Abstract
Arsenic is a ubiquitous element which may be found in surface water, groundwater, and drinking water. In higher concentrations, this element is considered genotoxic and carcinogenic; thus, its level must be strictly controlled. We investigated the concentration of total arsenic and arsenic species: As(III), As(V), MMA, DMA, and AsB in drinking water, surface water, wastewater, and snow collected from the provinces of Wielkopolska, Kujawy-Pomerania, and Lower Silesia (Poland). The total arsenic was analyzed by inductively coupled plasma mass spectrometry (ICP-MS), and arsenic species were analyzed with use of high-performance liquid chromatography inductively coupled plasma mass spectrometry (HPLC/ICP-MS). Obtained results revealed that maximum total arsenic concentration determined in drinking water samples was equal to 1.01 μg L−1. The highest concentration of total arsenic in surface water, equal to 3778 μg L−1 was determined in Trująca Stream situated in the area affected by geogenic arsenic contamination. Total arsenic concentration in wastewater samples was comparable to those determined in drinking water samples. However, significantly higher arsenic concentration, equal to 83.1 ± 5.9 μg L−1, was found in a snow sample collected in Legnica. As(V) was present in all of the investigated samples, and in most of them, it was the sole species observed. However, in snow sample collected in Legnica, more than 97 % of the determined concentration, amounting to 81 ± 11 μg L−1, was in the form of As(III), the most toxic arsenic species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic is widely distributed in surface water, groundwater, and drinking water. Its concentration in different types of water varies considerably. In some cases, it significantly exceeds expected mean values for arsenic and maximum permissible arsenic concentration allowed for drinking water, indicating a degree of pollution (Fowler et al. 2007).
Arsenic pollution is a worldwide problem many scientists have repeatedly expressed concern about. As a result, the biological and environmental consequences of its contamination are being studied in detail. Although most researchers focus on the arsenic originating from the natural sources, human activities (such as smelting of arsenic bearing minerals, the disposal of industrial waste, or burning of fossil fuels) can locally introduce a very high contamination (Bissen and Frimmel 2003; Matschullat 2000).
Issue of great importance is presence of arsenic in groundwater used as a source of drinking water. In recent years, cases of arsenic pollution have been reported in many countries such as the USA, China, Bangladesh, Pakistan, Taiwan, Chile, Argentina, Japan, Turkey, Thailand, Mexico, Vietnam, and India (Simsek 2013; He and Charlet 2013; Sorg et al. 2014; Smedley and Kinniburgh 2002; Berg et al. 2001; Gammons et al. 2005, Brahman et al. 2013). Due to permanent excessive level of arsenic, some countries, including Bangladesh, Mexico, Vietnam, and India, have considerably raised its level in drinking water. It is presumed that around 40 million people in Bangladesh live at immediate risk due to arsenic pollution (Bissen and Frimmel 2003). An analysis of 20,000 tube-well waters indicated that arsenic levels in drinking water are above the maximum permissible concentration limit of 10 μg L−1 (WHO 2011) in case of 62 % of tube-well waters. In some places, its concentration was as high as 3700 μg L−1 (Bagla and Kaiser 1996). There are also many papers reporting similar arsenic concentration, reaching several thousand μg L−1 (Berg et al. 2001; Brahman et al. 2013; Smedley et al. 2002; Farnfield et al. 2012; Gammons et al. 2005; Aiuppa et al. 2003; Bednar et al. 2004). It has been estimated that as many as 60–100 million people globally may be at risk of exposure to excessive levels of arsenic (Ng et al. 2003). Since arsenic contamination provides such a huge problem in many places in the world, it is worth to mention that various methods of its elimination from the aqueous environments are available. Besides conventional remediation techniques such as osmosis, ion exchange, or electrodialysis, for removing metal or metalloids from water, biosorption is characterized by the significant development in recent years [Basu et al. 2014; Abid et al. 2016]. One of the greatest advantages of this process is material of biosorbent, which is easy and commonly available, and inexpensive. A lot of different agricultural and food-industry biowastes such as coconut shell, coconut coir pith, mango leaf, rice polish, or tea waste have been examined as potential biosorbents for As-contaminated water. These various approaches are widely discussed in the paper of Shakoor et al. (2016). One of the most recent paper concerns As(V) biosorption with application of food processing biowastes, more specifically orange peel biowaste. This research revealed that with use of charged orange peel, it was possible to remove 98 % of As(V) from the solution containing 200 mg L−1 of As(V) using 4 g L−1 of biosorbent [Abid et al. 2016].
In most aquifers, bio-geo interactions probably dominate as the source of arsenic. Interaction of arsenic with organic and mineral colloids can elevate its concentration (He and Charlet 2013). Arsenic can exist in water in several organic and inorganic forms. Speciation of arsenic depends on pH, salinity, acid dissociation constant at logarithmic scale (pKa), and redox potential (Sugár et al. 2013). The toxicity of arsenic compounds decreases in the following order: arsines > inorganic arsenites > organic trivalent compounds (arsenooxides) > inorganic arsenates > organic pentavalent compounds > arsonium compounds > elemental arsenic (Adriano 2001; Fowler 1983; Mandal and Suzuki 2002). Arsenobetaine and arsenocholine are considered as nontoxic (Komorowicz and Barałkiewicz 2011).
It is well known that toxicological and environmental impact of arsenic strictly depends on the chemical form of this element (Cornelis et al. 2005; Brahman et al. 2013). Although there is a vast amount of information on the occurrence and concentration of total arsenic in different types of water, the data on the speciation of arsenic are limited (Sorg et al. 2014; Haque and Johannesson 2006). The shortage of information on the speciation of arsenic may be partially attributed to the complexity, cost, and time needed to perform arsenic speciation analyses (Sorg et al. 2014). Nevertheless, it is essential to develop sensitive and precise analytical procedures to identify and quantify arsenic species in water (Baig et al. 2010; Hirata and Toshimitsu 2005).
The USEPA has elaborated a document reviewing the science and technologies for monitoring arsenic in the environment (USEPA 2004). The methods approved by USEPA include inductively coupled plasma-mass spectrometry (ICP-MS), inductively coupled plasma atomic emission spectrometry (ICP-AES), graphite furnace atomic absorption (GFAAS), and hydride generation atomic absorption spectrometry (HGAAS), all of which may be characterized by method detection limits (MDL) ranging from 0.5 to 50 μg L−1 (USEPA 1999; Ma et al. 2014). The choice of an adequate analytical method is dictated by the purpose of our analysis, the level of analyte’s concentration in concrete matrix, and the type of compound in which analyte is present. The most often applied strategy is connection of HPLC separation with ICP-MS detection (Komorowicz and Baralkiewicz 2014; Bednar et al. 2004; Shraim et al. 2002; Deng et al. 2009; Jabłońska-Czapla et al. 2014) or AFS detection (Yu et al. 2014; Gong et al. 2006; Keller et al. 2014; Farias et al. 2015). Coupling of HG system to AAS (Berg et al. 2001; Affum et al. 2015); ICP-MS (Musil et al. 2014), ASF (Aiuppa et al. 2003; Deng et al. 2009; Musil et al. 2014), or ICP-AES (Smedley et al. 2002) is also quite popular. There are either reports about application of SPE system used for separation purposes before detection by WDXRF, ICP-MS, DLLME-SFO, GF-AAS, and ETAAS (Brahman et al. 2013; Baig et al. 2010; Shamsipur et al. 2014; Peng et al. 2015; Hagiwara et al. 2015).
Although a variety of analytical techniques have been already applied for arsenic species determination, as mentioned, HPLC separation which is followed by ICP-MS or HG-AFS undeniably belong to the most often used hyphenated techniques. ICP-MS technique is most commonly applied for speciation analysis, and this tendency is expected to continue to grow as the required instrumentation becomes more widely available. Said method provides reliable quantitative data for arsenic species at environmentally relevant levels in diverse matrices (Ma et al. 2014) as well as other significant advantages. These include the excellent separating power of HPLC, associated with a high degree of element specificity and very low detection limits of the ICP-MS technique. HPLC/ICP-MS can detect arsenic in inorganic and organic forms with high precision. Additionally, this system is totally integrated and automated (Liévremont et al. 2009; Tomlinson et al. 1995).
The objective of this study was to determine total arsenic and arsenic species concentration in drinking water, surface water, wastewater, and snow samples collected from Wielkopolska, Kujawy-Pomerania, and Lower Silesia provinces located in Poland. The last mentioned province is the As-affected area known for geogenic contamination with this element.
Experimental
Sampling sites
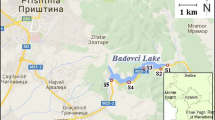
Samples were collected in the area of Poland from provinces of Wielkopolska, Kujawy-Pomerania, and Lower Silesia. Sample collection locations are presented in detail on the sampling map of study area (Fig. 1). One sample at each sampling point was collected. In total, 23 samples of drinking water, surface water, wastewater, and snow were submitted to analysis, three replicate measurements of each were made.
Drinking water and wastewater
Drinking water and wastewater samples were collected from places located in three different provinces of Poland: Wielkopolska, Kujawy-Pomerania, and Lower Silesia. Samples were collected at points of water intake, from water treatment stations or municipal sewage treatment plants.
Surface water
Surface water samples were collected from Lower Silesia province, which is the region definitely much richer in arsenic than Wielkopolska or Kujawy-Pomerania. Samples were taken from the Kaczawa River and the Trująca Stream from few different locations.
Kaczawa River
Kaczawa is the left-bank tributary of Odra River. Water supply point of drinking water for the city of Legnica is situated on the Kaczawa River, in Smokowice. Upper course of the Kaczawa River flows through farmlands; its lower and middle course flows through industrially-agricultural areas, which makes the river exposed to impurities flowing from the farmlands and cultivated fields. Supplies of the Kaczawa River may also be a great source of contamination (RRIEP 2006).
Trująca Stream
The Nysa Kłodzka River is the largest river of the Kłodzko Basin, and it starts in the Lower Silesia province. Nysa Kłodzka River enters Lower Silesia province below a confluence point with an outlet of the Trująca Stream and above the Otmuchowski Reservoir. The Nysa Kłodzka River in its upper course flows through mountainous areas, functioning mainly as a tourist area. The river and its supplies collect the water from nature preservation areas as well as from farm areas. There are industrial plants working in the river catchment area (RRIEP 2006).
Snow
Snow samples were collected in the city of Legnica situated in the Lower Silesia province and in the city of Poznań situated in the Wielkopolska province. The Lower Silesia province is an area recognized as significantly richer in arsenic in comparison to other provinces. During its history, a gold mine was operating in Złoty Stok City (Lower Silesia). Apart from gold mining, mine also produced an arsenic trioxide from the local ore (www.kopalniazlota.pl/pl/historia/historia-kopalni-zlota). Aforementioned sample was collected near the copper smelter and the refinery located near city of Legnica. Second sample of snow, collected from the center of Poznań, may be treated as a control sample.
Sample collection and preparation.
All samples were collected in polyethylene bottles previously cleaned with metal-free detergent, rinsed with deionized water, then soaked in 5 % nitric acid for 24 h and finally rinsed once again with deionized water. After collection, samples were promptly transported in a cool-box to the laboratory. Total arsenic and arsenic species determination were followed by water quality parameter’s investigation (pH, conductivity, COD, BOD, TOC) and determination of major inorganic ions (Ca 2+, Mg 2+, Na +, K +, Cl −, \( S{O}_4^{2-} \), \( P{O}_4^{3-} \), \( N{O}_3^{-} \)) concentration. Results regarding water quality parameters and basic anions and cations were partially provided by water treatment stations or municipal sewage treatment plants, from where some of the samples had been collected. For measurements aiming at the determination of total arsenic concentration, samples were acidified with 125 μL of nitric acid (suprapur nitric acid of 65 % (v/v)) on each 125 mL of sample. However, for arsenic speciation analysis samples were immediately frozen and thawed just before the analysis. Samples were then filtered through syringe filters with a pore size of 0.45 μm. The snow samples (Lower Silesia province and Wielkopolska province) were collected to the polyethylene bottles with use of polyethylene spatula. They were taken from the surface and 2 cm deep into the snow layer, so not only the dark layer of the atmospheric dust but also clean snow underneath was collected. After the sample melted, the water which arose from the melting snow was sampled.
Instrumentation
ELAN DRC II ICP-MS (PerkinElmerSCIEX, Ontario, Canada) was used for total arsenic determination as well as for arsenic species determination. In case of speciation analysis, ICP-MS was hyphenated with HPLC system. To collect the data, ions of arsenic at mass to charge ratio (m/z +) 75 were monitored. HPLC system consisted of a Perkin Elmer Series 200 HPLC Pump, Perkin Elmer Series 225 HPLC Autosampler and a Perkin Elmer Series 200 Column Oven. Autosampler was equipped with a Peltier Cooling Tray in order to keep the sample temperature equal to 4 °C. The anion exchange HPLC column PRP-X100 (4.6 × 150 mm) was used for arsenic species separation. The column was packed with 5 μm particles of styrene divinylbenzene copolymer with trimethylammonium exchange sites in PEEK hardware (Hamilton Company, Bonaduz, Switzerland). Applied column enabled to separate five arsenic species in 7 min using optimized HPLC conditions. Arsenic species eluted in the following order: AsB, As(III), DMA, MMA, and As(V).
The outlet of the HPLC column was connected via a switch, directly to the nebulizer of the ICP mass spectrometer. The data were collected using Chromera software (PerkinElmerSCIEX, Ontario, Canada). Operating conditions and optimal values of HPLC and ICP-MS parameters are presented in Table 1.
Total arsenic concentration was determined according to ISO 17294–2 (2003) standard; however, concentration of arsenic species was determined according to methodology developed and fully validated in our laboratory (Komorowicz and Baralkiewicz 2014).
Reagents
Chemicals and reagents used for total arsenic solutions and arsenic species (inorganic: arsenite (As(III)), and arsenate (As(V)); organic: arsenobetaine (AsB), monomethylarsenic acid (MMA), and dimethylarsenic acid (DMA)) solutions preparation, salts used for mobile phase preparation, buffer solution, reagents used for pH adjustment, as well as other chemicals used throughout the experiment, were described in details in the previously published work (Komorowicz and Baralkiewicz 2014). Particular steps of both analytical procedures—for total arsenic determination and for arsenic species determination—are presented on the Fig. 2 in the form of diagram.
Figures of merit
A lot of information in the scientific field is made on the basis of analytical measurements. Ensuring that obtained analytical results are reliable is extremely important therefore the process of quality control is a significant step of each analytical procedure (Konieczka and Namieśnik 2010). In our work, the analytical procedures, for total arsenic determination by ICP-MS technique and arsenic species determination by HPLC/ICP-MS technique, were characterized by the following parameters: selectivity, linearity, limit of detection, limit of quantification, precision, and trueness. Traceability was assured by analysis of certified reference material and analysis of spiked samples, for total arsenic and arsenic species, respectively. Measurement uncertainty of the analytical result for total arsenic and for each arsenic species was assessed by the modeling approach according to the Guide to the Expression of Uncertainty of Measurement (GUM 1993; Konieczka and Namieśnik 2010). Validation parameters together with uncertainty for total arsenic and arsenic species are presented in Table 2.
Total arsenic
Total arsenic concentration was determined with ICP-MS technique according to validated methodology described in ISO 17294–2 (2003) standard. The calibration curve for total arsenic was constructed at a concentration range of 0.2–20.0 μg L−1. In order to verify the linearity of calibration curves, approach based on drawing a graph of constant response for y/x values (where y denotes the instrumental signal and x is the concentration of analytes in the standard solution) with acceptable deviation within ±5 % was applied. The chosen operating range for each of the calibration curves was statistically verified by checking the homogeneity of variances using Snedecor’s F test. Precision calculated by analysis of CRM of river water SLRS-5 (National Research Council Canada, Ontario, Canada) amounted to 1.7 % (n = 10). The traceability for the total arsenic measurement was assessed by determining the % bias between the measured concentration (n = 10) and certified value of the mentioned certified reference material. Obtained value was equal to 0.4176 ± 0.0071 μg L−1; however, certified value amounted to 0.413 ± 0.039 μg L−1. Calculated recovery was equal to 101.1 ± 1.7 %, which confirms that sample matrix influence is negligible. Combined uncertainty of total arsenic was estimated taking into consideration the following sources of uncertainty: measurement repeatability, calibration, and recovery. Expanded uncertainty (U [%], k = 2) estimated for total arsenic using “bottom-up” approach was equal to 7.1 %. It may be applied to the concentration range of 0.2–20.0 μg L−1.
Arsenic species
Arsenic species concentration was determined by commercially available HPLC/ICP-MS technique according to developed and fully validated in our laboratory methodology described previously (ISO/IEC 2005; Komorowicz and Baralkiewicz 2014). “In-house” traceability defined as trueness was assured by analysis of spiked samples. Uncertainty budget for each arsenic species was estimated according to the bottom-up approach (GUM 1993).
Results and discussion
Total arsenic concentration in water media of Poland
Table 3 presents the concentration of TAs and speciated arsenic in drinking water, surface water, wastewater, and snow in provinces of Wielkopolska, Kujawy-Pomerania, and Lower Silesia. Results obtained for drinking water samples confirmed that total arsenic level was significantly lower than 10 μg L−1 which is the maximum permissible concentration of arsenic in drinking water, according to guidelines of the World Health Organization (WHO 2011), the US Environmental Protection Agency (USEPA 2009) and European Union (Official Journal of the EU 1998). In the majority of the drinking water samples, collected from regions of Wielkopolska, Kujawy-Pomerania, as well as from the area of Lower Silesia, concentration of total arsenic was significantly below 1 μg L−1. The highest concentration of total arsenic was found in a sample collected in Wałbrzych—0.249 ± 0.018 μg L−1.
Surface water samples were collected from the Kaczawa River and the Trująca Stream situated in the Lower Silesia province. Samples from the Kaczawa River were taken at three different points (from Wojcieszów Górny; above Świerzawa City; from water intake for Legnica city). Obtained results of total arsenic concentration for mentioned samples ranged from 0.928 ± 0.066 to 2.84 ± 0.20 μg L−1. However, total arsenic concentration in Trująca Stream was found to be much higher than these determined in Kaczawa River. In this case, samples were collected at two points. The first sample was collected from the upper reaches of the Trująca Stream, just above the Złoty Stok City. Second sample was taken close to the bridge located on the Błotnica-Topola road, at the close of a uniform water body. Obtained results were as follows: 10.94 ± 0.78 and 3778 ± 268 μg L−1. Such a high concentration of total arsenic in the second sample from Trująca Stream must be caused by the local geochemical structure, which includes deposits of arsenic. Złote Góry massif, within Trująca Stream is located, is mostly composed of mica slates of Proterozoic era relatively of the Lower Cambrian. Among them, there are slots of crystalline dolomite limestone with arsenic ores. In this area, the contact deposit with intrusive-hydrothermal character appears. Changes of dolomitic limestone in fine-grained diopsidic rocks through supplying the silica occurred during neighboring syenite intrusion. Afterward, diopsidic arsenic and gold rocks were introduced by hydrothermal silica. This process occurred fundamentally in contact rocks (http://zlotystok.salwach.pl/przemysl/wydobycie_rud_arsenu). Deposits of arsenic were primarily exploited in the area of Złoty Stok. While exploitation of arsenic ores, another deposit was found—gold. Gold, associated with the occurrence of arsenic ore, was most likely discovered in the seventh century, and the oldest record of mining operations conducted here from the thirteenth century. It is thus the oldest gold mine in Poland, where the exploitation of ore arsenic and recovery of gold continued until 1962 (http://www.kopalniazlota.pl/pl/historia/historia-wydobycia-zlota). According to opinion placed in the report about quality of rivers of Lower Silesia province prepared by Regional Inspectorate of Environmental Protection in Wrocław, the wastewater from mechanical-biological sewage treatment plant in Złoty Stok City, as well as from mechanical-biological sewage treatment plant of Material and Paint Plant is released to the Trująca Stream (RRIEP 2006, RIEPW 2001). However, in the plants mentioned above the wastewater comes under many processes and after sedimentation of deposit in the sedimentation tank refined wastewater is received. Obtained wastewater has reduced content of contaminants by approximately 90 to 96 %, and in this form, it gets to the channel which drains it to the Trująca Stream. Hence, the wastewater introduced to the Trująca Stream could not have considerable impact on increase of arsenic concentration in its water, so high concentrations of arsenic in this region are due to its geogenic characteristics.
Total arsenic concentration in the wastewater samples collected in the Wielkopolska and Kujawy-Pomerania was comparable with results obtained for drinking water samples. The highest concentration was determined in a sample collected from the wastewater treatment plant in Koziegłowy in which concentration of this element was equal to 1.82 ± 0.13 μg L−1.
Snow samples from two provinces were submitted to analysis. The first one was collected in the city of Legnica situated in Lower Silesia province. Sampling point was situated near the copper smelter and the refinery. Sample was characterized by a dark layer of atmospheric dust localized on its surface. Analysis revealed high concentration of arsenic equal to 83.1 ± 5.9 μg L−1. Almost all of arsenic in mentioned sample (97 %) was in the form of As(III), the most toxic arsenic species. Such a high concentration of arsenic could only come from particulate matter suspended in the air in Legnica, where, according to the report of the Regional Inspectorate for Environmental Protection in Wroclaw (RRIEP 2011), total arsenic concentration is the highest in comparison to other cities of Lower Silesia. High annual concentration of arsenic (5.9 ng m−3) but still placing within the permissible contamination level was observed only in Legnica. However, further potential increase only by 2 % would have caused exceeding permissible contamination level of arsenic (RRIEP 2011). At the same time, the second snow sample was collected in the centre of Poznań (the largest city in Wielkopolska province). Appearance of this sample was very similar to that collected in Legnica, its surface was also covered by dark atmospheric dust. However, the concentration of arsenic in the sample was below the limit of detection. Total arsenic concentration in all described samples is shown in Fig. 3.
Arsenic species concentration in water media of Poland
Samples dedicated to speciation analysis were analyzed directly; however, in the majority of samples, concentration of some species was below the limit of detection. We calculated the recovery values for each arsenic species by analysis of spiked samples due to lack of certified reference materials having certified concentration of arsenic species. Spike concentrations as well as the results of AsB, As(III), DMA, MMA, and As(V) determination in drinking water, surface water, wastewater, and snow are presented in Table 3 as an average of three measurements together with the expanded uncertainty.
From among species of our interest, only As(V) was present in all of the investigated samples. In more than 60 % of samples, As(V) was the only one occurring arsenic species. In two of the investigated samples, drinking water and snow ones collected in Wielkopolska, arsenic was not detected. In 35 % of samples, we found As(III), the most toxic arsenic species. The organic compound, MMA, was present in only one of all the investigated samples. Other organic compounds such as DMA and AsB were not detected in any sample, which may indicate a lack of the organic compounds responsible for methylation of inorganic species to organic forms.
Total and speciated arsenic concentration in the world
Table 4 demonstrates the results obtained for total arsenic and arsenic species in aqueous samples collected from many different regions in the world as well as the results obtained in this study. Besides concentration of arsenic and its species which is the crucial information, many other important issues such as sample sites and matrix, geogenic characteristics, sample pretreatment, and analytical techniques used are gathered in mentioned table. Majority of worldwide reports concerns groundwater samples as it often constitutes the primary source of drinking water. There are a lot of countries, such as: Vietnam, Pakistan, China, Argentina, Italy, India, Bangladesh, and Mongolia where reported concentration of arsenic amounts to even more than thousands of μg L−1 which is alarming (Berg et al. 2001; Brahman et al. 2013; Shakoor et al. 2015; Guo and Wang 2005; Smedley et al. 2002; Aiuppa et al. 2003; Rahman et al. 2015; Gong et al. 2006). Results regarding drinking water (Musil et al. 2014) are similar to these obtained in this work, significantly lower than 10 μg L−1 which is the maximum permissible concentration of arsenic in drinking water. In case of surface water samples, different levels of arsenic may be distinguished, which is also very similar to the results obtained by us. Starting from relatively low values not exceeding 20 μg L−1 (Baig et al. 2010) through hundreds of μg L−1 (Torrance et al. 2012) to these extremely high, determined exemplary in Argentina (up to 4780 μg L−1) (Gammons et al. 2005; Farnfield et al. 2012). The highest values are at the same level as result obtained by authors for surface water collected from Trująca Stream (up to 3778 μg L−1).
Conclusion
This study demonstrates the results of determination of total arsenic and arsenic species concentration in different types of water: drinking water, wastewater, surface water, and snow collected in Poland. The results point the following:
-
Concentration of total arsenic in examined drinking water samples was in the range of 0.14–1.01 μg L−1. In all investigated samples, As(V) was the dominant form, in the majority, the only one occurring.
-
High concentration of arsenic, up to 3778 μg L−1, was determined in surface water of the Trująca Stream. However, in samples collected from the Kaczawa River, situated in the same province as Trująca Stream, total arsenic concentration was in the range of 0.93–2.84 μg L−1. As(V) predominated, however As(III) was also present.
-
Concentration of total arsenic in wastewater samples was comparable with those determined in drinking water samples and ranged from 0.10 to 1.82 μg L−1.
-
Total arsenic concentration determined in a snow sample collected in Legnica was equal to 83.1 ± 5.9 μg L−1. More than 97 % of its concentration was confirmed to be As(III), the most toxic arsenic species. This high concentration was confirmed by the corresponding reports concerning air quality in Lower Silesia province.
Information relating total arsenic and its species concentration in different types of water samples collected in the area of Poland will help to broaden the knowledge in this field in the world, especially results regarding Trująca Stream which indicated high arsenic concentration caused by the local geochemical structure, which includes deposits of arsenic.
Abbreviations
- As(III):
-
Arsenite
- As(V):
-
Arsenate
- AsB:
-
Arsenobetaine
- CPE:
-
Cloud point extraction
- CRM:
-
Certified reference material
- DDTP:
-
Diethyldithiphosphate
- DMA:
-
Dimethylarsenic acid (dimethylarsinate)
- EDTA:
-
Ethylenediaminetetraacetic acid
- ETAAS:
-
Electrothermal atomic absorption spectrometry
- FI-HG-AAS:
-
Flow injection-hydride generation atomic absorption spectrometry
- FI-ICPMS:
-
Flow injection inductively coupled plasma mass spectrometry
- GF-AAS:
-
Graphite furnace atomic absorption spectrometry
- HG-AAS:
-
Hydride generation atomic absorption spectrometry
- HG-AFS:
-
Hydride generation atomic fluorescence spectrometry
- HG-CT-AFS:
-
Hydride generation-cryotrapping coupled to atomic fluorescence spectrometry
- HG-CT-ICPMS:
-
Hydride generation-cryotrapping coupled to inductively coupled plasma mass spectrometry
- HG-ICP-AES:
-
Hydride generation inductively coupled plasma atomic/optical emission spectrometry
- HPLC:
-
High-performance liquid chromatography
- HPLC/ICP-MS:
-
High-performance liquid chromatography inductively coupled plasma mass spectrometry
- iAs:
-
Inorganic arsenic
- ICP-AES:
-
Inductively coupled plasma atomic emission spectrometry
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- ICP-SF-MS:
-
Inductively coupled plasma sector field mass spectrometry
- INAA:
-
Instrumental neutron activation analysis
- LC:
-
Liquid chromatography
- LC-HGAFS:
-
Liquid chromatography hydride generation atomic fluorescence spectrometry
- MDL:
-
Method detection limit
- MMA:
-
Monomethylarsenic acid (methylarsonate)
- n.d.:
-
Not detected
- ORS:
-
Octopole reaction system
- PAs:
-
Particulate arsenic
- SPE:
-
Solid-phase extraction
- SPE-DLLME-SFO:
-
Solid-phase extraction coupled with dispersive liquid–liquid microextraction based on the solidification of floating organic drop
- TAs:
-
Total arsenic
- TMAO:
-
Trimethylarsine oxide
- USEPA:
-
US Environmental Protection Agency
- WDXRF:
-
Wavelength-dispersive X-ray fluorescence spectrometry
- WHO:
-
World Health Organization
References
Abid, M., Niazi, N. K., Bibi, I., Farooqi, A., Ok, Y. S., Kunhikrishnan, A., ad Ali, F., Ali, S., Igalavithana, A. D., & Arshad, M. (2016). Arsenic(V) biosorption by charred orange peel in aqueous environments. International Journal of Phytoremediation, 18, 442–449.
Acharya, R., Nair, A. G. C., & Reddy, A. V. R. (2009). Speciation and instrumental neutronactivationanalysis for arsenic in water samples. Journal of Radioanalytical and Nuclear Chemistry, 281, 279–282.
Adriano, D. C. (2001). Metals in the terrestrial environment. New York: Springer.
Affum, A. B., Osae, S. D., Nyarko, B. J. B., Afful, S., Fianco, J. R., Akiti, T. T., Adomako, D., Acquaah, S. O., Dorleku, M., Antoh, E., Barnes, F., & Affum, E. A. (2015). Total coliforms, arsenic and cadmium exposure through drinking water in the Western Region of Ghana: application of multivariate statistical technique to groundwater quality. Environmental Monitoring and Assessment, 187, 1–23.
Aiuppa, A., D’Alessandro, W., Federico, C., Palumbo, B., & Valenzia, M. (2003). The aquatic geochemistry of arsenic in volcanic groundwaters from southern Italy. Applied Geochemistry, 18, 1283–1296.
Bagla, P., & Kaiser, J. (1996). India’s spreading health crisis draws global arsenic experts. Science, 11, 174–175.
Baig, J. A., Kazi, T. G., Shah, A. Q., Kandhro, G. A., Afridi, H. I., Arain, M. B., Jamali, M. K., & Jalbani, N. (2010). Speciation and evaluation of arsenic in surface and ground water: a multivariate case study. Ecotoxicology and Environmental Safety, 73, 914–923.
Basu, A., Saha, D., Saha, R., Ghosh, T., & Saha, B. (2014). A review on sources, toxicity and remediation technologies for removing arsenic from drinking water. Research on Chemical Intermediates, 40, 447–485.
Bednar, A. J., Garbarino, J. R., Burkhardt, M. R., Ranville, J. F., & Wildeman, T. R. (2004). Field and laboratory arsenic speciation methods and their application to natural-water analysis. Water Research, 38, 355–364.
Berg, M., Tran, H. C., Nguyen, T. C., Pham, H. V., Schertenleib, R., & Giger, W. (2001). Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat. Environmental Science & Technology, 35, 2621–2626.
Bissen, M., & Frimmel, F. H. (2003). Arsenic—a review. Part I: occurrence, toxicity, speciation, mobility. Acta Hydrochimica et Hydrobiologica, 31, 9–18.
Brahman, K. D., Kazi, T. G., Afridi, H. I., Naseem, S., Arain, S. S., & Ullah, N. (2013). Evaluation of high levels of fluoride, arsenic species and other physicochemical parameters in underground water of two sub districts of Tharparkar, Pakistan: a multivariate study. Water Research, 47, 1005–1020.
Chakraborti, D., Rahman, M. M., Ahamed, S., Dutta, R. N., Pati, S., & Mukherjee, S. C. (2016a). Arsenic contamination of groundwater and its induced health effects in Shahpur block, Bhojpur district, Bihar state, India: risk evaluation. Environmental Science and Pollution Research. doi:10.1007/s11356-016-6149-8.
Chakraborti, D., Rahman, M. M., Ahamed, S., Dutta, R. N., Pati, S., & Mukherjee, S. C. (2016b). Arsenic ground water contamination and its health effects in Patna district (capital of Bihar) in the middle Ganga plain, India. Chemosphere, 152, 520–529.
Cornelis, L., Crews, H., Caruso, J., & Heumann, K. G. (2005). Handbook of elemental speciation II: species in the environment, food, medicine & occupational health. New York: Wiley.
Deng, Y., Wang, Y., Ma, T., & Gan, Y. (2009). Speciation and enrichment of arsenic in strongly reducing shallow aquifers at western Hetao Plain, northern China. Environmental Geology, 56, 1467–1477.
Farías, S. S., Londonio, A., Quintero, C., Befali, R., Soro, M., & Smichowski, P. (2015). On-line speciation and quantification of four arsenical species in rice samples collected in Argentina using a HPLC-HG-AFS coupling. Microchemical Journal, 120, 34–39.
Farnfield, H. R., Marcilla, A. L., & Ward, N. I. (2012). Arsenic speciation and trace element analysis of the volcanic río Agrio and the geothermal waters of Copahue, Argentina. The Science of the Total Environment, 433, 317–378.
Fowler, B. A. (1983). Biological and environmental effects of arsenic. New York: Elsevier.
Fowler, B. A., Chou, C. S. J., Jones, R. L., & Chen, C.-J. (2007). Handbook of the toxicology of metals (3rd ed.). Burlington: Elsevier.
Gammons, C. H., Wood, S. A., Pedrozo, F., Varekamp, J. C., Nelson, B. J., Shope, C. L., & Baffico, G. (2005). Hydrogeochemistry and rare earth element behavior in a volcanically acidified watershed in Patagonia, Argentina. Chemical Geology, 222, 249–267.
Gong, Z., Lu, X., Watt, C., Wen, B., He, B., Mumford, J., Ning, Z., Xia, Y., & Le, C. (2006). Speciation analysis of arsenic in groundwater from Inner Mongolia with an emphasis on acid-leachable particulate arsenic. Analytica Chimica Acta, 555, 181–187.
GUM (1993). Guide to the expression of uncertainty in measurement. Geneva: International Organization for Standardization.
Guo, H., & Wang, Y. (2005). Geochemical characteristics of shallow groundwater in Datong basin, northwestern China. Journal of Geochemical Exploration, 87, 109–120.
Hagiwara, K., Inui, T., Koike, Y., Aizawa, M., & Nakamura, T. (2015). Speciation of inorganic arsenic in drinking water by wavelength-dispersive X-ray fluorescence spectrometry after in situ preconcentration with miniature solid-phase extraction disks. Talanta, 134, 739–744.
Haque, S., & Johannesson, K. H. (2006). Arsenic concentrations and speciation along a groundwater flow path: the Carrizo sand aquifer, Texas, USA. Chemical Geology, 228, 57–71.
He, J., & Charlet, L. (2013). A review of arsenic presence in China drinking water. Journal of Hydrology, 492, 79–88.
Hirata, S., & Toshimitsu, H. (2005). Determination of arsenic species and arsenosugars in marine samples by HPLC-ICP-MS. Analytical and Bioanalytical Chemistry, 383, 454–460 http://kopalniazlota.pl/index.php/en/article/20 - History of Gold Mine in Złoty Stok City (in Polish). http://www.kopalniazlota.pl/pl/historia/historia-wydobycia-zlota - History of Gold Mine in Złoty Stok City (in Polish). http://zlotystok.salwach.pl/przemysl/wydobycie_rud_arsenu - Jerzy Tichanowicz. Arsenic ore mine in Złoty Stok City in Polish).
ISO 17294–2 (2003). Water quality. Application of inductively coupled plasma mass spectrometry (ICP-MS). Part 2: Determination of 62 elements.
ISO/IEC 17025:2005 (2005). General requirements for the competence of testing and calibration laboratories.
Jabłońska-Czapla, M., Szopa, S., Grygoyć, K., Łyko, A., & Michalski, R. (2014). Development and validation of HPLC-ICP-MS method for the determination inorganic Cr, As and Sb speciation forms and its application for Pławniowice reservoir (Poland) water and bottom sediments variability study. Talanta, 120, 475–483.
Keller, N. S., Stefánsson, A., & Sigfússon, B. (2014). Determination of arsenic speciation in sulfidic waters by ion chromatography hydride-generation atomic fluorescence spectrometry (IC-HG-AFS). Talanta, 128, 466–472.
Komorowicz, I., & Barałkiewicz, D. (2011). Arsenic and its speciation in water samples by high performance liquid chromatography inductively coupled plasma mass spectrometry—last decade review. Talanta, 84, 247–261.
Komorowicz, I., & Baralkiewicz, D. (2014). Arsenic speciation in water by high-performance liquid chromatography/ inductively coupled plasma mass spectrometry-method validation and uncertainty estimation. Rapid Communications in Mass Spectrometry, 28, 159–168.
Konieczka, P., & Namieśnik, J. (2010). Estimating uncertainty in analytical procedures based on chromatographic techniques. Journal of Chromatography A, 1217, 882–891.
Liévremont, D., Bertin, P. N., & Lett, M. C. (2009). Arsenic in contaminated waters: biogeochemical cycle, microbial metabolism and biotreatment processes. Biochimie, 91, 1229–1237.
Ma, J., Sengupta, M. K., Yuan, D., & Dasgupta, P. K. (2014). Speciation and detection of arsenic in aqueous samples: a review of recent progress in non-atomic spectrometric methods. Analytica Chimica Acta, 831, 1–23.
Mandal, B. K., & Suzuki, K. T. (2002). Arsenic round the world: a review. Talanta, 58, 201–235.
Matschullat, J. (2000). Arsenic in the geosphere—a review. Science of the Total Environment, 249, 297–312.
Musil, S., Matoušek, T., Currier, J. M., Styblo, M., & Dědina, J. (2014). Speciation analysis of arsenic by selective hydride generation-cryotrapping-atomic fluorescence spectrometry with flame-in-gas-shield atomizer: achieving extremely low detection limits with inexpensive instrumentation. Analytical Chemistry, 86, 10422–10428.
Ng, J. C., Wang, J., & Shraim, A. A. (2003). A global health problem caused by arsenic from natural sources. Chemosphere, 52, 1353–1359.
Official Journal of the European Union, Commission Directive 1998/83/EC, (1998). http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:330:0032:0054:EN:PDF.
Peng, H., Zhang, N., He, M., Chen, B., & Hu, B. (2015). Simultaneous speciation analysis of inorganic arsenic, chromium and selenium in environmental waters by 3-(2-aminoethylamino)propyltrimethoxysilane modified multi-wall carbon nanotubes packed microcolumn solid phase extraction and ICP-MS. Talanta, 131, 266–272.
Rahman, M. M., Dong, Z., & Naidu, R. (2015). Concentrations of arsenic and other elements in groundwater of Bangladesh and West Bengal, India: potential cancer risk. Chemosphere, 139, 54–64.
RRIEPW - Report of Regional Inspectorate for Environment Protection in Wrocław (2006). Evaluation of the quality of rivers in Lower Silesia province in the year 2005 (in Polish).
RRIEPW - Report of Regional Inspectorate for Environment Protection in Wrocław (2011). Evaluation of the air quality in Lower Silesia province in the year 2010 (in Polish).
RIEPW - Regional Inspectorate for Environment Protection in Wrocław (2001) Report about environment condition of Lower Silesia province in the year 2001 (in Polish).
Shakoor, M. B., Niazi, N. K., Bibi, I., Rahman, M. M., Naidu, R., Dong, Z., Shahid, M., & Arshad, M. (2015). Unraveling health risk and speciation of arsenic from groundwater in rural areas of Punjab, Pakistan. International Journal of Environmental Research and Public Health, 12, 12371–12390.
Shakoor, M. B., Niazi, N. K., Bibi, I., Murtaza, G., Kunhikrishnan, A., Seshadri, B., Shahid, M., Ali, S., Bolan, N. S., Ok, Y. S., Abid, M., & Ali, F. (2016). Remediation of arsenic-contaminated water using agricultural wastes as biosorbents. Critical Reviews in Environmental Science and Technology, 46, 467–499.
Shamsipur, M., Fattahi, N., Assadi, Y., Sadeghi, M., & Sharafi, K. (2014). Speciation of As(III) and As(V) in water samples by graphite furnace atomic absorption spectrometry after solid phase extraction combined with dispersive liquid-liquid microextraction based on the solidification of floating organic drop. Talanta, 130, 26–32.
Shraim, A., Sekaran, N. C., Anuradha, C. D., & Hirano, S. (2002). Speciation of arsenic in tube-well water samples collected from West Bengal, India, by high-performance liquid chromatography-inductively coupled plasma mass spectrometry. Applied Organometallic Chemistry, 16, 202–209.
Simsek, C. (2013). Assessment of naturally occurring arsenic contamination in the groundwater of Sarkisla Plain (Sivas/Turkey). Environment and Earth Science, 68, 691–702.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Smedley, P. L., Nicolli, H. B., MacDonald, D. M. J., Barros, A. J., & Tullio, J. O. (2002). Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Applied Geochemistry, 17, 259–284.
Sorg, T. J., Chen, A. S. C., & Wang, L. (2014). Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Research, 48, 156–169.
Sugár, É., Tatár, E., Záray, G., & Mihucz, V. G. (2013). Field separation-based speciation analysis of inorganic arsenic in public well water in Hungary. Microchemical Journal, 107, 131–135.
Tomlinson, M. J., Lin, L., & Caruso, J. A. (1995). Plasma mass spectrometry as a detector for chemical speciation studies. Analyst, 120, 583–589.
Torrance, K., Keenan, H., Munk, L., & Hagedorn, B. (2012). Arsenic speciation and mobility in surface water at Lucky Shot Mine, Alaska. Environmental Geochemistry and Health, 34, 711–723.
United States Environmental Protection Agency (1999). Analytical methods support document for arsenic in drinking water. http://www.epa.gov/ogwdw/arsenic/pdfs/methods.pdf
United States Environmental Protection Agency (2004). Monitoring arsenic in the environment: a review of science and technologies for field measurements and sensors, http://www.epa.gov/tio/download/char/arsenic_paper.pdf.
United States Environmental Protection Agency (2009). National primary drinking water regulations http://www.epa.gov/ogwdw000/consumer/pdf/mcl.pdf.
World Health Organization (2011). Guidelines for drinking water quality, 4th ed. Geneva, Switzerland.
Yu, X., Deng, T., Guo, Y., & Wang, Q. (2014). Arsenic species analysis in freshwater using liquid chromatography combined to hydride generation atomic fluorescence spectrometry. Journal of Analytical Chemistry, 69, 83–88.
Acknowledgments
This work was financially supported by the Research Project of the Polish Ministry of Science and Higher Education, MNiSzW N N204 140439.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Komorowicz, I., Barałkiewicz, D. Determination of total arsenic and arsenic species in drinking water, surface water, wastewater, and snow from Wielkopolska, Kujawy-Pomerania, and Lower Silesia provinces, Poland. Environ Monit Assess 188, 504 (2016). https://doi.org/10.1007/s10661-016-5477-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5477-y