Abstract
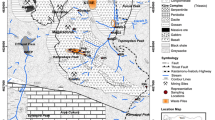
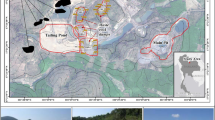
This study investigates the possibility of acid mine drainage (AMD) generation in active and derelict mine waste piles in Sarcheshmeh Copper Mine produced in several decades, using static tests including acid–base accounting (ABA) and net acid-generating pH (NAGpH). In this study, 51 composite samples were taken from 11 waste heaps, and static ABA and NAGpH tests were carried out on samples. While some piles are acid producing at present and AMD is discharging from the piles, most of them do not show any indication on their AMD potential, and they were investigated to define their acid-producing potential. The analysis of data indicates that eight waste piles are potentially acid generating with net neutralization potentials (NNPs) of −56.18 to −199.3, net acid generating of 2.19–3.31, and NPRs from 0.18 to 0.44. Other waste piles exhibited either a very low sulfur, high carbonate content or excess carbonate over sulfur; hence, they are not capable of acid production or they can be considered as weak acid producers. Consistency between results of ABA and NAGpH tests using a variety of classification criteria validates these tests as powerful means for preliminary evaluation of AMD/ARD possibilities in any mining district. It is also concluded that some of the piles with very negative NNPs are capable to produce AMD naturally, and they can be used in heap leaching process for economic recovery of trace amounts of metals without applying any biostimulation methods.






Similar content being viewed by others
References
Adam, K., Kourtis, A., Gazea, B., & Kontopoulos, A. (1997). Evaluation of static tests used to predict the potential for acid drainage generation at sulfide mine sites. Trans Inst Min Metall Sect A: Min Industry 106, January–April, A1–A8.
Akabzaa, T. M., Armah, T. E. K., & Baneong-Yakubo, B. K. (2007). Prediction of acid mine drainage generation potential in selected mines in the Ashanti metallogenic belt using static geochemical methods. Environ Geol, 52, 957–964.
Akcil, A., & Koldas, S. (2006). Acid mine drainage (AMD): Causes, treatment and case studies. J Cleaner Prod, 14, 1139–1145.
Azapagic, A. (2004). Developing a framework for sustainable development indicators for the mining and minerals industry. J Cleaner Prod, 12(6), 639–662.
Banisi, S., & Finch, J. A. (2001). Testing a floatation column at the Sarcheshmeh copper mine. Min Eng, 14(7), 785–789.
Bazin, D., Hübner, H., & Sjerp, A. (1968). Geological investigation in Kerman copper region: Geological survey of Iran. Internal report.
Bigham, J.M., & Nordstrom, D. K., (2000). Iron and aluminum hydroxysulfates from acid sulfate waters. In: Alpers, C. N., Jambor, J. L., & Nordstrom, D. K. (Eds.) Sulfate minerals crystallography, geochemistry, and environmental significance. Reviews Mineralogy and Geochemistry 40, 351–403.
Borden, R. K. (2002). Environmental geochemistry of the Bingham Canyon porphyry copper deposit, Utah. Environ Geol, 43, 752–758.
Canadian Mine Environment Neutral Drainage (MEND) (2001). MEND manual, 2, Sampling and analysis. MEND 5.4.2b: 111.
Castendyk, D. N., Mauk, J. L., & Webster, J. G. (2005). A mineral quantification method for wall rocks at open pit mines, and application to the Martha Au–Ag mine, Waihi, New Zealand. Appl Geochem, 20, 135–156.
Day, S. J. (1989). A practical approach to testing for acid-mine drainage in the mine planning and approval process. Thirteenth annual British Columbia Mine Reclamation Symposium. 7–9 June 1989, Vernon, British Columbia.
Egiebor, N. O., & Oni, B. (2007). Acid rock drainage formation and treatment: A review. Asia-Pac J Chem Eng, 2, 47–62.
Ehrlich, H. L. (2004). Beginnings of rational bioleaching and highlights in the development of biohydrometallurgy: A brief history. Eur J Miner Process Environ Prot, 4, 102–112.
Etminan, H. (1977). Le porphyre cuprifëre de Sar-Cheshmeh (Iran), rôle des phases fluidesdans les mechanisms d'altération et de mineralization. Sciences de la terre, Mem.34:78.
Ferguson, K. D., & Morin, (1991). The prediction of acid rock drainage—Lessons from the database. Proceedings of the Second International Conference on the Abatement of Acid Drainage, Montreal, Quebec, Vol 3
Greenhill, P. G. (2000). AMIRA International: AMD research through industry collaboration. In Proceedings from the Fifth International Conference on Acid Rock Drainage, ICARD 2000, Vol. 1, pp. 13–19.
Hezarkhani, A. (2006). Hydrothermal evolution of the Sar-Cheshmeh porphyry Cu–Mo deposit, Iran: Evidence from fluid inclusions. Journal of Asian Earth Sciences, 28, 409–422.
Ian Wark Research Institute (2002). ARD test handbook. AMIRA P387A Project; prediction and kinetic control of acid mine drainage. Melbourne: AMIRA International
Jambor, J. L., Dutrizac, J. E., & Chen, T. T. (2000). Contribution of specific minerals to the neutralization potential in static tests. In Proceedings of the Fifth International Conference on Acid Rock Drainage. Society for Mining, Metallurgy, and Exploration, Littleton, pp. 551–565
Lapakko, K. A. (1996). Characterization and static testing of ten gold mine tailings. In American Society for Surface Mining and Reclamation Meeting, Duluth, Minnesota, pp. 370–384
Lawrence, R. W. & Wang, Y. (1997). Determination of neutralization potential in the prediction of acid rock drainage. In: Proceedings from the Fourth International Conference on Acid Rock Drainage, Vancouver, pp. 451–464.
Lengke, M. F., Davis, A., & Bucknam, C. (2010). Improving management of potentially acid generating waste rock. Mine Water Environ., 29, 29–44.
Lottermoser, G. B. (2007). Mine wastes, characterization, treatment, environmental impacts (p. 304). Berlin, Heidelberg, New York: Springer.
Luptakova, A., Ubaldini, S., Macingova, E., Fornari, P., & Giuliano, V. (2012). Application of physical–chemical and biological–chemical methods for heavy metals removal from acid mine drainage. Process Biochemistry 47, 1633–1639.
Miller, S., Robertson, A., & Donahue, T. (1997). Advances in acid drainage prediction using the Net Acid Generation (NAG) test. In Proceedings fourth International Conference on Acid Rock Drainage, Vancouver, pp. 533–549
Modabberi, S. (2004). Environmental geochemistry and trace element anomaly in the Takab area and their impact on the Zarrineh Roud reservoir dam, with special reference to Zarshuran deposit. Ph.D. thesis, University of Shiraz.
Morin, A.K., & Hutt, N.M., (1998). Kinetic tests and risk assessment for ARD. In 5th annual BC metal leaching and ARD workshop, 9–10 Dec 1998, Vancouver, pp. 1–10
Nordstrom, D. K., & Alpers, C. N., (1999). Geochemistry of acid mine waters. In: Plumlee, G. S., & Logsdon, M. J. (Eds.), The Environmental Geochemistry of Mineral Deposits: Part A. Processes, Techniques, and Health Issues, Rev. Econ. Geol, vol. 6A, pp. 133– 160
Plumlee, G. (1999). The environmental geology of mineral deposits. In G. S. Plumlee & M. J. Logsdon (Eds.), The environmental geochemistry of mineral deposits. Part A: processes, techniques, and health issues. Vol. 6A, Chapter 3. Reviews in Economic Geology (pp. 71–116). Chelsea: Society of Economic Geologists.
Price, W. A., Errington, J., & Koyanagi, V. (1997). Guidelines for the prediction of acid rock drainage and metal leaching for mines in British Columbia: part I. General procedures and information requirements: MEND, Natural Resources Canada, Ottawa, Proceedings of the 4th International Conference on Acid Rock Drainage 1, p. 1–14.
Schafer, W. M. (2000). Use of the net acid generation pH test for assessing risk of acid generation. In Proceedings from the 5th international conference on acid rock drainage, ICARD 2000, Vol. 1, pp. 613–618
Schreck, P. (1998). Environmental impact of uncontrolled waste disposal in the mining and industrial areas in central Germany. Environ Geol, 35, 66–72.
Shahabpour, J. (1982). Aspects of alteration and mineralization at the Sar-Cheshmeh copper–molybdenum deposit, Kerman, Iran. Ph.D. thesis, Univ. Leeds, 342 p.
Shahabpour, J., & Kramers, J. D. (1987). Lead isotope data from the Sar-Cheshmeh porphyry copper deposit. Iran: Mineral Dep, 22, 278–281.
Smith, M. W., & Skema, V. W. (2001). Evaluating the potential for acid mine drainage remediation through remaining in the Tangascootack Creek watershed, Clinton County, Pennsylvania. Min Eng, 310, 41–48.
Sobek, A. A., Schuller, W. A., Freeman, J. R., & Smith, R. M. (1978). Field and laboratory methods applicable to overburdens and mine soils. Report EPA-600/z-78-054 (p. 203). Cincinnati: US Environmental Protection Agency.
SRK, “Steffen, Robertson, Kirsten”. (1989). Draft acid rock technical guide (Vol. 1). Vancouver: BC AMD Task Force.
Stromberg, B., & Banwart, S. A. (1999). Experimental study of acidity-consuming processes in mining waste rock: some influences of mineralogy and particle size. Appl Geochem, 14, 1–16.
Taylor, G. F. (1998). Overview: acid drainage: sources, impacts and responses. GROUNDWORK, Sept. 1998 contents page. Web posted and copyright 1998, Australian minerals and energy environment foundation.
Vanecek, M. (1994). Mineral deposits of the world, developments in economic geology, no 28. Amsterdam: Elsevier.
Warhurst, A., & Noronha, L. (2000). Environmental policy in mining: Corporate strategy and planning for closure. Boca Raton: Lewis.
Waterman, G. C., & Hamilton, R. L. (1975). The Sarcheshmeh porphyry copper deposit. Econ Geol, 70, 568–576.
White, W. & Lapakko, K. A. (2000). Preliminary indications of repeatability and reproducibility of the ASTM 5744–96 kinetic test for drainage pH and sulfate release rate. In: Proceedings from the Fifth International Conference on Acid Rock Drainage. SME, Littleton, pp. 621–630.
Acknowledgments
Authors would like to thank the National Iranian Copper Industries Company (NICICO) for financial support to this project according to student research support no. 946172; especially, Ms. E. Eslami and Mr. M. Adbollahi. Thanks are also due to the School of Geology, University of Tehran for providing laboratory facilities for part of this research. The authors also wish to thank Professor Mohammad Reza Ganjali, Dean of University College of Science and outstanding professor of the School of Chemistry of the University of Tehran for his advice and also Meysam Vaez zadeh and Hamid Reza Rahimi Lanji for their help with geochemical tests. The researchers would like to express their appreciation for the help of Dr. Rich Borden for his invaluable comments and advice. We would like to express our sincere thanks to anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modabberi, S., Alizadegan, A., Mirnejad, H. et al. Prediction of AMD generation potential in mining waste piles, in the sarcheshmeh porphyry copper deposit, Iran. Environ Monit Assess 185, 9077–9087 (2013). https://doi.org/10.1007/s10661-013-3237-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3237-9