-0648-2
Lim, S. L., Lee, L. H., & Wu, T. Y. (2016). Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. Journal of Cleaner Production, 111, 262–278. https://doi.org/10.1016/j.jclepro.2015.08.083
Markovac, J., & Goldstein, G. W. (1988). Picomolar concentrations of lead stimulate brain protein kinase C.
Mavroidis, C., & Dubey, A. (2003). Biomimetics: from pulses to motors. Nature Materials, 2(9), 573–574. https://doi.org/10.1038/nmat973
Mitchell, R., Chang, B., Huang, C., & De Master, E. (1971). Inhibition of mitochondrial energy-linked functions by arsenate. Evidence for a nonhydrolytic mode of inhibitor action. Biochemistry, 10(11), 2049–2054. https://doi.org/10.1021/bi00787a013
Nesci, S., Trombetti, F., Pirini, M., Ventrella, V., & Pagliarani, A. (2016). Mercury and protein thiols: stimulation of mitochondrial F1FO-ATPase and inhibition of respiration. Chemico-Biological Interactions, 260, 42–49. https://doi.org/10.1016/j.cbi.2016.10.018
Nowatari, T., Fukunaga, K., & Ohkohchi, N. (2012). Regulation of signal transduction and role of platelets in liver regeneration. International Journal of Hepatology, 2012, Article ID 542479, 8 pages, 1, DOI: https://doi.org/10.1155/2012/542479.
Owojori, O. J., Reinecke, A. J., & Rozanov, A. B. (2008). Effects of salinity on partitioning, uptake and toxicity of zinc in the earthworm Eiseniafetida. Soil Biology & Biochemistry, 40(9), 2385–2393. https://doi.org/10.1016/j.soilbio.2008.05.019
Owojori, O. J., Reinecke, A. J., & Rozanov, A. B. (2009). The combined stress effects of salinity and copper on the earthworm Eiseniafetida. Applied Soil Ecology, 41(3), 277–285. https://doi.org/10.1016/j.apsoil.2008.11.006
Pelicano, H., Feng, L., Zhou, Y., Carew, J. S., Hileman, E. O., Plunkett, W., Keating, M. J., & Huang, P. (2003). Inhibition of mitochondrial respiration a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. Journal of Biological Chemistry, 278(39), 37832–37839. https://doi.org/10.1074/jbc.M301546200
Reinecke, A., Reinecke, S., & Lambrechts, H. (1997). Uptake and toxicity of copper and zinc for the African earthworm, Eudriluseugeniae (Oligochaeta). Biology and Fertility of Soils, 24(1), 27–31. https://doi.org/10.1007/BF01420216
Roberts, B. L., & Dorough, H. W. (1985). Hazards of chemicals to earthworms. Environmental Toxicology and Chemistry, 4(3), 307–323. https://doi.org/10.1002/etc.5620040306
Robertson, R. P., Harmon, J., Tran, P. O., Tanaka, Y., & Takahashi, H. (2003). Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes, 52(3), 581–587. https://doi.org/10.2337/diabetes.52.3.581
Sadasivam, S., Manikam, A. (2008). Biochemical methods (3rd ed). New Delhi: New Age International (P) Ltd.
Schiller, C. M., Fowler, B. A., & Woods, J. S. (1977).Effects of arsenic on pyruvate dehydrogenase activation.Environmental health perspectives, 19, 205.
Shak, K. P. Y., Wu, T. Y., Lim, S. L., & Lee, C. A. (2014). Sustainable reuse of rice residues as feedstocks in vermicomposting for organic fertilizer production. Environmental Science and Pollution Research, 21(2), 1349–1359. https://doi.org/10.1007/s11356-013-1995-0
Sinha, R. K. (2009). Earthworms: the miracle of nature (Charles Darwin’s ‘unheralded soldiers of mankind & farmer’s friends’): Springer.
Steffenson, M. M., Formanowicz, D. R., & Brown, C. A. (2014). Autotomy and its effects on wolf spider foraging success. Ethology, 120(11), 1128–1136. https://doi.org/10.1111/eth.12288
US Environmental Protection Agency (EPA). Air quality criteria for lead. Research Triangle Park, NC, 1986 (Report EPA-600/8-83/028F). https: nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=20009CUO.TXT. Accessed 15 Dec 2017.
Vidal, D., & Horne, A. (2003). Mercury toxicity in the aquatic oligochaete Sparganophilus pearsei II: autotomy as a novel form of protection. Archives of Environmental Contamination and Toxicology, 45(4), 462–467. https://doi.org/10.1007/s00244-003-2119-5
Vliegenthart, A. D. B., Tucker, C. S., Pozo, J. D., & Dear, J. W. (2014). Zebrafish as model organisms for studying drug-induced liver injury. British Journal of Clinicl Pharmacology, 78(6), 1217–1227.
Weinberg, J., Harding, P., & Humes, H. (1982). Mitochondrial bioenergetics during the initiation of mercuric chloride-induced renal injury. I. Direct effects of in vitro mercuric chloride on renal mitochondrial function. Journal of Biological Chemistry, 257(1), 60–67.
World Health Organization (WHO) (1979). Sodium chlorides and conductivity in drinking water. EURO reports and studies.No. 2. Copenhagen, Denmark: WHO Regional Office for Europe.https://www.ircwash.org/resources/sodium-chlorides-and-conductivity-drinking- water-report-who-working-group-hague-1-5-may. Accessed 15 Dec 2017.
Wrinn, K. M., & Uetz, G. W. (2008). Effects of autotomy and regeneration on detection and capture of prey in a generalist predator. Behavioral Ecology, 19(6), 1282–1288. https://doi.org/10.1093/beheco/arn077
Wu, T. Y., Lim, S. L., Lim, P. N., & Shak, K. P. Y. (2014). Biotransformation of biodegradable solid wastes into organic fertilizers using composting or/and vermicomposting. Chemical Engineering Transactions, 39, 1579–1584.
Zhang, C., Willett, C., &Fremgen, T. (2003). Zebrafish: an animal model for toxicological studies. Current Protocols in Toxicology, 1.7.1–1.7. 18.
Acknowledgements
Authors thank Manonmaniam Sundaranar University, Tirunelveli, India for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 1508 kb)
Rights and permissions
About this article
Cite this article
Yesudhason, B.V., Kanniah, P., Subramanian, E.R. et al. Exploiting the unique phenotypes of the earthworm Eudrilus eugeniae to evaluate the toxicity of chemical substances. Environ Monit Assess 190, 145 (2018). https://doi.org/10.1007/s10661-018-6477-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6477-x