Abstract
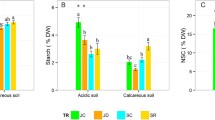
Trees in cutaway peatland are growing in difficult conditions. Fertilization with nutrient-rich wood ash helps improve growth conditions. Photosynthesis and carbohydrate concentration along leaf anatomy were studied on plots treated with 10 and 5 t ha−1 wood ash (WA10 and WA5) and on untreated (Control) plot to explain the physiological background of the differences in tree growth. The leaves from WA10 had the largest leaf area, total thickness, the thickest mesophyll and also significantly larger average values of all anatomical parameters of the shoots. The photosynthetic assimilation was significantly higher on treated plots at 200 and 400 ppm CO2 levels. In leaves on the treated plots, the sucrose concentration was lower while that of starch was higher than in trees on untreated soil. The differences in the maximum photosynthesis were relatively small. At unit ground, the leaf area provided for a wood ash-treated tree an efficient surface for CO2 assimilation, light interception and some starch storage during the growing period.





Similar content being viewed by others
References
Aalto, T., & Juurola, E. (2002). A three-dimensional model of CO2 transport in airspaces and mesophyll cells of a silver birch leaf. Plant, Cell and Environment, 25, 1399–1409.
Amtmann, A., Troufflard, S., & Armengaud, P. (2008). The effect of potassium nutrition on pest and disease resistance in plants. Physiologia Plantarum, 133(4), 682–691.
Armengaud, P., Sulpice, R., Miller, A. J., Stitt, M., Amtmann, A., & Gibon, Y. (2009). Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots. Plant Physiology, 150(2), 772–785.
Ashraf, M., & Harris, P. J. C. (2013). Photosynthesis under stressful environments. Photosynthetica, 51(2), 163–190.
Benlloch-González, M., Arquero, O., Fournier, J. M., Barranco, D., & Benlloch, M. (2008). K+ starvation inhibits water-stress-induced stomatal closure. Journal of Plant Physiology, 165, 623–630.
Black, K., & Gallagher, G. (2010). The greenhouse gas balance of peatland forest. Environ. 11, c/o COFORD, Department of Agriculture, Fisheries and Food.
Bozzola, J.J., & Russell, L.D. (1992). Electron microscopy. Jones & Bartlett Learning, LLC.
Campbell, D. R., Lavoie, C., & Rochefort, L. (2002). Wind erosion and surface stability in abandoned milled peatlands. Canadian Journal of Soil Science, 82, 85–95.
Ceulemans, R., & Mousseau, M. (1994). Effects of elevated atmospheric CO2 on woody plants. New Phytologist, 27, 425–446.
Chaves, M. M., Flexas, J., & Pinheiro, C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany, 103, 551–560.
Dodd, I. C. (2003). Hormonal interactions and stomatal responses. Journal of Plant Growth Regulation, 22, 32–46.
Einig, W., Lauxmann, U., Hauch, B., Hampp, R., Landolt, W., Maurer, S., & Matyssek, R. (1997). Ozone-induced accumulation of carbohydrates changes enzyme activities of carbohydrate metabolism in birch leaves. New Phytologist, 137, 673–680.
Ericsson, T., & Kähr, M. (1995). Growth and nutrition of birch seedlings at varied relative addition rates of magnesium. Tree Physiology, 15, 85–93.
Gielen, B., & Ceulemans, R. (2001). The likely impact of rising atmospheric CO2 on natural and managed Populus: a literature review. Environmental Pollution, 115, 335–358.
Groeneveld, E., & Rochefort, L. (2002). Nursing plants in peatland restoration: on their potential use to alleviate frost heaving problems. Suoseura, 53(3-4), 73–85.
Hermans, C., Hammond, J. P., White, P. J., & Verbruggen, N. (2006). How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science, 11(12), 610–617.
Hölttä, T., Mencuccini, M., & Nikinmaa, E. (2009). Linking phloem function to structure: analysis with a coupled xylem-phloem transport model. Journal of Theoretical Biology, 259(2), 325–337.
Hopkins, W. G., & Hüner, N. P. A. (2009). Introduction to plant physiology. Hoboken: Wiley.
Huber, S. C. (1984). Biochemical basis for effects of K-deficiency on assimilate export rate and accumulation of soluble sugars in soybean leaves. Plant Physiology, 76(2), 424–430.
Huotari, N., Tillman-Sutela, E., & Kubin, E. (2009). Ground vegetation exceeds tree seedlings in early biomass production and carbon stock on an ash-fertilized cut-away peatland. Biomass and Bioenergy, 33, 1108–1115.
Hytönen, J. (1995). Effect of fertilizer treatment on the biomass production and nutrient uptake of short-rotation willow on cutaway peatlands. Silva Fennica, 29, 21–40.
Hytönen, J. (2005). Effects of liming on the growth of birch and willow on cut-away peat substrates in greenhouse. Baltic Forestry, 11(2), 68–74.
Hytönen, J., & Aro, L. (2012). Biomass and nutrition of naturally regenerated and coppiced birch on cutaway peatland during 37 years. Silva Fennica, 46, 377–394.
Hytönen, J., & Saarsalmi, A. (2009). Long-term biomass production and nutrient uptake of birch, alder and willow plantations on cut-away peatland. Biomass and Bioenergy, 33, 1197–1211.
Ingestad, T. (1962). Macroelement nutrition in pine, spruce and birch seedlings in nutrient solutions. Medd statens skogsforskningsinst, 51, 1–150.
Itoh, R., Yamagishi, J., & Ishii, R. (1997). Effects of potassium deficiency on leaf growth, related water relations and accumulation of solutes in leaves of soybean plants. Japanese Journal of Crop Science, 66(4), 691–697.
Jacobson, S. (2003). Addition of stabilized wood ashes to Swedish coniferous stands on mineral soils—effects on stem growth and needle nutrient concentrations. Silva Fennica, 37(4), 437–450.
Kaunisto, S., & Aro, L. (1996). Forestry use of cutaway peatlands. In H. Vasander (Ed.), Peatlands in Finland (pp. 130–134). Helsinki: Finnish Peatland Society.
Kenzo, T., Ichie, T., Yoneda, R., Kitahashi, Y., Watanabe, Y., Ninomiya, I., & Koike, T. (2004). Interspecific variation of photosynthesis and leaf characteristics in canopy trees of five species of Dipterocarpaceae in a tropical rain forest. Tree Physiology, 24, 1187–1192.
Kikamägi, K., & Ots, K. (2010). Stimulating the growth of trees with ashes of various biofuels (wood, peat) on a cutaway peatland. Forestry Studies, 52, 60–71.
Kikamägi, K., Ots, K., & Kuznetsova, T. (2013). Effect of wood ash on the biomass production and nutrient status of young silver birch (Betula pendula Roth.) trees on cutaway peatlands in Estonia. Ecological Engineering, 58, 17–25.
Kikamägi, K., Ots, K., Kuznetsova, T., & Pototski, A. (2014). The growth and nutrients status of conifers on ash-treated cutaway peatland. Trees, 28(1), 53–64.
Kleczewski, N. M., Herms, D. A., & Bonello, P. (2012). Nutrient and water availability alter belowground patterns of biomass allocation, carbon partitioning, and ectomycorrhizal abundance in Betula nigra. Trees, 26, 525–533.
Klõšeiko, J., Mandre, M., & Korsjukov, R. (2006). Needle carbohydrate concentrations in Norway spruce as affected by wood ash application to soil. Proceedings of the Estonian Academy of Sciences: Biology Ecology, 55(2), 123–136.
Koch, K. (2004). Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology, 7(3), 235–246.
Lavoie, C., Saint-Louis, A., & Lachance, D. (2005). Vegetation dynamics on an abandoned vacuum-mined peatland: 5 years of monitoring. Wetlands Ecology and Management, 13, 621–633.
Lukjanova, A., & Mandre, M. (2009). The effect of wood ash fertilisation on the anatomy and localisation of lignin in Scots pine (Pinus sylvestris L.) needles. Baltic Forestry, 15(2), 177–185.
Lukjanova, A., & Mandre, M. (2010). Effects of alkalization of the environment on the anatomy of Scots pine (Pinus sylvestris) needles. Water, Air, and Soil Pollution, 206(1–4), 13–22.
Maier, C. A., Palmroth, S., & Ward, E. (2008). Short-term effects of fertilization on photosynthesis and leaf morphology of field-grown loblolly pine following long-term exposure to elevated CO2 concentration. Tree Physiology, 28, 597–606.
Mandre, M., Korsjukov, R., & Ots, K. (2004). Effect of wood ash application on the biomass distribution and physiological state of Norway spruce seedlings on sandy soils. Plant and Soil, 265(1–2), 301–314.
Merilo, E., Heinsoo, K., & Kull, O. (2005). Leaf photosynthetic properties in willow (Salix viminalis and Salix dasyclados) plantation in response to fertilization. European Journal of Forest Research, 125(2), 93–100.
Moilanen, M., Hytönen, J., & Leppälä, M. (2012). Application of wood ash accelerates soil respiration and tree growth on drained peatland. European Journal of Soil Science, 63, 467–475.
Norby, R. J., Wullschleger, S. D., Gunderson, C. A., Johnson, D. W., & Ceulemans, R. (1999). Tree responses to rising CO2 in field experiments: implications for the future forest. Plant, Cell and Environment, 22, 683–714.
Pääkkönen, E., Holopainen, T., & Kärenlampi, L. (1995). Ageing-related anatomical and ultrastructural changes in leaves of birch (Betula pendula Roth.) clones as affected by low ozone exposure. Annals of Botany, 75, 285–294.
Paavilainen, E., & Päivänen, J. (1995). Peatland forestry: ecology and principles (Ecological studies 111). Berlin: Springer.
Pallardy, S. G. (2008). Physiology of woody plants (3rd ed.). Amsterdam: Academic.
Puijalon, S., Piola, F., & Bornette, G. (2008). Abiotic stresses increase plant regeneration ability. Evolutionary Ecology, 22, 493–506.
Ramst, R., Orru, M., Salo, V., Halliste, L. (2006). Revision of Estonian cutaway peatlands. The second stage: Ida-Viru, Lääne-Viru, Jõgeva, Järva ja Tartu County. Geological survey of Estonia, Tallinn (in Estonian).
Renou, F., & Farrell, E. P. (2005). Reclaiming peatlands for forestry: the Irish experience. In J. A. Stanturf & P. A. Madsen (Eds.), Restoration of boreal and temperate forests (pp. 541–557). Boca Raton: CRC Press.
Renou-Wilson, F., & Farrell, E. P. (2007). The use of foliar and soil information for optimising the nutrition of Sitka spruce and Norway spruce on cutaway peatlands. Silva Fennica, 41, 409–424.
Rey, A., & Jarvis, P. G. (1997). Growth response of young birch trees (Betula pendula Roth.) after four and half years of CO2 exposure. Annals of Botany, 80, 809–816.
Rey, A., & Jarvis, P. G. (1998). Long-term photosynthetic acclimation to increased atmospheric CO2 concentration in young birch (Betula pendula) trees. Tree Physiology, 18, 441–450.
Riikonen, J., Oksanen, E., Peltonen, P., Holopainen, T., & Vapaavuori, E. (2003). Seasonal variation of physiological characteristics of two silver birch (Betula pendula) clones in the field. Canadian Journal of Forest Research, 33, 2164–2176.
Riipi, M., Ossipov, V., Lempa, K., Haukioja, E., Koricheva, J., Ossipova, S., & Pihlaja, K. (2002). Seasonal changes in birch leaf chemistry: are there trade-offs between leaf growth and accumulation of phenolics? Oecologia, 30, 380–390.
Ruzin, E.S. (1999). Plant microtechnique and microscopy. Oxford University Press, 322 p.
Sellin, A., Õunapuu, E., Kaurilind, E., & Alber, M. (2012). Size-dependent variability of leaf and shoot hydraulic conductance in silver birch. Trees, 26(3), 821–831.
Steen, E., & Larsson, K. (1986). Carbohydrates in roots and rhizomes of perennial grasses. New Phytologist, 104(3), 339–346.
Terashima, I., Miyazawa, S.-I., & Hanba, T. Y. (2001). Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. Journal of Plant Research, 114, 93–105.
Thomas, P. A., & Packham, J. R. (2007). Ecology of woodlands and forests: description dynamics and diversity. Cambridge: Cambrige University Press.
Uri, V., Lõhmus, K., Kiviste, A., & Aosaar, J. (2009). The dynamics of biomass production in relation to foliar and root traits in a grey alder (Alnus incana (L.) Moench) plantation on abandoned agricultural land. Forestry, 82(1), 61–74.
Wallenda, T., Schaeffer, C., Einig, W., Wingler, A., Hampp, R., Seith, B., George, E., & Marschner, H. (1996). Effects of varied soil nitrogen supply on Norway spruce (Picea abies [L.] Karst.). II. Carbon metabolism in needles and mycorrhizal roots. Plant and Soil, 186(2), 361–369.
Wang, T., Tigerstedt, P. M. A., & Viherä-Aarnio, A. (1995). Photosynthesis and canopy characteristics in genetically defined families of silver birch (Betula pendula). Tree Physiology, 15, 665–671.
Acknowledgments
This work was supported by the institutional research funding IUT21-4 (B21004) of the Estonian Ministry of Education and Research, by the Environmental Investment Centre (Projects Nos 29 and 3708) and by the State Forest Management Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguraijuja, K., Klõšeiko, J., Ots, K. et al. Effect of wood ash on leaf and shoot anatomy, photosynthesis and carbohydrate concentrations in birch on a cutaway peatland. Environ Monit Assess 187, 444 (2015). https://doi.org/10.1007/s10661-015-4681-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4681-5