Abstract
The authors present the changes of the As, Cd, Fe, Mn, Pb and Zn contents in two plant-considered metallophytes: common bent Agrostis capillaris (blades) and birch Betula pendula (leaves and seeds), recorded in a Zn-Pb industrial region of Olkusz (Poland) in 1994 and 2014. The highest amounts of Cd (12 ppm) and Zn (2524 ppm) in the common bent occur in the vicinity of the mining and metallurgical works ‘Bolesław’ in Bukowno. However, these values are significantly lower than those in 1994: Cd by 87 % and Zn by 52 %. The highest contents of Fe (2674 ppm), Mn (130 ppm) and Pb (334 ppm) in this grass species are in the vicinity of the closed Olkusz mine. These contents have increased in comparison with the 1994 figures: Fe by 56 %, Mn by 120 % and Pb by 6 %. In the birch leaves, the metal contents averaged for four sites are the following: As 2.1, Cd 6.5, Fe 261, Mn 110, Pb 70 and Zn 1657 ppm, being lower from the figures in 1994. The highest As, Fe and Pb contents of birch leaves occur in the habitat closest to the former Olkusz mine, while those of Cd, Mn and Zn in the habitat distant at 100 m from the active plant in Bukowno. The common bent grass generates better resistance mechanisms against the metals than does the birch. In the birch leaves, the contents of the metals are significantly lower than those of the grass blades, but higher from those of the birch seeds collected from the same tree individuals. It is a proof of good functioning of the mechanisms preventing excessive metal amounts from the cell metabolism and of the presence of physiological barriers protecting birch seeds as the generative organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mining and metallurgy of Zn-Pb ores are examples of the industrial activities most devastating the environment (Verner et al. 1996; Kicińska 2011; Cabała 2009). Many years of geotechnical, hydrological and chemical transformations of the soil and plant cover, emissions of industrial dusts that are carriers of metals, for instance Zn, Pb, Cd, Tl and As, and accumulation of industrial waste disposed of on-mine dumps and in flotation-tailing ponds have led to permanent changes of biocenoses, particularly marked in the vegetation growing in the areas located close to Zn-Pb mines and processing plants (Landberg and Greger 1996; Kicińska-Świderska 2003; Larcher 2003). Clear effects of these changes have been recorded as inherited mechanisms of elevated tolerance to stress factors, which can be observed in common plant species (for instance, Betula pendula—further referred to as Bp, Salix L., Agrostis capillaris—further referred to as Ac) growing in the areas of Zn-Pb industrial activities and because of that highly polluted with heavy metals (Kayzer et al. 2011; Kicińska 2009; Szarek-Łukaszewska 2009). Such organisms, called metallophytes, are forced to adapt to environmental conditions they live in and become with time subject to microevolutionary changes. Thus, they show a significant diversification of their ontogenetic traits not only within a given taxon group but also within the same species (Nowak et al. 2011; Rivelli et al. 2014). Considering long-term impact of the metals accumulated in soils, the plants are expected to evolve into new forms and even taxa (Dueck et al. 1984; Ernst 2006; Wu et al. 1975).
A particular resistance and tolerance to high contents of metals in the environment are characteristic of grass (among others Agrostis capillaris, Silene vulgaris), moss (among others Pleurozium schreberi, Sphagnum fallax) and some pioneer tree species (among others Betula pendula, Pinus silvestris). The study carried out by Czerniak and Poszyler-Adamska (2006) shows that the assimilation systems of the Bp are predisposed to cumulate dioxins. Therefore, this species is often applied to monitor strongly polluted environments (Kayzer et al. 2011). The anatomical features of leaves, for example, their size, mass and the presence of necroses, may also be biomarkers of pollution with heavy metals and also of a water and/or nutrition deficit (Dmuchowski et al. 2011).
The amounts of elements accumulated in plants result from combined processes of their intake from soil solutions or a bedrock and absorption by aboveground plant parts. These processes are controlled, among others, by cation exchange through cellular membranes, intracellular ion transport, metabolism and finally the presence and interaction of particular ions in the rhizosphere (Kuo and Harsh 1997; Kabata-Pendias and Pendias 1999). However, essential in the studies on the impact of metals on plants in the areas highly contaminated by the Zn-Pb industry is not only analysing the total metal content of plants, but determining the bioavailable metal forms present in the environment and their ability to form complexes with some fractions of organic matter due to biosorption and cumulation processes (Chojnacka et al. 2005). A continuous monitoring of emissions polluting industrial areas allows measuring the annual deposition of contaminants in the sites more proximal or distant from ore mining and processing installations. However, the metals already accumulated in soils may still significantly affect living organisms for many years to come, despite limiting the environmental nuisance of current ore mining and processing (Astel et al. 2007).
An access to the European Union imposed on new country members introducing many legislative corrections, including the necessity of adapting industrial production to environmental requirements. It has brought about a significant, positive change of the volume and type of pollutants discharged into the environment. Therefore, the authors based on chemometric analyses to evaluate the changes of the chemical composition that took place during the last 20 years in the aboveground parts of two plants (Bp and Ac), commonly occurring in a close vicinity and in an areas more distant from the mining and metallurgical plants of the Olkusz ore area (OOA) of Poland. This study was focused on the following: (a) determining the current metal contents of selected plant species growing in the industrial areas affected by ages of intensive mining and metallurgical activity, (b) chemometric comparing the changes recorded over the last 20 years in the habitats of two common plant species—the Ac and the Bp and (c) identifying major contemporary sources of the metal-polluting soil-plant systems.
Brief outline of Olkusz area of Zn-Pb ores
The OOA hosts one of the world-scale Zn-Pb deposits (Mississippi Valley type). It is located in the Silesia-Cracow industrial region of south-central Poland. The ore minerals of the OOA occur in ore-bearing dolostones in two forms: as primary sulphides ZnS—sphalerite, PbS—galena, FeS2—pyrite and marcasite, and secondary ‘oxides’ (carbonates, silicates, etc.) called also galmei ores (Sass-Gustkiewicz 1997). Mining of the Pb and Zn ores has proceeded in the region since the twelfth century; therefore, we face today the historical and contemporary environmental impacts of ore mining and processing. The area contains surface ore exposures and also disposal sites of flotation tailings, old dumps of slags and also active mining and metallurgical works ‘Bolesław’ further referred to as ZGH that produce, among others, lead products, electrolytic zinc and galvanization alloys. The whole OOA is exposed to a strong impact of that industry that affects its soil-plant system, surface waters, groundwaters and atmosphere. Emissions of SO2 and PM10 usually exceed standard values (DPE 2008/50/EC; DPE 2004/107/EC) and are really nuisances in the autumn and winter months (Table 1). Monitoring of the particulate matter that has been carried out since 2007 indicates its long-term and above-standard emissions, particularly of the PM10 fraction, but despite that, monitoring of the deposition of metals was interrupted some 15 years ago. The results of such measurements conducted in the 1990s by SANEPID (an independent domestic sanitary and epidemiological research and control institution) showed the following maximum values in the OOA (in mg/m2): Zn 2822, Cd 22, Pb 309 and Mn 62, also 7.2 g/m2 Fe2O3 (Table 2). This high annual load of metals falling onto the OOA soils must have exerted a considerable impact on all the organisms living within the area. A quality of habitats is particularly important for plants; the organisms that cannot change their locations. A growth of plants strongly depends on the living conditions, mainly stress factors (climate, macro- and microelements in the system, competitiveness of other organisms, etc.).
Study site and methods
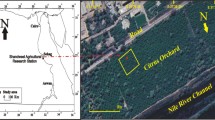
The sites of the investigations carried out at the end of September 2014 were exactly the same as those of the analogue investigations that had been conducted in 1994 (Fig. 1, points I–IV); it allowed us to record long-term changes of the metal contents of some plants occupying these four sites. The habitats differ one from another first of all in their distance from and direction (wind rose) against major emitters (ZGH, flotation-tailing ponds and major transport routes). The research material collected in each of the sites included the following: 30 aboveground parts of the grass species Ac (symbols of samples G/I–G/IV), 1 kg of leaves of the tree species Bp (symbols BL/I–BL/IV) and 200–300 g seeds of the even-aged individuals of the pioneer tree Bp (symbols BS/I–BS/IV). The leaves of the birch trees from each habitat were divided into two parts. One of them was washed three times with 300 ml distilled water and next dried (symbol of samples BLw/I–BLw/IV); the other was not washed (symbol of samples BLuw/I–BLuw/IV).The contents of metals present on and inside the leaves were analysed. The load of metals (Cd, Fe, Mn, Pb and Zn) on leaves of the betula trees in 1994 and 2014 was calculated for the specified habitats as the difference between the metal contents of the unwashed and washed leaves (BLUW–BLW).
The total decomposition of plant tissue (both washed and not washed) was carried out with 7 ml of 65 % HNO3 and 1 ml of 30 % H2O2 in a microwave oven (high pressure-segmented rotor HPR 1000/10 s at 200 °C, microwave power up to 1000 W). Analytical weighed plant amounts were 0.5 g each. This methodology allows determining the total contents of metals in plants. The concentrations of metals (Zn, Pb, Cd, Fe and Mn) in solutions were measured with an ICP-MS 6100 analyser (Elan) at the detection limits of elements equal to 2°10−5 mg/dm3. Statistical analyses were carried out using the Statistica version 10 software (StatSoft Inc 2011).
Results and discussion
Metal contents of grass (common bent Ac)
The contents of As, Cd, Fe, Mn, Pb and Zn in samples of the common bent grass analysed in 2014 show significant differences among the four sites and low differences within a site (Table 3). The highest contents of Cd and Zn, 12 and 2524 ppm, respectively, were found in habitat no. III located in the closest distance about 100 m from ZGH. These figures compared with those obtained in 1994 are, however, lower by as much as 87 % for Cd and 52 % for Zn. The highest 2014 contents of Fe (2674 ppm), Mn (130 ppm) and Pb (334 ppm) have the grass samples from site no. II located in the closest distance from the Olkusz mine (open in 1962 and closed in 2003). Compared with those obtained in 1994, they are higher by 56, 120 and 6 %, respectively. In case of Zn and Pb, the 2014 figures for the Ac grass exceed significantly toxic limits (Table 3). The As content is the highest (17.8 ppm) in habitat no. I, which is 5 % higher than that analysed in 1994. In this site also higher are the 2014 contents of Mn (14 %) and Pb (12 %) in relation to the 1994 data. The lowest contents of the metals considered, ten times at the average if compared to three other sites, are those in the Ac growing in habitat no. IV located some 750 m from ZGH and to SW from the main pollution emitter.
All the Ac samples have their As, Cd, Pb and Zn contents many times higher than the upper limits of the so-called natural grass contents (Kabata-Pendias and Pendias 1999). In the Ac from habitat nos. I, II and III are also exceeded the toxic limits of As and Zn that are 5 and 400 ppm, respectively. In the case of Pb with the toxic limit for grass 300 ppm, this value is exceeded (334 ppm, Pb) only in the Ac from the habitat no. II. In the Ac from habitat no. IV, the Fe content is below the upper natural value; while in the case of Mn, its values do not exceed such a value for all the four habitats. These results indicate significant adaptiveness and accumulation of metals in the species Ac.
In comparison with the 1994 data, the 2014 contents of metals in the aboveground Ac parts are significantly lower, with two exceptions only. The highest is the metal decrease in Ac (by 86 % on the average) in habitat no. IV, and the second in turn (a decrease of 70 % on the average) is habitat no. III. The lowest improvement (a decrease of only fifteen or so percent) has been found in habitat no. I. In the year 2014, the change of the habitat whose Ac had previously been most burdened with metals was also noted. It is habitat no. III, in which the significant improvement (70 % quoted above) is an effect of installing modern filters in the chimneys of the mining metallurical plant located nearby, due to which emission of industrial dusts has been considerably limited. The increases but in the cases of three metals and two habitats only in 2014 show the contents of Fe, Mn and Pb in habitats nos. I and II. This situation can be explained by a secondary deflation from large-size industrial dumps and old flotation tailing ponds. These waste materials still contain significant amounts of metals (Kicińska and Wójcik 2011; Gruszecka and Wdowin 2013), and the fine particles drift mainly to NE, i.e. in the prevailing direction of the wind rose, where habitat nos. I and II are located.
Metal contents in birch trees Bp
The contents of metals in the leaves of the Bp analysed in 2014 are considerably lower than those obtained in 1994 (the same trees were sampled and at the same time of the year (Table 3); they are also lower from the metal contents of the grass Ac (Table 4).
The highest 2014 metal contents of the birch Bp leaves are As 2.1, Cd 6.5, Fe 261, Mn 110, Pb 70 and Zn 1657 ppm. In the case of As, Fe and Pb, these values refer to habitat no. II, located in the closest distance from the Olkusz mine (closed in 2003), whereas in the case of Cd, Mn and Zn to habitat no. III, located only 100 m from ZGH. The lowest metal contents of the Bp leaves are in habitat no. IV; it is worth mentioning that also the lowest are metal contents of the Ac grass in the same habitat. Dmuchowski et al. (2011) analysed in 2010 the leaves of birch trees growing in the OOA and determined 0.11–9.95 ppm As (with an average for the whole OOA of 0.37 ppm). Their data correspond well with the figures presented here for the year 2014 (Table 4). The determinations of selected metals were also carried out in the birch leaves in 2005 (Gruszecka 2007); she found in the area of waste ponds the maximum contents of As 25, Cd 9.3, Cr 3.1, Pb 460 and Zn 1569 ppm. In both papers, the highest contents of As are noted in the birch leaves collected between ZGH and the former Olkusz mine.
A significant decrease of the As, Cd, Fe, Mn, Pb and Zn contents of the Bp leaves is the most important conclusion resulting from the comparison of the 1994 and 2014 data. The highest improvement is in the case of As, whose averaged content for all the habitats dropped by 93 %. For other metals, such drops are the following: Cd by 84 %, Fe by 71 %, Pb by 66 %, Zn by 64 % and Mn by 52 %. It is a desirable tendency of environmental changes noted in the 20-year period.
Seeds were the other research material collected from the birch trees. The highest contents of the metals in the seeds occur in habitat no. III (Table 5). They are as follows (the italicised values in parentheses show corresponding contents of leaves): Cd 5.4 ppm (6.5), Mn 96 ppm (110), Pb 46 ppm (65) and Zn 848 ppm (1657). Although these seed contents of Cd, Fe, Pb and Zn are lower (even by around 90 %) in comparison to the 1994 figures, habitat no. III has been still the most contaminated of the four sites tested. In contrast, in the seeds of habitat no. II, the contents of Pb increased by 9 % and those of Mn even by 91 %, referred to the 1994 figures, but the remaining elements noted significant drops: Cd by 91 %, Fe by 46 % and Zn by 57 %. The lowest metal contents of the birch tree seeds are in habitat no. IV.
Considering the metal contents of the birch seeds averaged for all the habitats, the 1994:2014 decreases are high: Pb by 99 %, Cd by 97 %, Zn by 86 %, Fe by 80 % and Mn by 37 %.
The contents of the metals in the birch seeds are two to three times on the average lower if compared with the metal contents of the leaves collected from the same trees (Table 4). It is a proof of a predominance of a passive (non-metabolic) transport of elements, particularly of Zn and Pb, over the active transport, based mainly on the diffusion of Pb2+ and Zn2+ ions from a soil solution into the endodermic cells of the plant roots, from which they are transported into the aboveground plant parts by a transpiratory water movement. A differentiation of the contents of elements in the generative and vegetative plant parts results from a metabolic (active) uptake of metals by root systems. The differences may be due to either the lack of a plant biologic barrier, which leads to a fast adaptation of such a species, or the inherited resistance strategy of a plant organism based on retaining metals in the roots. There are practically two mechanisms of detoxication acting in root systems. In the first of them, the metals are accumulated in the cell walls; in the other, they are bound by phenol compounds in the vacuoles of tannin cells. Such mechanisms were also found in birch trees by Ciarkowska et al. (2009), who determined that the roots of the birches growing on the substrate highly polluted with flotation tailings from the OOA took up 8 % Zn, 4 % Pb and 1 % Cd of the metals present in the substrate, whereas the analogue uptake of these metals by birch sprouts was lower by about 1 %. When the birch trees grew on a non-polluted substrate, the roots retained 75 % Zn, 24 % Pb and 72 % Cd% present in their substrate, and the respective uptakes of the three metals in the aboveground birch parts was significantly lower.
In the case of the Bp, high amounts of metals taken up from soil solutions are stopped by both the tree root systems and the physiological barriers due to which the metals are immobilised in the vegetative plant organs, while the generative organs, i.e. seeds, are protected. This process has been traced using the 1994 and 2014 data by calculating the transport index T: it is a quotient of the content of a given element in the birch leaves (vegetative parts) to such content in the birch seeds (generative parts). The values of the index T ≤ 1 indicate that the physiological barriers in the aboveground birch parts do not retain excessive amounts of elements. If T > 1, the retaining mechanisms are active in the roots. The results of such calculations (Table 6) show that in 1994 the worst situation was in habitat no. III, for which the T indices took on the values significantly below 1. It is particularly worrying in the case of Cd and Pb with the T = 0.13. Such a low index indicates a weak action of the mechanisms protecting plants from excessive amounts of toxic elements. The highest T indices have been calculated for habitat no. IV in the case of Cd and Zn.
Considering the 2014 data, the T index is improved for all the metals, with the highest change of its values even by 1.77 units. Such results point to developing and fixing by the birch trees protecting mechanisms adequate to the existing environmental conditions.
Current sources of metals affecting the OOA plant species
Up to now, two sources of metals affecting the plants growing in the OOA have been indicated. The first is the so-called soil load, understood not only as the total content of metals, but as the bioavailable forms of ions present in the soil environment. High amounts of metals in the soil affect the morphology of roots: they dry, get deformed, have the growth limited and are deprived of symbiotic mycelia. These are results of a disturbed histogenesis, improper development of lateral roots or unbalanced tissue growth (Cabała 2009). The other source is represented by the so-called atmospheric load, understood as the total of various substances: polar (gases and dust) and nonpolar (mainly organic compounds) polluting the air (Poborski 1991; Kicińska-Świderska 2004). If deposited on the surfaces of the aboveground plant fragments, they can enter their inner parts via a stomatal and/or extrastomatal uptake and be included in (or partly excluded from) plant metabolic processes. Mechanisms of a fast excluding the excessive, free ions that enter plant cells via a metabolic route result in the formation of its tolerance to some elements. Such a process depends on a plant species, its ontogenic traits and the type of the element (Ernst 2006). The amounts of metals that can be present on aboveground plant parts were calculated comparing the metal contents in washed and not washed samples of birch leaves. The results for the years 1994 and 2014 (Fig. 2) indicate the following:
-
a few times (from 2 to 5) decrease of the Cd and Zn amounts present on birch leaves between 1994 and 2014,
-
habitat no. III, in which the highest decrease of the contents Pb and Cd both in washed and not washed leaves was recorded between 1994 and 2014 and
-
habitat nos. I and II, in which the contents of Fe and Mn in the not washed leaves increased in 2014 in relation to those in 1994.
These results point to a long-lasting and intensive deflation resulting in drifting the dusts containing high contents of metal compounds (Fe, Mn) from the flotation tailing dried ponds located in proximity to the habitats tested (Kicińska-Świderska 1999; Gruszecka and Wdowin 2013). The samples from the closest vicinity of the ZGH (habitat nos. III and IV, Fig. 1) indicate a significant environmental improvement, i.e. a decrease of the amounts of the deflated dusts containing metals and deposited onto soils. It confirms positive effects of the actions limiting the environmental impact of the mining and processing ZGH installations. To obtain a better visualisation of the inflow direction of pollutants via the atmosphere, the Wafer 3 W (XYZ) plots have been prepared, ascribing the grid location coordinates of the habitats adequate rectangular coordinates. The load of metals on leaves of the betula trees in 1994 and 2014 was calculated for the specified habitats as the difference between the metal contents of the unwashed and washed leaves (Fig. 3). The graphs show the direction(s) of the maximum inflow of the pollutants deposited on plants.
In the year 1994, the highest loads of atmospheric pollutants affected the plants growing in the eastern part of the OOA. The most polluted with Zn, Pb, Mn, Fe and Cd were the aboveground parts of the plants of habitat no. II, located in the closest distance from the active then Olkusz mine. In the case of Pb and Cd, their additional source was represented by secondary dusts transported by the western winds prevailing in the OOA region. It can be thus assumed that these pollutants originated due to operations of the ZGH. In the year 2014, an important change was recorded, i.e. a decrease of the Zn and Cd amounts deposited on aboveground parts of the plant. The plants of habitat no. III were exposed most on the deposition of these two metals. The remaining metals were transported by winds from the north, which is a new direction, in which old ponds of flotation tailings are located and are currently a significant source of metals that pollute also plants nearby. In the case of Fe and Mn analysed in 2014, their contents are higher than those recorded in the 1990s. The least polluted in 1994 was habitat no. IV, which has also now been the cleanest of the four habitats. Although located 700 m from ZGH, this site is situated south of the Zn-Pb works that is upwind from a major pollution source.
Conclusions
The metal contents in the common bent Ac were significantly higher in 2014 than the values published as the so-called natural contents. In all habitats, these values also exceed the contents regarded as toxic ones. It is a proof that the Ac grass has developed permanent tolerance particularly to high contents of Zn, Pb and Cd. Despite considerable metal amounts, the Ac grass individuals have not revealed visible changes of their aboveground morphology, and the in situ inspections have shown their normal growth.
The Ac grass individuals also contain higher metal contents in comparison to those of the pioneer Bp birch tree, which suggests that the resistance mechanisms against pollution of grass varieties exceed those of trees. The metal contents of the birch are higher than those of the birch seeds collected from the same trees. It is, in turn, a sign of a proper functioning of the mechanisms that prevent excessive metal amounts from entering into the cell metabolic system and of the presence of physiological barriers protecting the generative organs of trees. A comparison of the 1994 and 2014 results shows that a long-lasting impact of stress factors on plants results in their developing and fixing some protection mechanisms that become genetically transferred to next generations. The fact that the active metal transport is dominating in the case of generative parts of plants indicates that concentrations of ions in soil solutions and amounts of elements in atmospheric dusts significantly decreased between 1994 and 2014.
Preventive measures and legal regulations pertaining to the environmental protection and introduced in the last 20 years have considerably improved a state of the environment in the Olkusz ore area (OOA). The changes are clearly marked in vicinity of the ZGH plant, whose emissions have been limited. Of the four habitats studied, the soil-plant structure of the habitat located in the shortest distance from ZGH has changed the most and its pollution distinctly dropped. However, the study has identified new sources of metal emissions: they come from unprotected (or insufficiently protected) old ponds of flotation tailings and slag dumps. These are the sites currently contaminating the regions located to the south and southeast of them, i.e. in the direction of dominant winds that deflate fine, metal-containing dust particles.
References
Astel, A., Walna, B., & Kurzyca, I. (2007). Chemometrics in the assessment of local and trans-boundary air pollution. International Journal of Environment and Health, 1(1), 1–12.
Cabała, J. (2009). Heavy metals in ground soil environment of the Olkusz area of Zn-Pb ore exploatation. Katowice: Silesia Univeristy Press. in Polish.
Chojnacka, K., Chojnacki, A., Górecka, H., & Górecki, H. (2005). Bioavailability of heavy metals form polluted soils to plants. Science of the Total Environment, 337, 175–182.
Ciarkowska, K., Hanus-Fajerska, E., & Gajewski, Z. (2009). The usefulness of common birch to stabilization of grounds strongly contaminated by heavy metals. Environmental Protection and Natural Resources, 41, 449–455.
Czerniak, A., & Poszyler-Adamska, A. (2006). The content of dioxins and heavy metals in the camera assimilation of scots pine (Pinius sylvestris L.) and silver birch (Betula pendula Roth.) growing in the vicinity of a local landfill. Zeszyty Problemowe Postepów Nauk Rolniczych, 515, 476–54. in Polish.
Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air (Dz. U. UE L 23). http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:023:0003:0016:EN:PDF.
Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe (Dz. U. UE L 152). http://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:32008L0050.
Dmuchowski, W., Gozdowski, D., Baczewska, A., & Brągoszewska, P. (2011). Evaluatyion of the arsenic environmental pollution on the basis of different bioindicative methods. Environmental Protection and Natural Resources, 48, 391–402.
Dueck, T. H. A., Ernst, W. H. O., Faber, J., & Pasman, F. (1984). Heavy metal immission and genetic constitution of plant population in the vicinity of two metal emission sources. Angewandte Botanik, 58, 47–59.
Ernst, W. H. O. (2006). Evolution of metal tolerance in higher plants. Forest Snow and Landscape Research, 80(3), 251–274.
Gruszecka, A. (2007). Heavy metals in waters and river sediments and soils in the vicinity of landfill mining and metallurgical Bukowno (Poland) and Mansfeld (Germany) - an environmental risk assessment. Doctoral thesis, AGH, Kraków. in Polish.
Gruszecka, A., & Wdowin, M. (2013). Characteristics and distribution of analyzed metals in soil profiles in the vicinity of a postflotation waste site in the Bukowno region, Poland. Environmental Monitoring and Assessment, 185, 8157–8168.
Kabata-Pendias, A., & Pendias, H. (1999). Biogeochemistry of trace elements. Warszawa: Wydawnictwo Naukowe PWN. in Polish.
Kayzer, D., Czerniak, A., & Poszyler-Adamska, A. (2011). Effect of trace elements on the morphometric parameters of assimilation apparatus in white birch. Infrastructure and Ecology of Rural Areas, 2, 261–273.
Kicińska, A. (2009). Arsenic and thallium in soils and plants in the Bukowno area. Environmental Protection and Natural Resources, 40, 199–208.
Kicińska, A. (2011). Occurrence and mobility of zinc, lead and cadmium in soils polluted by mining and metallurgical industries. Environmental Protection and Natural Resources, 49, 152–162.
Kicińska, A., & Wójcik, R. (2011). The influence of Zn-Pb flotation waste & slag on plants-soils system, Acta Environmentalica Universitatis Comenianae. Bratislava, 19(1), 164–168.
Kicińska-Świderska, A. (1999). Heavy metals in soils and plants in selected areas impact the steel industry. Doctoral thesis, AGH, Kraków. in Polish.
Kicińska-Świderska, A. (2003). The zinc resistance of the grass Agrostis capillaris growing on surrounding of Zn-smelter at Bukowno area. Archives of Environmental Protection, 29(3), 149–158.
Kicińska-Świderska, A. (2004). Influence of mineralogical and geochemical compositions on the mobility of heavy metals from industrial dusts from ZGH “Bolesław” in Bukowno. Geology: Quarterly of AGH – University of Science and Technology Press, 30(2), 191–205.
Kuo, S., & Harsh, J.B. (1997). Physicochemical characterization of metals in soil and their plant availability.In: Adriano D.C., Chen Z-S., Yang S-S. & Iskandar I.K. (Eds.) Biogeochemistry of trace metals, (pp. 75–120). Science Reviews.
Landberg, T., & Greger, M. (1996). Differences in uptake and tolerance to heavy metals in Salis from unpolluted and polluted areas. Applied Geochemistry, 11(1–2), 175–180.
Larcher, W. (2003). Physiological plant ecology. Berlin, Springer. monitoring.krakow.pios.gov.pl/stacje/stacja/10. Accessed 29 September 2015.
Nowak, T., Kapusta, P., Jędrzejczyk-Korycińska, M., Szarek-Łukaszewska, G., & Godzik, B. (2011). The vascular plants of the Olkusz Ore-bearing Region. Kraków.
Poborski, P.S. (1991). Downloading air pollution from the plant foliage. In: Contaminated environment and the physiology of plants. Warszawa.
Rivelli, A. R., Puschenreiter, M., & De Maria, S. (2014). Assessment of cadmium uptake and nutrient content in sunflower plants grown under Cd stress. Plant Soil Environment, 60(2), 80–86.
Sass-Gustkiewicz, M. (1997). Revised and completed paragenetic order of minerals in the Pomorzany lead-zinc deposit. Upper Silesian Region, Poland, Mineralogica Polonica, 28, 46–80.
StatSoft Inc. 2011. STATISTICA (Data analysis software system). ver. 10.0. www.statsoft.com.
Szarek-Łukaszewska, G. (2009). Vegetation of reclaimed and spontaneously vegetated Zn-Pb mine wastes in southern Poland. Polish Journal of Environmental Studies, 18(4), 717–733.
Verner, J. F., Ramsey, M. H., Helios-Rybicka, E., & Jędrzejczyk, B. (1996). Heavy metal contamination of soils around a Pb-Zn smelter in Bukowno, Poland. Applied Geochemistry, 11(1–2), 11–16.
Wu, L., Bradshaw, A. D., & Thurman, D. A. (1975). The potential for evolution of heavy metal tolerance in plants. III The rapid evolution of copper tolerance in Agrostis stolonifera. Heredity, 34, 165–187.
Acknowledgments
This work was financed within statutory research of AGH grant no. 10.10.140.199.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kicińska, A., Gruszecka-Kosowska, A. Long-term changes of metal contents in two metallophyte species (Olkusz area of Zn-Pb ores, Poland). Environ Monit Assess 188, 339 (2016). https://doi.org/10.1007/s10661-016-5330-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5330-3