Abstract
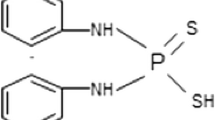
Dianilinodithiophosphoric acid (DDA) is widely used as sulfide mineral flotation collector in China. It is necessary to investigate the biodegradability of DDA to provide the fundamental knowledge to assess the environmental fate in the risk assessment of DDA and to design and operate the DDA flotation wastewater biological treatment plant. In the present study, the primary and ready aerobic biodegradations of DDA were studied and the primary biodegradation kinetic model of DDA was developed. The results show that DDA displays a good primary biodegradability and its biodegradation ratio reaches 99.8 % in 7 days. In contrast, DDA is not easily ready biodegradable; hence, it is a partially biodegradable organic compound. The primary aerobic biodegradation kinetics can be described using the first-order reaction kinetics equation: C = 19.72191e−0.01513t.






Similar content being viewed by others
References
Araujo, D. M., et al. (2010). Biodegradation studies on fatty amines used for reverse flotation of iron ore. International Biodeterioration & Biodegradation, 64, 151–155.
Chen, S. H., et al. (2010). Experimental study on the biodegradability of alkyl xanthate collectors. Acta Scientiarum Naturalium Universitatis Pekinensis, 46, 351–357.
Chen, S., et al. (2011a). Primary biodegradation of sulfide mineral flotation collectors. Minerals Engineering, 24, 953–955.
Chen, S. H., et al. (2011b). Evaluation of biodegradability of alkyl xanthates flotation collectors. Journal of Central South University(Science and Technology), 42, 546–554.
Contrera, R. C., et al. (2014). First-order kinetics of landfill leachate treatment in a pilot-scale anaerobic sequence batch biofilm reactor. Journal of Environmental Management, 145, 385–393.
Deo, N., & Natarajan, K. A. (1998). Biological removal of some flotation collector reagents from aqueous solutions and mineral surfaces. Minerals Engineering, 11, 717–738.
Hao, Y., et al. (2012). Degradation and influence factors of ammonium butyl aerofloat in paddy soil. Acta Scientiae Circumstantiae, 32, 1454–1458.
Houot, R. (1982). Beneficiation of phosphatic ores through flotation: review of industrial applications and potential developments. International Journal of Mineral Processing, 9(4), 353–384.
Li, H. F. (1990). Flotation wastewater and tailing treatment. Beijing: The Chinese Society for Metals.
Li, X. M., et al. (2009). Study on the environmental pollution and control of organic chemicals in the mine beneficiation. Journal of Anhui Agricultural Sciences, 37, 5086–5087. 5116.
Lotter, N. O., & Bradshaw, D. J. (2010). The formulation and use of mixed collectors in sulphide flotation. Minerals Engineering, 23, 945–951.
Mei, C. F., et al. (2015). A comparative study of biodegradability of a carcinogenic aromatic amine (4,4′-diaminodiphenylmethane) with OECD 301 test methods. Ecotoxicology and Environmental Safety, 111, 123–130.
Momoh, O. L. Y., et al. (2013). Development of simplified anaerobic digestion models (SADM’s) for studying anaerobic biodegradability and kinetics of complex biomass. Biochemical Engineering Journal, 79, 84–93.
OECD. (1992). Ready biodegradability. 301 B: CO 2 evolution (modified Sturm test). Pairs: OECD.
Pedrazzani, R., et al. (2012). Biodegradability, toxicity and mutagenicity of detergents: integrated experimental evaluations. Ecotoxicology and Environmental Safety, 84, 274–281.
Qin, Y., et al. (2005). Primary aerobic biodegradation of cationic and amphoteric surfactants. Journal of Surfactants and Detergents, 1, 55–58.
Qin, Y., et al. (2006). Primary aerobic biodegradation of linear and oxo alcohol alkylpolyglucosides (APG). Journal of Surfactants and Detergents, 9, 227–230.
Reuschenbach, P., et al. (2003). A critical comparison of respirometric biodegradation tests based on OECD 301 and related test methods. Water Research, 37, 1571–1582.
Richterich, K., & Steber, J. (2001). The time-window-an inadequate criterion for the ready biodegradability assessment of technical surfactants. Chemosphere, 44, 1649–1654.
Rubio, J., et al. (2002). Overview of flotation as a wastewater treatment technique. Minerals Engineering, 15, 139–155.
Sathian, S., et al. (2014). Performance of SBR for the treatment of textile dye wastewater: optimization and kinetic studies. Alexandria Engineering Journal., 53, 417–426.
Shaohua, C., et al. (2011). Primary biodegradation of sulfide mineral flotation collectors. Minerals Engineering, 24, 953–955.
She, Z., et al. (2012). Study on the aerobic biodegradability and degradation kinetics of 3-NP; 2,4-DNP and 2,6-DNP. Journal of Hazardous Materials, 241–242, 478–485.
Sun, S. Y., et al. (2001). Study on the sulphide ore flotation wastewater purification and recycling. Nonferroius Metals, 4, 33–37.
Swigert, J. P., et al. (2014). Aquatic hazard and biodegradability of light and middle atmospheric distillate petroleum streams. Chemosphere, 108, 1–9.
Wei-feng, S., et al. (2012). Co-metabolism characteristics of aniline aerofloat with different substrates in flotation wastewater. The Chinese Journal of Nonferrous Metals., 22, 2090–2096.
Wen, Q., et al. (2015). Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere, 132, 63–69.
Xie, G. Y., et al. (2002). Treatment and reuse of wastewater from a sulphide ore flotation plant. Chinese Journal of Environmental Engineering., 2, 67–70.
Xinping, P., et al. (2010). Research on lead-zinc sulfide ore dressing wastewater treatment and reuse technologies. Hunan Nonferrous Metals, 26(2), 40–42.
Yan, H. Z., et al. (2010). Evaluation of biodegradability of amine collectors. Advanced Materials Research, 113–116, 267–271.
Youquan, L., & Rongshang, L. (1994). Study on properties of aniline aerofloat. Mining and Metallurgy, 3, 50–54.
Zhao, J., et al. (2006). Aerobic biodegradation of alkylphenol ethoxylates. Bioresource Technology, 97, 2478–2480.
Acknowledgments
This work was supported by the Education Special Funds of the University Discipline Construction of Guangdong Province (Grant No. 2014KTSP022), Nature Science Foundation of Guangdong (2015A030303003), and Science and Technology project of Guangdong (2014B020216009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, W., Sun, S., Xu, P. et al. Evaluating the primary and ready biodegradability of dianilinodithiophosphoric acid. Environ Monit Assess 188, 232 (2016). https://doi.org/10.1007/s10661-016-5242-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5242-2