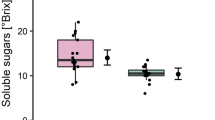
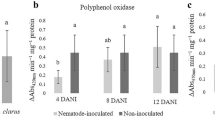
Abstract
The aim of this study was at investigating biochemical changes and plant nutrient alterations in roots and leaves of apple rootstock MM106 following nematode infection. The pathogenic potential of Pratylenchus vulnus at increasing inoculum doses was also investigated. Three tests were carried out using apple plants of different ages (3 months, 1 year, and 3 years) under greenhouse conditions. The enzymatic activity of Catalase (CAT), Peroxidase (POX), Polyphenol-Oxidase (PPO), and Ascorbate peroxidase (APX) were measured in roots of 3-month-old apple plants at 7 days post-inoculation (dpi). Total phenol, total protein content, the lipid peroxidation marker (MDA) and the content of N, P, K, Fe, and Zn were quantified in roots of 3- and 36-month-old plants by atomic spectrometry at 135 dpi and 11 months after inoculation, respectively. The activity of POX, PPO and APX increased after pathogen infection in 3-month-old plants whereas CAT activity decreased. Total protein content and MDA augmented, but total phenols were reduced in 3- and 36-month-old plants inoculated with 1000 or 5000 nematodes. The content of P, K, and Zn in leaves decreased but Fe content increased at the highest dose tested (5000 nematodes/plant). Changes in the enzymatic activities induced in apple roots in response to P. vulnus are part of the defense mechanisms of the host against nematode attack. The nematode reproduced in rootstocks MM106, MM111 and Ba29 and population densities increased on rootstock MM106 as the initial dose did.

Similar content being viewed by others
References
Abd-Allah, A. S. E. (2006). Effect of spraying some micro and macronutrients in fruit set, yield and fruit quality of Washington navel orange tree. Applied Science - Research, 11, 1059–1063.
Acedo, J. (1968). Dissertation. Univ. of Massachusetts Amherst.
Ahuja, S., & Ahuja, S. P. (1980). Effects of root-knot nematode Meloidogyne incognita infection on the peroxidase and polyphenoloxidase activities in the roots of selected vegetables crops. Nematologia Mediterranea, 8, 207–210.
Almagro, L., Gómez, L. V., Belchi-Navarro, S., Bru, R., Ros-Barceló, A., & Pedreño, M. A. (2009). Class III peroxidases in plant defence reactions. Journal of Experimental Botany, 60(2), 377–390.
Arrigoni O. (1979). A biological defense mechanism in plants. Dans: Lamberti, F. & Taylor, C. E. (Eds) Root-knot nematodes (Meloidogyne species). Systematics, biology and control. London: Academic Press, 457–467.
Askary, T. H., Banday, S. A., Iqbal, U., Khan, A. A., Mir, M. M. & Waliullah, M. I. S. (2012). Plant parasitic nematode diversity in pome, stone and nut fruits, in Lichtfouse, E. (Eds), Agroecology and strategies for climate change: Sustainable agriculture reviews 8, Springer, Dordrecht.
Avallone, S., Guiraud, J. P., Brillouet, J. M., & Teisson, C. (2003). Enzymatic browning and biochemical alterations in black spots of pineapple. Current Microbiology, 47, 113–118.
Baldridge, G. D., O’Neill, N. R., & Samac, D. A. (1998). Alfalfa (Medicago sativa L.) resistance to the root-lesion nematode, Pratylenchus penetrans: Defense-response gene mRNA and isoflavonoid phytoalexin levels in roots. Plant Molecular Biology, 38, 999–1010.
Bonner, J. (1950). Plant Biochemistry (p. 537). Academic Press.
Borden, S., & Higgins, V. J. (2002). Hydrogen peroxide plays a critical role in the defense response of tomato to Cladosporium fulvum. Physiological and Molecular Plant Pathology, 61, 227–236.
Bradford, M. M. (1976). A rapid and sensitive method for quantitation or microgram quantities of protein utilizing the principle of protein-dye hinding. Analytical Biochemistry, 72, 248–254.
Castillo, P. & Vovlas, N. (2007). Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management. Vol. 6. Brill, Leiden-Boston. Pp. 529.
Chihani-Hammas, N., Hajji- Hedfi, L., Regaieg, H., Larayedh, A., Badiss, A., Qing, Y., & Horrigue-Raouani, N. (2018). First report of Pratylenchus vulnus associated with apple in Tunisia. Journal of Nematology., 50(4), 579–586.
Culver, D., Ramm, D. W., & Mckenry, M. V. (1989). Procedures for field and greenhouse screening of Prunus genotypes for resistance and tolerance to root-lesion nematode. Journal of the American Society for Horticultural Science, 114, 30–35.
De Grisse, A. T. (1969). Redescription ou modification de quelques techniques utilisées dans l’étude des nématodes phytoparasitaires. Mededelingen Rijksfaculteti der Landbouveten Gent, 34, 351–359.
Dhindsa, R. H., Dhindsa, R. P., & Thorpe, T. A. (1981). Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. Journal of Experimental Botany, 32, 93–101.
El-Beltagi, H. S., Farahat, A. A., Alsayed, A. A., & Mahfoud, N. M. (2012). Response of antioxidant substances and enzymes activities as a defense mechanism against root knot nematode infection. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 40(1), 132–142.
Feldman, A. W., & Hanks, R. W. (1964). Quantitative changes in the free and protein amino acids in roots of healthy, Radopholus similis infected, and recovered grapefruit seedling. Phytopathology, 54, 1210–1215.
Fernández, C., Pinochet, J., & Dolcet, R. (1992). Host parasite relationship of Pratylenchus vulnus on apple and pear rootstocks. Nematropica, 22, 227–236.
Filipjev, I. N. (1936). On the classification of the Tylenchinae. Proceedings of the Helminthological Society of Washington, 3, 80–82.
Fosu-Nyarko, J., & Jones, M. G. K. (2016). Advances in understanding the molecular mechanisms of root lesion nematode host interactions. Annual Review of Phytopathology, 54, 253–278.
Foyer, C. H., & Noctor, G. (2005). Oxidant and antioxidant signaling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment, 28, 1056–1071.
Giebel, J. (1970). Phenolic content in roots of some Solanaceae and its influence on IAA-oxidase activity as an indicator of resistance to Heterodera rostochiensis. Nematologica, 16, 22–32.
Giebel, J. (1982). Mechanism of resistance to plant nematodes. Annual Review of Phytopathology, 20, 257–279.
Glorieux, C., & Calderon, P. B. (2017). Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biological Chemistry, 398, 1095–1108.
Hajji, L., Elouaer, M. A., Regaieg, H., M’Hamdi-Boughalleb, N., & Horrigue-Raouani, N. (2017). Biochemical and plant nutrient alterations induced by Meloidogyne javanica and Fusarium oxysporum f. sp. radicis lycopersici co-infection on tomato cultivars with differing level of resistance to M. javanica. European Journal of Plant Pathology, 148, 463–472.
Haraguchi, H., Saito, T., Okamura, N., & Yagi, A. (1995). Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Medica, 61(4), 333–336.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives in Biochemistry and Biophysics, 125, 189–198.
Huber, D. M., & Graham, R. D. (1999). The role of nutrition in crop resistance and tolerance to diseases. In Z. Rengel (Ed.), Mineral nutrition of crops: Fundamental mechanisms and implications (pp. 169–206). Food Products Press.
Jaffee, B. A., & Mai, W. F. (1979). Growth reduction of apple seedlings by Pratylenchus penetrans as influenced by seedling and age at inoculation. Journal of Nematology, 11(2), 163–165.
Jaffee, B. A., Abawi, G., & Mai, W. (1982). Role of soil microflora and Pratylenchus penetrans in an apple replant disease. Phytopathology, 72, 247–251.
Janssen, T., Karssen, G., Orlando, V., Subbotin, S. A. & Bert, W. (2017). Molecular characterization and species delimiting of plant-parasitic nematodes of the genus Pratylenchus from the Penetrans group (Nematoda: Pratylenchidae). Molecular Phylogenetics and Evolution, 117, 30–48. Available at: https://doi.org/10.1016/j.ympev.2017.07.027
Jones, J. T., Haegeman, A., Danchin, E. G. J., Gaur, H. S., Helder, J., Jones, M. G. K., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J. E., Wesemael, W. M. I., & Perry, R. N. (2013). Top 10 plant- parasitic nematodes in molecular plant pathology. Molecular Plant Pathology, 14(9), 946–961.
Kirkpatrick, J. D., Mai, W. F., Parker, T. C. G., & Fisher, E. G. (1964). Effect of phosphorus and potassium nutrition of sour cherry on the soil population levels of five plant-parasitic nematodes. Phytopathology, 54, 706–712.
Kubalt, K. (2016). The role of phenolic compounds in plant resistance. Biotechnology and Food Sciences, 80(2), 97–108.
Lamberti, F., & Baines, R. C. (1969). Pathogenicity of four species of Meloidogyne on three varieties of olive trees. Journal of Nematology, 1, 111–115.
Leeman, M., den Ouden, F. M., van Pelt, J. A., Dirkx, F. P. M., Steijl, H., Bakker, P. A. H. M., & Schippers, B. (1996). Iron availability affects induction of systemic resistance to fusarium wilt of radish by Pseudomonas fluorescens. Phytopathology, 86, 149–155.
Linsell, K. J., Riley, I. T., Davies, K. A., & Oldach, K. H. (2014). Characterization of resistance to Pratylenchus thornei (nematoda) in wheat (Triticum aestivum): Attraction, penetration, motility, and reproduction. Phytopathology, 104, 174–187.
Mai, W. F., & Parker, K. G. (1967). Root diseases of fruit trees in New York State. I: Populations of Pratylenchus penetrans and growth of cherry in response to soil treatment with nematicides. Plant Disease Reporter, 51, 398–401.
Mai, W. F., & Parker, K. G. (1972). Root diseases of fruit trees in New York State. IV." Influence of preplant treatment with a nematicide, charcoal from burned brush, and complete mineral nutrition on growth and yield of sour cherry trees and numbers of Pratylenchus penetrans. Plant Disease Reporter, 56, 141–145.
Malik, C. P., & Singh, M. B. (1980). Plant enzymology and histo enzymology (pp. 30–84). New Delhi: Kalyani publishers.
Matern, U., & Kneusel, R. E. (1988). Phenolic compounds in plant disease resistance. Phytoparasitica, 16, 153–170.
McDonald, S., Prenzler, P. D., Autolovich, M., & Robards, K. (2001). Phenolic content and antioxidant activity of olive extracts. Food Chemistry, 73, 73–84.
Mellersh, D. G., Foulds, I. V., Higgins, V. J., & Heath, M. (2002). H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant Journal, 29, 257–268.
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., & Mittler, R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment, 33, 453–467.
Molinari, S. (2001). Inhibition of H2O2-degrading enzymes in the response of Mi-bearing tomato to root- knot nematodes and salicylic acid treatment. Nematologia Mediterranea, 29(2), 235–239.
Moody, E. H., Lownsbery, B. F., & Ahmed, J. M. (1973). Culture of the root lesion nematode Pratylenchus vulnus on carrot disks. Journal of Nematology, 19, 125–134.
Nakano, Y., & Asada, K. (1981). Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant and Cell Physiology, 22, 867–880.
Nico, A., Jiménez-Dı́az, R. M., & Castillo, P. (2003). Host Suitability of the Olive Cultivars Arbequina and Picual for Plant-Parasitic Nematodes. Journal of Nematology, 35(1), 29–34.
Pinochet, J., Raski, D. J., & Goheen, A. C. (1976). Effects of Pratylenchus vulnus and Xiphinema index singly and combined on vine growth of Vitis vinifera. Journal of Nematology, 8, 330–335.
Pinochet, J., Verdejo, S., Soler, A., & Canals, J. (1992). Host range of a population of Pratylenchus vulnus in commercial fruit, nut, citrus, and grape rootstocks in Spain. Journal of Nematology, 24(4S), 693–698.
Pinochet, J., Camprubí, A., & Calvet, C. (1993). Effects of the root lesion nematode Pratylenchus vulnus and the mycorrhizal fungus Glomus mosseae on the growth of EMLA-26 apple rootstocks. Mycorrhiza, 4, 79–83.
Pinochet, J., Calvet, C., Camprubi, A., & Fernández, C. (1995). Interaction between the root-lesion nematode Pratylenchus vulnus and the mycorrhizal association of Glomus intraradices and Santa Lucia 64 cherry rootstock. Plant and Soil, 170, 323–329.
Pinochet, J., Anglés, M., Dalmau, E., Fernandez, C., & Felipe, A. (1996). Prunus rootstock evaluation to root-knot and lesion nematodes in Spain. Journal of Nematology, 28, 616–623.
Pinta, M. (1973). Méthodes de références pour détermination des éléments dans végétaux : Détermination des éléments Ca, Mg, Fe, Mn, Zn et Cu par absorption atomique. Oléagineuse, 28, 87–92.
Pitcher, R. S., Patrick, Z. A., & Mountain, W. B. (1960). Studies on the host-parasite relations of Pratylenchus penetrans (Cobb) to apple seedlings. I. Pathogenicity under Sterile Conditions. Nematologica, 5, 309–314.
Pourcel, L., Routaboul, J. M., Cheynier, V., Lepiniec, L., & Debeaujon, I. (2006). Flavonoid oxidation in plants: From biochemical properties to physiological functions. Plant Science, 12(1), 1360–1385.
Prabhu, A. S., Fageria, N. K., Berni, R. F., & Rodrigues, F. A. (2007). Phosphorus and plant disease. In L. E. Datnoff, W. H. Elmer, & D. M. Huber (Eds.), Mineral Nutrition and Plant Disease (pp. 45–55). APS Press.
Schmid-Siegert, E. S., Stepushchenko, O., Glauser, G., & Farmer, E. E. (2016). Membranes as structural antioxidants: Recycling of malondialdehyde to its source in oxidation-sensitive chloroplast fatty acids. The Journal of Biological Chemistry, 291(25), 13005–13013.
Sharma, P., Jha, A. B., Dubey, R. S & Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 1, 1–26. Available at: https://doi.org/10.1155/2012/217037
Siddiqui, I. A., Shaukat, S. S., & Hamid, M. (2002). Role of zinc in rhizobacteria mediated suppression of root-infecting fungi and root-knot nematode. Journal of Phytopathology, 150, 569–575.
Sogut, M. A., Devran, Z., Arici, S. E., San, B., & Yildirim, A. N. (2013). Host reactions of root lesion nematodes (Pratylenchus spp.) on the rootstocks of pome and stone fruits. Turkish Journal of Entomology, 37(2), 239–248.
Stewart, R. J., Sawyer, B. J. B., & Robinson, S. P. (2002). Blackheart development following chilling in fruit of susceptible and resistant pineapple cultivars. Australian Journal of Experimental Agriculture, 42, 195–199.
Sundararaj, P., & Mehta, U. K. (1991). Effect of Pratylenchus zeae Graham, 1951 on the enzymes and amino acid content of sugarcane roots. Indian Journal of Nematology, 21(1), 78–84.
Townshend, J. L., & Stobbs, L. (1981). Histopathology and histochemistry of lesions caused by Pratylenchus penetrans in roots of forage legumes. Canadian Journal of Plant Pathology, 3, 123–128.
Vaganan, M. M., Ravi, I., Nandakumar, A., Sarumathi, S., Sundararaju, P., & Mustaffa, M. M. (2014). Phenylpropanoid enzymes, phenolic polymers and metabolites as chemical defenses to infection of Pratylenchus coffeae in roots of resistant and susceptible bananas (Musa spp.). Indian Journal of Experimental Biology, 52, 252–260.
Valette, C., Andary, C., Geiger, J. P., Sarah, J. L., & Nicole, M. (1998). Histochemical and cytochemical investigations of phenols in roots of banana infected by the burrowing nematode Radopholus similis. Phytopathology, 88, 1141–1148.
Whish, J. P. M., Thompson, J. P., Clewett, T. G., Lawrence, J. L., & Wood, J. (2014). Pratylenchus thornei populations reduce water uptake in intolerant wheat cultivars. Field Crops Research., 161, 1–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial interests
The authors declare they have no financial interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declares that they has no conflict of interest or competing interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chihani-Hammas, N., Verdejo-Lucas, S. & Horrigue-Raouani, N. Pratylenchus vulnus infecting apple rootstock MM106: Defense reaction, plant nutrient alterations and pathogenic potential. Eur J Plant Pathol 167, 169–181 (2023). https://doi.org/10.1007/s10658-023-02694-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02694-z