Abstract
Seed coating is an effective method for delivering beneficial microorganisms to the soil. Additionally, coated seeds are protected against pathogens or microbial contaminants during storage. This work aimed to evaluate the effect of seed coating with the beneficial fungus Trichoderma koningiopsis against fungal contaminants of sweet sorghum (Sorghum bicolor (L.) Moench) during storage. Sorghum-treated seeds with three concentrations of Trichoderma koningiopsis were stored at two temperatures (5 °C and 18 °C) over 12 months to evaluate physiological-, and sanitary quality of seeds. The concentrations of the most prevalent contaminant fungi Diaporthe melonis, Curvularia lunata and Penicillium polinocum were monitored. The viability of Trichoderma koningiopsis was evaluated every three months and correlated with the seed quality variables. The results indicate that beneficial fungi can protect seeds against fungal contamination during storage avoiding the application of a chemical fungicide. The coating procedure did not affect the seed germination over the 12 months of storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sweet sorghum (Sorghum bicolor (L.) Moench) is an important species for its sugar accumulation ability in the stem parenchyma tissue, making it a valuable source for grain, sugar, ethanol, and especially forage production (Bernal, 2014). This species also has high biomass production potential, high regrowth capacity, favorable leaf/stem ratio, and forage quality (Coronado et al., 2019). Sweet sorghum plants can be used either for fresh or dry consumption, this crop is positioned as an ideal nutritional supplement for animals, mainly in cattle (Mejía-Kerguelén et al., 2019). Due to its efficient water management, sweet sorghum easily adapts to different types of soil and adverse climatic conditions such as drought. It requires a lower amount of hydric resources compared to other cereals, being a sustainable agricultural system (Coronado et al., 2019; Getachew et al., 2016).
Seed storage is important for food security, biodiversity conservation, and agricultural sustainability among others. The success of seed storage depends on the initial quality of the seeds. Poor storage conditions could generate negative biochemical and physical effects compromising seed vigor (Hartmann et al., 2017). During the post-harvest process, several factors influence the degree of seed deterioration, such as moisture content, environmental conditions and the intrinsic characteristics of the seeds. Due to the natural seed respiration process, the physiological quality tends to decrease during storage; however, the adoption of appropriate management practices contributes to their longevity and quality assurance.
Among the most important factors for the optimal storage of seeds are the moisture content and the storage temperature of the seeds. Appropriate moisture content during storage extends the shelf-life of the seeds, while reducing the risks of microorganism activity (Bernardes et al., 2020). Low storage temperatures extend the shelf-life of seeds and could mitigate the negative effects of high moisture content (Hartmann et al., 2017).
Fungi constitute the largest group of seed-borne pathogens (Özer & Coşkuntuna, 2016). It is estimated that approximately 10–20% of the stored seeds become deteriorated by fungi (Kumar et al., 2023). Many fungal pathogens infect seeds during the development and maturation stages reducing seed yield and quality by limiting or inhibiting germination and seedling emergence (Bailly et al., 2019). Fungi cause different types of diseases and disorders such as abortion, size reduction, rot, necrosis, seed discoloration, reduction or elimination of germination capacity, and physiological alterations in the seeds (Özer & Coşkuntuna, 2016).
Among these organisms, fungi of the genera Diaporthe, Curvularia and Penicillium can cause the most economically important seed diseases in species such as sorghum. Heavy infestation of Diaporthe can lead to yield losses of up to 100% and a 90% reduction in seed germination (Li et al., 2023). Seed germination loss due to different Curvularia species ranged from 24.4 to 25.9% (Girish et al., 2011). Losses due to Penicillium spp. rotting disease are estimated to be approximately 15%–45% (El-Samawaty et al., 2021).
Different strategies have been used to control seed-borne pathogens including chemical, biological, regulatory, and cultural methods (Bisen et al., 2020; Chrapačienė et al., 2022). Seed treatment could reduce the damage caused by these pathogens (Bailly et al., 2019; Mancini & Romanazzi, 2014). Currently, seed treatment with chemical synthesis products such as fungicides is still the most widely used approach by farmers to control these diseases (Bisen et al., 2020). However, the growing concerns about their negative impact on the environment, non-target organisms and human health, as well as the increasing resistance of pathogens has encouraged the exploration of sustainable alternatives (Bisen et al., 2020; Chrapačienė et al., 2022).
The use of microorganisms for agricultural disease management is one of the safest alternatives compared to conventional management practices that have severely affected the environment and agroecosystems (Bisen et al., 2020). Plant Beneficial Microorganisms (PBM) provide several benefits to plants, such as nutrient uptake and distribution, stress tolerance, pathogens protection, plant immune system stimulation and growth promotion among others (Paravar et al., 2023). The mobility of microorganism in soil is low, therefore microorganism should be close to the site of action, in the rhizosphere. Seed coating has been used as a delivery system for microbes reducing the amount of inoculant needed to achieve the desired effect and is considered a precision agriculture technique (Ma, 2019).
Trichoderma koningiopsis is a fungus with excellent biocontrol activity against phytopathogens such as: Botrytis cinerea, Sclerotium cepivorum, Sclerotinia sclerotiorum, Sclerotinia minor, Fusarium oxysporum and Rhizoctonia solani (Cotes, 2018). In Colombia, the biofungicide Tricotec® WG based on the strain T. koningiopsis Th003 has been developed at the Corporación colombiana de investigación agropecuaria (AGROSAVIA, 2020) and it is widely used by farmers. In this work we explore seed coating with T. koningiopsis Th003 to preserve S. bicolor seeds during storage and protect them from common contaminants.
Materials and methods
Location
This research was conducted at Tibaitatá Research Center in AGROSAVIA (Corporación colombiana de investigación agropecuaria) located in Mosquera, Colombia (N 4º41′43.1349’’, W 74º12′18.7666’’, 2600 m).
Plant material
Sweet sorghum seeds AGRVSR1 used for the experiment were obtained from the seed multiplication fields located in El Espinal – Tolima, Colombia.
Seed coating procedure and storage
Sorghum seeds (200 g) were coated using a fluidized bed (Uni-glatt 8512 Glatt® GmbH, Binzen, Germany) in a Wurster configuration. The inlet air temperature to fluidize the seeds was 35 °C. The microorganism suspension (T. koningiopsis Th003) prepared from the product Tricotec® WP (AGROSAVIA) with a biopolymer was sprayed onto the seeds using a peristaltic pump and a compressed air spray gun at 13.8 kPa. Three concentrations of T. koningiopsis were applied (D1: 1 × 105 CFU seed−1, D2: 5 × 105 CFU seed−1 and D3: 1 × 106 CFU seed−1). A chemical control (V) was included (Carboxin + Thiram 200 g a.i./kg) and was applied by manual shaking of the seeds and the product in plastic bags. Untreated seeds corresponded to absolute control (T). Coated seeds were packed in silver trilaminate bags (15X20 cm) separately for each dose, temperature (5 °C and 18 °C), and sampling times (0, 3, 6, 9, and 12 months).
Trichoderma koningiopsis viability over the seeds
The viability of T. koningiopsis conidia over seeds (colony forming units UFC seed−1) was determined using the plate counting technique. Ten seeds were placed in 10 mL of 0.1% Tween 80 to wash off conidia. Serial dilutions were conducted, and 100 µL were poured onto Petri dishes with PDA medium and Triton 100X, and incubated at 25 ± 2 °C. The colonies were counted after 5 days.
Physiological seed quality evaluation
Physiological quality was assessed by the germination percentage for each treatment. The methodology proposed by the "International Seed Testing Association" (ISTA) for sorghum seed was used; germination was considered as the proportion of seeds that produced seedlings classified as normal under specific conditions and time periods (ISTA, 2016). The experimental unit consisted of 200 seeds and four replicates (50 seeds per replicate) placed between germination paper in a growth chamber, incubated at ± 25 °C and HR ± 80% for 10 days. The initial count was carried out on the 5th day after the start of the test and the final count on the 10th day. In each sampling time, the number of non-germinated or dead seeds, normal seedlings, and abnormal seedlings was evaluated. The results for each component were expressed as percentages.
Molecular identification
In order to guarantee the identity of the most prevalent contaminants and to determine if seed treatment with T.koningiopsis was selective against those fungal contaminants, pure-culture isolates of Diaporthe melonis, Curvularia lunata, and Penicillium polinocum were molecularly identified. DNA extraction was carried out according to the method proposed by Griffith and Shaw (1998). Subsequently, PCR amplification was done using the primers ITS1F-ITS4 (ITS1-F 5ʹ-CTTGGTCATTTAGAGGAAGTAA-3ʹ and ITS4 5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) and using the following program: 35 cycles of steps 1 to 4, (1) initial denaturation for 2 min at 95 °C, (2) denaturation 1 min at 94 °C, (3) annealing for 30 s at 55 °C, (4) extention for 3 min at 72 ºC and (5) a final extension for 7 min at 72 ºC.
The resulting sequences were compared with the corresponding ITS1-5.8S-ITS2 region (Schoch et al., 2012) of the sequences in the GenBank database of the National Center for Biotechnology Information (NCBI, 2022). The isolated fungi biotypes were preserved in PDA medium at -4 °C (three copies of each isolate) and cryopreserved at -80 °C using a solution of Glycerol 20% and Peptone 0.5%.
Sanitary seed quality evaluation
A modified blotter test was employed to evaluate the prevalence of fungal contaminants in the seeds of each treatment (Gaur, et al., 1997; Tsedaley, 2015). In 150 X 25 mm plastic Petri dishes humid chamber conditions were generated using sterile napkins (absorbent towels in Z 24 cm × 25 cm), and sterile distilled water. On the wet napkin, 50 seeds of each treatment were placed separately, and four repetitions per treatment were employed. The seeds were incubated at 25 °C ± 3 °C and RH ~ 90% ± 5% for seven days (Ordon et al., 2009). After seven days the containers were checked to evaluate fungi mycelial growth on the seeds (Ordon et al., 2009). The emerging mycelium on the surface of the seeds was subsequently seeded in PDA medium pH 4.5 (Adjusted with Lactic Acid). The isolates were incubated in the dark at 25 °C for 7 days in the same culture medium.
At the end of the germination process in humid chambers, seven days after sowing, the percentage of total germination, healthy seeds, germinated healthy seeds, and contaminating fungi prevalence differentiated by genus was evaluated (Chrapačienė et al., 2022; Ordon et al., 2009; Wain-Tassi et al., 2012).
Experimental design and statistical analysis
A completely randomized design with four repetitions and a two-level factorial arrangement was used. The first level corresponded to the storage temperature: 5 °C or 18 °C. The second factor referred to the seed treatment, which included three concentrations of T. koningiopsis koningiopsis (D1, D2, and D3), compared with the chemical control (V) and the absolute control of untreated seeds (T). The experimental units used to evaluate the sanitary and physiological quality were the Petri dish with 50 seeds, and the roll of germination paper with 50 seeds respectively. For the viability of T. koningiopsis over the seeds, the experimental unit consisted of 10 seeds of each treatment. Viability results were expressed on a log base.
The data were analyzed using the Analysis of Variance—ANOVA procedure of the statistical program R Studio® (R Core Team, 2020). In addition, Tukey’s mean comparison test was performed with a significance level of 5%. Furthermore, multivariate analysis was performed comparing distribution profiles of each of the response variables.
Results and discussion
Trichoderma koningiopsis viability over the seeds
The viability of microorganisms on the seed is a critical factor in the development of coating formulations. The PBM must remain viable during seed storage period for subsequent establishment in the soil (Sarkar et al., 2021). Table 1 shows the viability results of T. koningiopsis at the three doses applied to sorghum seeds stored for 12 months at temperatures of 5 °C and 18 °C. It is observed that at the lowest dose (D1) there was a drastic reduction in the viability of the microorganism after 3 months of storage at the two temperatures evaluated, on the other hand, in the doses D2 and D3, it was observed that the storage temperature of 18 °C caused a greater reduction in viability. The only treatment in which the viability remained stable during the 12 months of storage was at the higher dose (D3) of the microorganism stored at 5 °C (S1). This effect on the viability of the microorganism over the seed as a function of the initial concentration and the coating formulation composition had been previously reported (Cortés-Rojas et al., 2021). However, the minimum concentration needed for a protection effect against contaminant fungus on stored seeds had not been evaluated. Probably, the mechanism involved is related to mycoparasitism, the production of secondary metabolites with antibiotic activity, and nutrient competition between the beneficial- and the contaminant fungus (Saldaña-Mendoza et al., 2023). Consequently, it is important to maintain the minimum effective viability of the beneficial fungi to avoid seed contamination over the storage time required. Harmful conditions over the seed such as temperature gradients, UV radiation, moisture, extreme drought, and seed exudates could affect the microorganism survival during storage, therefore a proper coating formulation that protects microorganisms should be developed et al., 2019). When viable microorganisms are delivered into the soil, endophytism and rhizosphere colonization are promoted, therefore seed preservation during storage and plant growth promotion could be achieved using coated seeds with T. koningiopsis (Ma, 2019).
Physiological seed quality evaluation
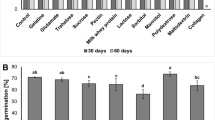
The germination results (Fig. 1) indicate that the coating process with the microorganism did not affect the germination process of the seed for most of the treatments. However, it was not possible to establish a correlation between treatments and seed storage under different temperature conditions.
A homogeneous distribution of the germination data population was obtained at 0 and 3 months of sampling, a greater variation was observed at 6 and 9 months. The results of D2 at 9 months at 18 °C correspond to atypical data, which could be related to an uneven initial physiological seed quality of this replicate.
Germination studies on seeds treated with T. koningiopsis suggest that variations in plant germination may be related to the form of application, the plant species, and the strain of T. koningiopsis used for the test. Results on seeds of other cereal species such as corn that were treated with Trichoderma extracts, showed a reduction in germination rate, but not in the final rate of seed germination. However, when seeds were treated with the spore suspension the germination was inhibited due to the colonization of the seed surface by the microorganism (Hajieghrari, 2010). The opposite occurs with chili and soybean seeds treated with different strains of Trichoderma, which showed a positive effect on germination rates (Mukhtar et al., 2012).
The germination percentage of the chemical treatment was the highest and the most stable over time at temperatures of 18 °C and 5 °C until the sixth month. In the subsequent measurement (9 months) at 5 °C a slight decrease was observed but without significant differences. Conversely, the control (untreated seeds) after 3 months of evaluation, showed a decrease in germination percentages at both temperatures. This result is consistent with the report by Trafane et al. (2017) on soybean, who recommended seed treatment to protect seeds from biotic and abiotic factors that affect quality. Owolade et al. (2019), found that sorghum seeds germination is preserved at low-temperature conditions if the initial moisture content is between 10.5 and 12.5%. Moisture values above 12.5% and high-temperature conditions significantly affect seed germination after 6 and 9 months of storage.
Germination rate of treated and untreated sorghum seeds stored at 5 °C and 18 °C for 0 months 3 months, 6 months, 9 months, and 12 months as a function of three doses (D1, D2, D3) of T. koningiopsis applied, a chemical control (V) and untreated seeds (T). Same letters indicate no significant difference for the same treatment over time at the same temperature (Tukey p > 0.05)
Molecular identification
Six species of filamentous fungi were molecularly identified in sorghum seed. Five of the identified species showed a percentage of identity and a query cover of 100%. One of the species found, Diaporthe melonis, had an identity percentage of 87% (S2). All the genera found have been previously reported as contaminants of sorghum seed in storage (Islam et al., 2009; Patiño-Moscoso et al., 2023).
Sanitary seed quality evaluation
Contaminating fungi on sorghum seeds varied significantly depending on the T. koningiopsis dose and time elapsed. In general, a greater reduction of contaminating fungi was evidenced at lower temperatures and with higher doses of T. koningiopsis (Fig. 2).
During the 12 months of storage, the seeds treated with T. koningiopsis showed in most cases a lower percentage of contamination compared to the absolute- and chemical controls. At storage temperature of 5 °C, the highest doses of T. koningiopsis (5 × 105 and 1 × 106 CFU seed−1) significantly reduced the presence of contaminating fungi compared to untreated seeds. At the highest storage temperature (18 °C), the efficacy of biological control was lower, however significant reductions in contamination were observed compared to controls. It is important to clarify that in each of the measurements, there were no significant differences in the incidence of fungi in seeds for the same treatment at the two storage temperatures.
The chemical control was effective in reducing contaminating fungi and its efficacy remained stable over time and under storage conditions. For the T. koningiopsis treatments, the biocontrol effect could be achieved at the first months of storage through the production of secondary metabolites with antibiotic activity and this effect could persist over time even when the viability of the microorganism decreased especially at 18 °C.
Fungal contamination of treated and untreated sorghum seeds stored at 5 °C and 18 °C for 0 months, 3 months, 6 months, 9 months, and 12 months as a function of three doses (D1, D2, D3) of T. koningiopsis applied, a chemical control (V) and untreated seeds (T). Same letters indicate no significant difference for the same treatment over time at the same temperature (Tukey p > 0.05)
The results obtained provide concrete information on the efficacy of T. koningiopsis as a biological control agent for the reduction of contaminating fungi in stored sorghum seeds. The data indicate that the use of T. koningiopsis can be a viable strategy to ensure seed quality, and thus contribute to the reduction of losses due to fungal contamination during storage.
The efficacy of T. koningiopsis was not related to the slowing of contaminant fungal growth and metabolism at low temperatures, as the behavior was similar at both temperatures. However, the effect of seed treatment with T. koningiopsis is remarkable at all sampling temperatures and sampling times (Table 1), which shows that T. koningiopsis is providing seed protection over time.
The efficacy of biological control was influenced by the dose of T. koningiopsis applied. The highest doses (5 × 105 and 1 × 106 CFU seed−1) resulted in a greater reduction of contaminating fungi compared to the lowest dose (1 × 105 CFU seed−1).
The use of T. koningiopsis as a biological control agent offers significant advantages in comparison with chemical control. Firstly, T. koningiopsis is a beneficial organism with low toxicity to non-target organisms, making it a safer and more sustainable option compared to chemically synthesized fungicides. In addition, the use of T. koningiopsis can reduce the risk of developing fungal resistance, which is a common problem associated with the repeated use of chemical fungicides (Tyśkiewicz et al., 2022).
Of the six species of contaminating filamentous fungi, the highest prevalence was found for Diaporthe melonis, Curvularia lunata, and Penicillium polonicum (S3, S4 and S5). The genera Penicillium and Curvularia have been previously reported as storage- and field fungi respectively (Islam et al., 2009), however, D. melonis has not been reported as affecting seed or sorghum plants (Sorghum bicolor) (Patiño-Moscoso et al., 2023).
According to Montes-García et al. (2010) in S. bicolor the seed quality losses could be attributed to the pathogen C. lunata, especially when the grain-filling phase coincides with high temperatures and high relative humidity. On the other hand, Prom (2004) evaluated the effect of common fungi on sorghum seeds and found that C. lunata was the most frequent contaminant with significant negative correlations with germination potential. Other studies support the effectiveness of Trichoderma in controlling pathogens such as Curvularia lunata and Diaporthe in rice and avocado production systems (Ramírez et al., 2019; Infante et al., 2009; Vijitrpanth et al., 2023).
The principal component analysis (PCA) presented in a biplot (Fig. 3), showed the relationship between the different variables evaluated. The biplot provided a visual representation of the relationships between the variables and how the data were grouped based on the different storage conditions and the treatments applied to the seeds (Fig. 3). Dimensions 1 and 2 explained 76.2% of the variability of the data, dimension 1 representing physiological quality variables while dimension 2 sanitary quality variables.
Principal component analysis of physiological and sanitary variables: Germination rate (PG), Abnormal seedling (PA), Contaminated seeds (SC), Prevalence of Penicillium polonicum (PTP), Prevalence of Curvularia lunata (PTC), Prevalence of Diaporthe melonis (PTD) of treated and untreated sorghum seeds stored at 5 °C and 18 °C (A.) for 0 months, 3 months, 6 months, 9 months, and 12 months (B.) as a function of three doses (D1, D2, D3) of T. koningiopsis applied, a chemical control (V) and untreated seeds (T) (C.)
The effect of the variables contaminated seeds (SC), seeds contaminated by Penicillium polonicum (PTP), seeds contaminated by Curvularia lunata (PTC) and seeds contaminated by Diaporthe melonis (PTD) are in the same direction as axis 1 indicating a high positive correlation with each other. Consecutively, a negative correlation is observed between the percentage of germination and the percentage of abnormal plants. However, no clear correlation was observed between the seed germination and its contamination. This suggests that the germination capacity of the seeds is not directly related to their level of contamination by fungi. However, these contaminant fungi can generate negative alterations in the subsequent development of the crop.
These results contrast with those reported by Prom et al., (2003) who affirm that germination of sorghum seeds is significantly reduced by fungi such as C. lunata and F. thapsinum. Similarly, Yago et al., (2011) indicate that the low germination rate in sorghum is correlated with the presence of microorganisms such as Alternaria alternata, Aspergillus flavus, Curvularia lunata, and Fusarium moniliforme,which affect the growth of new emerging sprouds. These fungi can affect germination by altering seed embryo (Worang et al., 2008). In the present study it was not determined if the fungi were superficial or if they had reached the embryo, however, it could be inferred that the isolates identified as contaminant fungi did not reach the embryo and did not affect germination. In this regard, Yago et al., (2011) states that some fungi are only saprophytic in nature and could be easily eliminated by disinfection, but there are also seed-borne fungi that could penetrate the inner layer of the seeds. Usually, the endosperm and embryo infection by fungi occurs during the seedling stage.
The biplot analysis shows that the results of the absolute control are found close to and in the same direction of the variables related to seed contamination, indicating similarities in their multivariate profiles. This behavior indicates that the absence of any treatment on the seeds contributes to the appearance of contaminating fungi. Accordingly, the chemical treatment is associated with higher positive values of germination compared to other treatments. Furthermore, as the dose of T. koningiopsis increases, a decrease in the percentage of seed contamination is observed, confirming the protective effect of T. koningiopsis against fungal contamination. This result agrees with that reported by Corallo Fabiano et al. (2021) who state that sorghum seeds treated with T. asperellum can remain healthy and protect themselves from pathogens such as Fusarium nygamai. The isolates of Trichoderma spp. have shown the ability to endophytically colonize and protect sorghum plants (Corallo Fabiano et al., 2021; Coninck et al., 2020). This suggests their potential as promising sources of antagonists’ application in biological control.
Regarding the influence of storage time and temperature on the evaluated variables, it is observed (Fig. 3) that the highest values of total contamination of seed (for each fungal species) are associated with the control treatment (control) and with some results of the first 3 months of storage, especially when the seeds were stored at 5 °C. However, there is no clear pattern that shows a significant effect of sampling times or storage temperatures on the response to the variables evaluated. These results contrast with the study by Owolade et al., (2019) who show that the prevalence of sorghum-contaminating fungi on seeds was significantly higher when the seeds are stored at room temperature but is independent of the type of packaging and storage time.
Seed moisture increases during storage and high infection rates can lead to substantial loss of sorghum seed; therefore, control of temperature and relative humidity during storage is important. Moisture content in stored seeds affects both quality and storability, the water activity promotes microbial growth, which increases the rate of seed damage (Abdulsalaam & Shenge, 2011). The effect of coating formulations on moisture content and hygroscopicity should be monitored over storage time.
The results obtained in this investigation showed that sorghum seed coated with T. koningiopsis-based formulations could be stored for approximately one year at temperatures ranging between 18 °C and 5 °C. This approach significantly reduces the presence of fungal contaminants both from the field and from storage, all without compromising the physiological quality of the seeds, as reflected in their germination capacity.
Conclusions
The coating process with the beneficial fungus T. koningiopsis did not affect the germination process of sorghum. The viability of the microorganism on the seeds depended on the concentration applied and the storage temperature, at 18 °C a sharp reduction was observed specially at lower doses, however a concentration of 1 × 103 UFC seed−1 was enough to protect seeds of contaminant fungi. A dose dependent correlation was observed for contaminated seeds and sanitary quality of seeds, at the highest concentration (D3) of T. koningiopsis applied, lower percentage of contaminated seeds were obtained. Seed preservation using beneficial fungi could be used instead of a fungicide against contaminant fungi. In future studies it would be interesting to evaluate the effect on plant growth promotion of the coated seeds with T. koningiopsis after storage and the prevalence of field diseases considering the resistance induction effect.
Data availability
All datasets generated for this study are included in the article/Supplementary Material.
References
Abdulsalaam, S., & Shenge, K. S. (2011). Seed borne pathogens on farmer-saved sorghum (Sorghum bicolor L.) seed. Journal of Stored Products and Postharvest Research, 2(2), 24–28.
Bailly, C., Bousquet, A., Braun, V., Buitink, J., Desbois-Vimont, C., Durand Tardif, M., & Szambien, M. (2019). Towards seed protection using biocontrol strategies hal-02931599. https://hal.archives-ouvertes.fr/hal-02931599
Bernal, J. H., Rincón, A., Guevara, E., Hernández, R., & Flórez, H. (2014). Sorgo forrajero Corpoica JJT-18 Boletín técnico Producción tecnológica Corporación colombiana de investigación agropecuaria Agrosavia. http://hdl.handle.net/20.500.12324/1068
Bisen, K., Singh, V., Keswani, C., Ray, S., Sarma, B. K., & Singh, H. B. (2020). Use of biocontrol agents for the management of seed-borne diseases. In R. Kumar & A. Gupta (Eds.), Seed-borne diseases of agricultural crops: Detection diagnosis & management (1st ed., pp. 651–663). Singapore. https://doi.org/10.1007/978-981-32-9046-4_22
Chrapačienė, S., Rasiukevičiūtė, N., & Valiuškaitė, A. (2022). Control of Seed-Borne Fungi by Selected Essential Oils. Horticulturae, 8(3), 220. https://doi.org/10.3390/horticulturae8030220
Coninck, E., Scauflaire, J., Gollier, M., Liénard, C., Foucart, G., Manssens, G., & Legrève, A. (2020). Trichoderma atroviride as a promising biocontrol agent in seed coating for reducing Fusarium damping-off on maize. Journal of Applied Microbiology, 129(3), 637–651. https://doi.org/10.1111/jam.14641
Corallo Fabiano, A. B., Bettucci Rossi, L. J., & Tiscornia Córdoba, S. M. (2021). Trichoderma strains selection for biological control of Fusarium nygamai in sorghum ( Sorghum bicolor L. Moench). Agricultural Biotechnology, 8(1), 4064. https://doi.org/10.23850/24220582.4064
Coronado, M., Martínez, D., Vásquez, J., Medina, F., & Córdova, A. (2019). Evaluación del programa “paquetes tecnológicos de sorgo forrajero” en el DDR 143 de SAGARPA Moctezuma Sonora. Revista Mexicana de Agronegocios, 45, 355–369. https://www.redalyc.org/articulo.oa?id=14162394008
Cortés-Rojas, D. F., Beltrán-Acosta, C. R., Zapata-Narvaez, Y., Chaparro, M., & Cruz-Barrera, M. (2021). Seed coating as a delivery system for the endophyte Trichoderma koningiopsis Th003 in rice (Oryza sativa). Applied Microbiology and Biotechnology, 105, 1889–1904.
Corporación colombiana de investigación agropecuaria [AGROSAVIA] (2020, March 2021) Sorgo dulce para forraje Corpoica JJT-18. Retrieved March 10 2021, from: https://www.agrosavia.co/productos-y-servicios/oferta-tecnol%C3%B3gica/l%C3%ADnea-pecuaria/ganader%C3%ADa-y-especies-menores/material-reproductivo-vegetal-semillas/302-sorgo-dulce-corpoica-jjt-18.
Cotes, A. M. (2018). Control biológico de fitopatógenos, insectos y ácaros (Vol. 2). Corporación colombiana de investigación agropecuaria (AGROSAVIA).
El-Samawaty, A. E. M. A., El-Wakil, D. A., Alamery, S., & Mahmoud, M. M. H. (2021). Potency of plant extracts against Penicillium species isolated from different seeds and fruits in Saudi Arabia. Saudi Journal of Biological Sciences, 28(6), 3294–3302. https://doi.org/10.1016/j.sjbs.2021.02.074
Gaur, S. N., Agarwal, G., & Gupta, S. K. (1997). Use of LPC antagonist, choline, in the management of bronchial asthma. The Indian Journal of Chest Diseases & Allied Sciences, 39(2), 107–113.
Getachew, G., Putman, D., De Ben, C., & De Peters, E. (2016). Potential of Sorghum as an Alternative to Corn Forage. American Journal of Plant Sciences, 7, 1106–1121. https://doi.org/10.4236/ajps.2016.77106
Girish, G. A., Baig, M. M. V., Anitha, K., & Chakrabarty, S. K. (2011). Curvularia species detected in sorghum seeds collected from Marathwada Region of Maharashtra. Indian Journal of Plant Protection, 39(4), 299–303.
Griffith, G. W., & Shaw, D. S. (1998). Polymorphisms in Phytophthora infestans: Four mitochondrial haplotypes are detected after PCR amplification of DNA from pure cultures or from host lesions. Applied and Environmental Microbiology, 64(10), 4007–4014. https://doi.org/10.1128/aem.64.10.4007-4014.1998
Hajieghrari, B. (2010). Effects of some Iranian Trichoderma isolates on maize seed germination and seedling vigor. African Journal of Biotechnology, 9(28), 4342–4347.
Hartmann, H.T., Kester, D.E., Davies, F. T., Geneve, R. L., & Wilson, S. B. (2017). Hartmann and Kester’s Plant Propagation Principles and Practices (9th ed.). Pearson Education Inc.
Infante, D., Martínez, B., González, N., & Reyes, Y. (2009). Mecanismos de acción de Trichoderma frente a hongos fitopatógenos. Revista Protección Vegetal, 24(1), 14–21.
International Seed Testing Association [ISTA]. (2016). International Rules for Seed Analysis 2016. ISTA.
Islam, S. M. M., Masum, M. M. I., & Fakir, M. G. A. (2009). Prevalence of seed-borne fungi in sorghum of different locations of Bangladesh. Scientific Research Essays, 4(3), 176–179. https://doi.org/10.5897/SRE.9000767
Kumar, N., Khurana, S. M. P., & Pandey, V. N. (2023). Deciphering of seed Health of common food grains (wheat, rice) of North Eastern UP and Gurgaon Haryana. India. Sci Rep, 13, 8480. https://doi.org/10.1038/s41598-023-34510-3
Li, S., Smith, J. R., & Zhang, L. (2023). Evaluation of exotic soybean accessions and their use in developing improved soybean lines with resistance to Phomopsis seed decay. PLoS ONE, 18(6), e0286519. https://doi.org/10.1371/journal.pone.0286519
Ma, Y. (2019). Seed coating with beneficial microorganisms for precision agriculture. Biotechnology Advances, 37, 107423. https://doi.org/10.1016/j.biotechadv.2019.107423
Mancini, V., & Romanazzi, G. (2014). Seed treatments to control seedborne fungal pathogens of vegetable crops. Pest Management Science, 70(6), 860–868. https://doi.org/10.1002/ps.3693
Mejía-Kerguelén, S., Tapia-Coronado, J. J., Atencio-Solano, L. M., & Cadena-Torres, J. (2019). Producción y calidad nutricional del forraje del sorgo dulce en monocultivo e intercalado con maíz y frijol. Pastos y Forrajes, 42(2), 1–15.
Montes-García, N., Prom, L. K., Montes-Rodríguez, N., García-Gracia, M. Á., Pecina-Quintero, V., & Díaz-Franco, A. (2010). Efecto de fungicidas sistémicos en el control de la micobiota parasítica del grano de sorgo (Sorghum bicolor (L.) Moench). Revista Mexicana de Fitopatología, 28(2), 156–158.
Mukhtar, I., Hannan, A., Atiq, M., & Nawaz, A. (2012). Impact of Trichoderma species on seed germination in soybean. Pakistan Journal of Phytopathology, 24(2), 159–162.
Ordon, F., Habekuss, A., Kastirr, U., Rabenstein, F., & Kühne, T. (2009). Virus resistance in cereals: Sources of resistance, genetics, and breeding. Phytopathology, 157(9), 535–545. https://doi.org/10.1111/j.1439-0434.2009.01540.x
Owolade, O. F., Olasoji, J. O., & Afolabi, C. G. (2019). Effect of storage temperature and packaging materials on seed germination and seed-borne fungi of sorghum (Sorghum bicolor L. Moench.) in Southwest Nigeria. International Journal of Agroforestry and Silviculture, 7(9), 1–5.
Özer, N., & Coşkuntuna, A. (2016). The biological control possibilities of seed-borne fungi. In P. Kumar, V. Gupta, A. Tiwari, & M. Kamle (Eds.), Current trends in plant disease diagnostics and management practices (1st ed., pp. 83–403) https://doi.org/10.1007/978-3-319-27312-9_17
Patiño-Moscoso, M. A., Osorio-Guerrero, K. V., Flórez-Gómez, D. L., Sarmiento-Moreno, L. F., & Vargas-Ramírez, D. N. (2023). Identificación molecular y prevalencia de contaminantes fúngicos en semillas de cultivos semestrales. Science Agropecuaria, 14(3), 347–358. https://doi.org/10.17268/sci.agropecu.2023.030
Prom, L. K., Waniska, R. D., Kollo, A. I., & Rooney, W. L. (2003). Response of eight sorghum cultivars inoculated with Fusarium thapsinum Curvularia lunata and a mixture of the two fungi. Crop Protection, 22(4), 623–628. https://doi.org/10.1016/S0261-2194(02)00246-6
R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org/
Rocha, I., Ma, Y., Souza-Alonso, P., Vosátka, M., Freitas, H., & Oliveira, R. S. (2019). Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Frontiers in Plant Science, 10, 1357. https://doi.org/10.3389/fpls.2019.01357
Saldaña-Mendoza, S. A., Pacios-Michelena, S., Palacios-Ponce, A. S., Chávez-González, M. L., & Aguilar, C. N. (2023). Trichoderma as a biological control agent: Mechanisms of action, benefits for crops, and development of formulations. World Journal of Microbiology and Biotechnology, 39, 269.
Sarkar, D., Singh, S., Parihar, M., & Rakshit, A. (2021). Seed bio-priming with microbial inoculants: A tailored approach towards improved crop performance, nutritional security, and agricultural sustainability for smallholder farmers. Current Research in Environmental Sustainability, 3, 100093. https://doi.org/10.1016/j.crsust.2021.100093
Schoch, C. L., Seifert, K. A., Huhndorf, S., Robert, V., Spouge, J. L., Levesque, C. A., & Schindel, D. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences of the United States of America, 109, 6241–6246. https://doi.org/10.1073/pnas.1117018109
Tsedaley, B. (2015). A review on disease detection, pathogen identification and population genetics in fungi. Journal of Biology, Agriculture and Healthcare, 5(1), 6–20.
Trafane, L., da Motta, X., Suarez, C., da Silva, A., Géri, E., & Madruga, L. (2017). Tratamiento de semillas de soja y su influencia sobre la calidad fisiológica a lo largo del almacenamiento. Agrociencia (Uruguay), 21(1), 58–68.
Tyśkiewicz, R., Nowak, A., Ozimek, E., & Jaroszuk-Ściseł, J. (2022). Trichoderma: The Current Status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. International Journal of Molecular Sciences, 23(4), 2329. https://doi.org/10.3390/ijms23042329
Vijitrpanth, A., Jantasorn, A., & Dethoup, T. (2023). Potential and fungicidal compatibility of antagonist endophytic Trichoderma spp. from rice leaves in controlling dirty panicle disease in intensive rice farming. BioControl, 68, 61–73. https://doi.org/10.1007/s10526-022-10161-7
Wain-Tassi, A. L., Santos, J. F., Cássia Panizzi, R., & Vieira, R. D. (2012). Seed-borne pathogens and electrical conductivity of soybean seeds. Scientia Agricola, 69(1), 19–25. https://doi.org/10.1590/S0103-90162012000100004
Worang, R. L., Dharmaputra, O. S., Syarief, R., & Miftahudin. (2008). The quality of physic nut (Jatropha curcas L.) seeds packed in plastic material during storage. Biotropia, 15, 25–36.
Yago, J. I., Roh, J. H., Bae, S. D., Yoon, Y. N., Kim, H. J., & Nam, M. H. (2011). The Effect of Seed-borne mycoflora from sorghum and foxtail millet seeds on germination and disease transmission. Mycobiology, 39(3), 206–218. https://doi.org/10.5941/MYCO.2011.39.3.206
Acknowledgements
The authors would like to thank to the Ministerio de Agricultura y Desarrollo Rural of Colombia (MADR), the Corporación colombiana de investigación agropecuaria (AGROSAVIA) for their participation and financing of this research, under the framework of project ID 1001876 “Conservation and production of quality seed and plant material for AGROSAVIA’s corporate Technological Offers in order to activate escalation and commercial linkage processes (Phase II).” Jaime Andrés Rocha, David Vargas, Alexandra Santacruz, Yolanda Castro, and Luisa Sarmiento of AGROSAVIA, who were always willing to collaborate in all the activities of this research.
Funding
Open Access funding provided by Colombia Consortium
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interests.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osorio-Guerrero, K.V., Patiño-Moscoso, M.A., Flórez-Gómez, D.L. et al. Trichoderma koningiopsis applied as seed coating protects sweet sorghum (Sorghum bicolor (L.) Moench) from fungal contaminants during storage. Eur J Plant Pathol 169, 581–591 (2024). https://doi.org/10.1007/s10658-024-02855-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-024-02855-8