Abstract
Sclerotinia stem rot caused by Sclerotinia sclerotiorum is one of the most important diseases of oilseed rape in the world. Because of the absence of resistant varieties and the disadvantages of chemical control, application of antifungal microbes has become an eco-friendly and effective measure to control this disease. In this study, Bacillus subtilis strain RSS-1, isolated from soil samples, was identified based on morphological, physiological and biochemical tests, and DNA gyrase subunit A (gyrA), gyrB, DNA-directed RNA polymerase subunit beta (rpoB) and rpoC gene sequence analysis. It significantly inhibited mycelial growth and sclerotial production of S. sclerotiorum in vitro. In greenhouse experiments, all three tested concentrations (106, 107, 108 cfu mL−1) of cell fermentation broth and culture filtrate significantly reduced the severity of sclerotinia stem rot on oilseed rape (P < 0.05). RSS-1 was more effective at reducing disease severity when applied 24 h before inoculation with S. sclerotiorum than at 24 h post inoculation, suggesting that RSS-1 should be applied as a prophylactic rather than a curative biological agent. Colonization tests indicated that the population density of RSS-1 on rapeseed leaves significantly decreased (P < 0.05) over 6 days. However, RSS-1 could stably colonize in rhizospheric soil of rapeseed over 30 days. Challenge inoculation tests showed that RSS-1 significantly inhibited the activities of polygalacturonase and cellulase and accumulation of oxalic acid during the S. sclerotiorum infection. These results suggest that RSS-1 was a potential biological agent for controlling sclerotinia stem rot caused by S. sclerotiorum on oilseed rape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sclerotinia stem rot caused by Sclerotinia sclerotiorum (Lib.) de Bary is one of the most important diseases of oilseed rape (Brassica napus L.) in the rapeseed producing regions of the world (Boland & Hall, 1994; Liang & Rollins, 2018). S. sclerotiorum has a wide host range, and has been reported to infect more than 450 plant species, including several agronomic important crops, such as oilseed rape, soybean, sunflower, tobacco and peanut, and a range of vegetables, including lettuce, bean, cabbage, cauliflower, carrot and potato (Boland & Hall, 1994). Sclerotinia stem rot has been considered the most important disease of oilseed rape in rapeseed production areas in China because it causes great economic losses in China, especially in the rapeseed production regions of the Yangtze River Basin where the temperature and moisture are favorable for epidemics of this disease (Li et al., 2006; Wang et al., 2020). The annual incidence rate of sclerotinia stem rot in this region was estimated at 15% ~ 30% on average and reached up to 80% in serious epidemic years, with yield losses estimated at 1.8 × 108 kg, which accounts for approximately 60% of the total losses of oilseed rape in the Yangtze River Basin (Li et al., 2006; Yang et al., 2009).
Sclerotinia sclerotiorum overwinters in soil as sclerotia that can generally germinate carpogenically or myceliogenically according to environmental conditions (Le Tourneau, 1979). Mycelia or airborne ascospores from these sclerotia can directly penetrate the host stems, leaves, flowers and siliques and easily spread to adjacent plants (McCartney et al., 2001). Due to enormous economic losses on rapeseed and other important crops, control of this notorious pathogen has received extensive attention.
The control of sclerotinia stem rot of oilseed rape is a difficult assignment because of the pathogen’s wide host range in association with its persistent dormant structures, sclerotia, produced after mycelial growth encounters a nutrient scarce environment. These sclerotia provide a long term survival mechanism for S. sclerotiorum and can remain dormant for up to 8 years in soil (Willetts & Wong, 1980). Although some available traditional disease management practices such as crop rotation, application of chemical fungicides and other cultural measures have been used to control of diseases caused by S. sclerotiorum, their effectiveness may be very limited because of problems associated with cost, absence of resistant varieties as well as environmental and health concerns (Bolton et al., 2006; Li et al., 2008). Therefore, highly efficient and environmentally sound alternative methods to control sclerotinia stem rot of oilseed rape are desirable.
Application of beneficial microbial agents to control plant pathogens is considered an eco-friendly, economic and effective component of integrated management measures (Chen et al., 2020). In the search for beneficial microbes, several different taxonomic groups of microorganisms, including fungi (Li et al., 2005; Silva et al., 2022), bacteria (Balthazar et al., 2022; Hu et al., 2014; Li et al., 2020), actinomyces (Baharlouei et al., 2011; Yang et al., 2022) and viruses (Yu et al., 2010; Zhang et al., 2020), have been demonstrated effectively as potential biocontrol agents for managing sclerotinia stem rot caused by S. sclerotiorum. Biological control is a sustainable measure for long-term management of plant diseases in the field. Li et al. (2005) reported that Coniothyrium minitans could effectively suppress sclerotinia seed rot of alfalfa caused by S. sclerotiorum in a 3-year field trial, and the effectiveness of disease control was comparable to that achieved with the fungicide benomyl. Furthermore, C. minitans could also efficiently parasitize sclerotia in the field (Gerlagh et al., 1999). Several bacterial strains of Bacillus subtilis have been studied for controlling the diseases caused by S. sclerotiorum on rapeseed and other important crops (Hu et al., 2005; Zhang and Xue, 2010; Monteiro et al., 2013; Vinodkumar et al., 2017). B. subtilis strain Tu-100 significantly reduced the incidence of disease caused by S. sclerotiorum on oilseed rape, and also promoted the growth of rapeseed plants in dry weight and length based on a 2-year field experiment (Hu et al., 2005). Previously, a field study on the foliar application of a broad antimicrobial activity B. subtilis strain NJ-18 showed a reduction in sclerotinia stem rot incidence and severity on oilseed rape, and its effectiveness was as high as 77.1% (Yang et al., 2009). It can be seen that biological control of plant sclerotinia diseases has received attention as an alternative to the concentrated application of chemically synthesized products.
Although several biocontrol agents have been studied for controlling sclerotinia stem rot in different crops, including rapeseed, more diverse biocontrol resources are urgently needed for effective management of sclerotinia stem rot. The multifaceted capacity of Bacillus species is suitable for sustainable management of this obstinate disease (Choudhary & Johri, 2009; Kloepper et al., 2004). The main objectives of this study were to identify the bacterial strain RSS-1, determine its inhibitory ability against S. sclerotiorum in vitro, perform a preliminary analysis of the possible biocontrol mechanism of RSS-1, and evaluate the potential of RSS-1 against S. sclerotiorum under greenhouse conditions.
Materials and methods
Isolation and identification of antagonistic bacteria against S. sclerotiorum
Rhizospheric soil samples of oilseed rape were collected from Hefei (E117°15′48″, N31°52′5″) and Xuancheng (E118°46′4″, N30°56′49″) in Anhui Province, China. The samples were air-dried naturally in the laboratory. To obtain bacterial strains, 20 g rhizospheric soil was gently diluted in 50 mL distilled water and shaken at 180 r‧min−1 for 30 min in an incubator at 25 °C. One hundred microliter tenfold dilution of the homogenates was uniformly spread on nutrient agar medium (NA; beef extract, 3 g L−1; peptone, 10 g L−1; NaCl, 5 g L−1; agar, 15 g L−1; pH 7.0) to isolate bacteria. Selected colonies were streaked on Luria–Bertani medium (LB; yeast extract, 5 g L−1; sodium chloride, 10 g L−1; typtone, 10 g L−1; agar 15.0 g L−1 and the pH adjusted to 7.0). The strains were checked for purity and preserved in 30% glycerin (v/v) at -20 °C for long term storage.
The inhibitory activity of selected strains against S. sclerotiorum was determined by performing a dual culture bioassay (Wang et al., 2020). The bacterial strains with high antagonistic activity were selected for further identification and biocontrol tests. The phenotypic properties, including morphology, physiological and biochemical characteristics, were investigated following standard procedures (Dong & Cai, 2001), and molecular identification was conducted by using BLAST (Basic Local Alignment Search Tool) of the DNA gyrase subunit A (gyrA), gyrB, DNA-directed RNA polymerase subunit beta (rpoB), and rpoC gene sequences. The partial sequence of gyrB gene was amplified by polymerase chain reaction (PCR) utilizing the universal primer pair UP-1/UP-2r (UP-1: 5'-GAAGTCATCATGACCGTTCTGCAYGCNGGNGGNAARTTYGA-3'; UP-2r: 5'-AGCAGGGTACGGATGTGCGAGCCRTCNACRTCNGCRTCNGTCAT-3') (Yamamoto & Harayama, 1995). The partial sequence of gyrA, rpoB, and rpoC genes were amplified by PCR using primer pairs BsgyrA-499F/-1073R (BsgyrA-499F: 5'-CTGCTCGTGAACGGTGCT-3'; BsgyrA-1073R: 5'-TGATGGTCAAGGTAATGCTCC-3'), BsrpoB-1497F/-1687R (BsrpoB-1497F:5'-GGATGTATCGCCTAAGCAG-3'; BsrpoB-1687R: 5'-TAACAGCGGCACCAGAGT-3'), and BsrpoC-1305F/-1990R (BsrpoC-1305F: 5'-AATCCGTCTTCACCCGCT-3'; BsrpoC-1990R: 5'-CGATGTCAGAAACCCCAAC-3'), with an expectant PCR product length of 575, 191, and 686 bp, respectively. The three aforementioned primer pairs were designed in this study based on gyrA, rpoB, and rpoC genes of B. subtilis subsp. subtilis type strain 168. The DNA fragments were amplified under the following conditions: 94 °C for 5 min, 35 cycles of 94 °C for 45 s, 56 °C (gyrB, and rpoC) or 55 °C (gyrA and rpoB) for 45 s and 72 °C for 100 s and a final extension at 72 °C for 10 min. The amplified DNA fragments were sequenced using primers BsgyrA-499F/-1073R, UP-1S/UP-2Sr (UP-1S: 5'-GAAGTCATCATGACCGTTCTGCA-3'; UP-2Sr: 5'-AGCAGGGTACGGATGTGCGAGCC-3') (Yamamoto & Harayama, 1995), BsrpoB-1497F/-1687R, and BsrpoC-1305F/-1990R, respectively, and submitted to GenBank. The gyrA, gyrB, rpoB, and rpoC gene sequences of B. subtilis and other Bacillus strains from the NCBI (National Center for Biotechnology Information) database used for construction of the phylogenetic tree in this study are listed in Supplementary Table S1. Pseudomonas syringae pv. actinidiae strain ICMP 18,884 was used as an out-group. Multiple sequence alignments were performed using the Clustal program (Chenna et al., 2003). Molecular evolutionary analyses were conducted using MEGA Version 4.0 (Kumar et al., 2008). The phylogenetic tree was constructed using the Neighbor-Joining algorithm and maximum likelihood analyses, with bootstrap values calculated from 1000 replicate runs using the software routines included in the MEGA software (Kumar et al., 2008).
Antagonistic activity of RSS-1 in vitro
A highly pathogenic S. sclerotiorum isolate NGA4, which was isolated from a diseased rapeseed plant in Ningguo, was used to assess antagonistic activity in this study. The fungal isolate was incubated on potato dextrose agar medium (PDA) at 25 °C, and stored on PDA slants at 4 °C. The antagonistic activity of RSS-1 against mycelial growth and sclerotial production of S. sclerotiorum was evaluated using dual-culture experiments. One hundred microliter overnight RSS-1 cell fermentation broth was inoculated into 100 mL LB liquid medium in 250 mL flask and incubated at 25 °C for 24 h in a 180 r‧min−1 shaking incubator. A filter paper strip (20 mm × 2 mm) was immersed in the bacterial suspension (approximately 1 × 108 cfu mL−1) for 30 s and placed on complete medium (CM) (Talbot et al., 1993) at the centre of a 9 cm diameter Petri dish. Two 6 mm agar plugs containing 2-day-old actively growing mycelia of S. sclerotiorum were placed on the plate 2.5 cm from the bacterized filter paper strip, one on each side. A CM plate, inoculated with S. sclerotiorum alone, was served as positive control. The effects of bacterial cell-free filtrate on mycelial growth and sclerotial production of S. sclerotiorum were examined on PDA using a well diffusion assay. The cell-free filtrate of bacterial strain RSS-1 was obtained by centrifugation of a cell fermentation broth dilution with 1 × 108 cfu mL−1 at 12,000 r‧min−1 and passing the supernatant through a sterile 0.22 μm filter (Millipore Corporation). In the well diffusion assay, a 6 mm agar disc containing 2-day-old actively growing mycelia of S. sclerotiorum was placed on PDA at the center of a 9 cm diameter Petri dish. Following this, 100 μL of cell-free filtrate was deposited in each of four 6-mm diameter wells made into the PDA plates at a distance of 2.5 cm from the agar disc. A PDA plate containing cell-free filtrate, but 100 μL of sterile LB broth and inoculated with a S. sclerotiorum mycelial agar disc alone served as a positive control. The plates were cultivated at 25 °C in darkness. The experiment was performed thrice, each with five replicates. The inhibitory activity of each treatment was calculated according to the following formula at five days post inoculation (dpi), and the sclerotial number was counted at 14 dpi.
Here, DT stands for the diameter of the clear zone between two S. sclerotiorum plugs in the treatment plates (mm); PC stands for the diameter of the clear zone between two S. sclerotiorum plugs in control plates (mm) and 50 is the distance between two agar plugs (mm). The experiments were repeated thrice, and the data presented in this paper are the averages of three experiments.
Biocontrol efficacies of RSS-1 against sclerotinia stem rot on detached leaves
A local rapeseed cultivar Deyou 7 (Hefei Fengle Seed Co., Ltd., moderately susceptible to sclerotinia stem rot) was used to evaluate biocontrol efficacies of RSS-1 against sclerotinia stem rot on detached leaves. Five seeds were sowed in polypropylene flowerpots (10 × 10 × 9 cm) filled with sterilized cultivation soil mixture containing loamy soil and vermiculite (2:1, v/v). The planted pots were placed under a 16 h photoperiod at 25 °C in a greenhouse. The assay was performed according to the procedures described by Zhang and Xue (2010). Briefly, 45-day-old leaves of rapeseed were surface-sterilized for 3 min with 75% (v/v) ethylalcohol, and then the leaves were rinsed three times in sterile distilled water and air dried on sterile filter paper. Finally, the surface-sterilized leaves were divided into seven groups (at least eight leaves per group). To obtain cell fermentation broths and cell-free culture filtrate, RSS-1 was inoculated in flasks containing LB broth medium and cultured in an incubator shaker at 180 r‧min−1 for 24 h at 25 °C. The cell density of the fermentation broth was adjusted to 108 cfu mL−1. The cell fermentation broths of 106 and 107 cfu mL−1 were obtained by serial dilution of the cell fermentation broth of 108 cfu mL−1, and cell-free culture filtrate was acquired by centrifuging the cell fermentation broth of 108 cfu mL−1 and filtering the supernatant with sterile 0.22 μm filters (Millipore Corporation) as mentioned above. Four sets of leaves were individually immersed in the cell fermentation broths (approximately 106, 107, 108 cfu mL−1, respectively) and cell-free culture filtrate described above for 30 s and placed on a sterile salver. Two sets of leaves were inoculated with S. sclerotiorum at 24 h before or post soaking with RSS-1 cell fermentation broth (108 cfu mL−1). The last set was soaked with LB medium which served as a positive control. All the treated leaves above were inoculated with 6 mm mycelial discs, cut from the 2-day-old colony margin of S. sclerotiorum on PDA plates. The salvers with inoculated leaves were then kept in an incubator (100% RH and 25 °C) in the dark. Biocontrol efficacies of different treatments of RSS-1 against sclerotinia stem rot on detached leaves were assessed according to the lesion diameters.
Biocontrol efficacies of different treatments of RSS-1 against sclerotinia stem rot
Sixty-day-old plants of rapeseed (Deyou 7, moderately susceptible to sclerotinia stem rot) were cultivated as mentioned above, and a hyphal suspension for plant infection was prepared following procedures previously described by Zhang and Xue (2010). To assess the biocontrol efficacies of different treatments of RSS-1 against sclerotinia stem rot under greenhouse conditions, three concentrations of the cell fermentation broth (106, 107 and 108 cfu mL−1) and cell-free culture filtrate were sprayed on pot-grown plants to run-off using a hand-held atomizer (Worth Garden Company, Shanghai, China). The treated rapeseed plants were air-dried for 2 h and inoculated with the hyphal suspension by spraying to runoff. Two time intervals between bacterial application and pathogen inoculation were conducted as mentioned above. Plants treated with the hyphal suspension alone were used as the control. Each treatment containing 20 plants had three replicates, and the experiment was repeated twice in a greenhouse. Disease severity was investigated at 1, 3, 5, 7 and 15 dpi respectively based on a 0–5 grade of severity scale as previously described by Zhang and Xue (2010). In each disease assessment, the mean disease severity per pot was calculated. A disease index and the control efficacy of each treatment were measured using the following formula.
Here, n is the number of diseased plants at that grade, i is the grade of disease severity, and N is the total number of investigated plants. DCK is the disease index of the control group, and DT is the disease index of the treatment group. The experiments were repeated twice, and the data presented here are the averages of two experiments.
Enzyme activities and oxalic acid accumulation during infection
Five hundred milligrams of rapeseed leaf tissue (fresh weigh, FW), cut from the areas of rapeseed leaves with different treatments, including marginal zones of the lesion that were treated with RSS-1 and inoculated with S. sclerotiorum, zones at the edge of the lesion inoculated with S. sclerotiorum alone and healthy rapeseed leaf tissue, were collected at 6, 12, 24, 48, 72, 96 h post inoculation (hpi), ground in liquid nitrogen with mortar and pestle until a fine powder resulted, and then homogenized in 2 mL extraction buffer consisting of 1.0 mol L−1 NaCl and 20 mmol L−1 Tris–HCl (pH 7.4). After extraction, the homogenate was centrifuged for 20 min at 12,000 r‧min−1 in an Eppendorf 5417R centrifuge at 4 °C. The supernatant liquid was used to serve as a crude enzyme source of total polygalacturonases (PGs) and cellulase.
Polygalacturonase activity was determined by measuring the amount of reducing saccharides released from polygalacturonic acid using the method of dinitrosalicylic acid reagent (DNS) as described previously (Miller, 1959), and using D-galacturonic acid as a standard. The reaction mixture consisted of 1.0 mL of 0.1% (w/v) polygalacturonic acid (dissolved in 50 mmol L−1 citrate buffer, pH 5.0), 1.0 mL of 50 mmol L−1 citrate buffer (pH 5.0) and 0.1 mL of crude enzyme at 50 °C for 30 min in a water bath. Reactions were stopped by the addition of 2.9 mL of DNS. Tubes were placed in a boiling-water bath for 5 min (Riou et al., 1991). One unit of PG activity was defined as the amount of enzyme required to release 1.0 mg of D-galacturonic acid per hour at 50 °C at pH 5.0. The enzyme activity was expressed as units per gram fresh weight per milliliter (U g−1 FW mL−1).
Cellulase activity was determined by measuring the release of reducing groups using DNS assay (Miller, 1959) and glucose as the standard. The reaction mixture consisted of 1.0 mL of 1.0% (w/v) carboxymethylated cellulose (CMC) (dissolved in 50 mmol L−1 citrate buffer, pH 5.0), 1.0 mL of 50 mmol L−1 citrate buffer (pH 5.0) and 0.1 mL of crude enzyme at 50 °C for 30 min in a water bath. Reactions were stopped by the addition of 2.9 mL of DNS. Tubes were placed in a boiling-water bath for 10 min (Moyo et al., 2003). One unit of cellulase activity was defined as the amount of enzyme required to release 1.0 mg of D-glucose per hour at 50 °C at pH 5.0. The enzyme activity was expressed as units per gram fresh weight per milliliter (U g−1 FW mL−1).
The absorbance of reaction products mentioned above was determined using an ultraviolet spectrophotometer (Thermo Fisher Scientific, Waltham, MA) at 540 nm. The experiments were repeated three times.
Two grams of rapeseed leaf tissue (fresh weigh, FW), cut from areas of rapeseed leaves with different treatments as mentioned above, was collected at 12, 24, 48, 72, 96 hpi and was ground in liquid nitrogen with mortar and pestle until a fine powder resulted. It was then homogenized in 5 mL of distilled water and 10 mL of diluted hydrochloric acid, and boiled for 15 min. After this the cooled homogenate was transferred to a 100-mL volumetric flask, diluted to 25 mL and cultured in an incubator shaker at 180 r‧min−1 overnight at 25 °C. The homogenate was filtered through four layers of cheesecloth, and centrifuged for 20 min at 12,000 r‧min−1 in an Eppendorf 5417R centrifuge at 4 °C, and the precipitate was washed twice (Baker, 1952). The supernatant liquid was used to serve as a crude extraction source of total oxalate.
The extraction liquid was placed in a water-bath at 80 °C for 5 min and the oxalic acid was titrated with 0.02 mol L−1 potassium permanganate (Baker, 1952). The experiments were repeated twice. One milliliter of 0.02 mol L−1 fresh potassium permanganate which was consumed during the titration is equivalent to 0.90 mg of oxalic acid.
Colonization of RSS-1 population on rapeseed leaves and in rhizospheric soil
In this study, a double-resistant mutant (streptomycin, Str; rifampicin, Rif) of B. subtilis strain RSS-1 was spontaneously generated on LB plates by the antibiotic-taming method (Str & Rif-LB; 0.1, 0.5, 1, 2, 5, 10, 20, 50, 100, and 200 μg mL−1, respectively). Briefly, 100 μL overnight cell fermentation broth (108 cfu mL−1) of RSS-1 was initially spread on LB plates containing 0.1 μg mL−1 Str and incubated at 37 °C in the dark. After incubation, the growing colonies were transferred onto LB plates containing a two- to five-fold increased concentration of Str. After 7 to 10 rounds of selection at each increasing concentration of antibiotic, the mutant was isolated and transferred to Str-free LB plates when colonies stably grew from the LB plates containing 200 μg mL−1 Str. Str-resistant mutant (200 μg mL−1) of RSS-1 was used for obtaining the Rif-resistant mutant as described above. Finally, the mutant with a growth rate on 200 μg mL−1 Str & Rif-LB similar to the wild-type strain was used for further study.
The dynamics of the RSS-1 population both on plant leaves and in rhizospheric soil were monitored in greenhouse conditions. The rapeseed plants were sprayed with the RSS-1 cell fermentation broth at the concentration of 108 cfu mL−1 or were poured with the RSS-1 cell fermentation broth with the same rate in rhizospheric soil. Plants treated with the distilled water served as negative control. The bacterial population was recovered by collecting leaf or rhizospheric soil at 2, 4, 6, 10, 20, 30 days after bacterization. One gram of leaves or rhizospheric soil was homogenized in a sterile flask containing 10 mL of sterilized distilled water and shaked at 180 r‧min−1 for 30 min. Serial dilutions of the resulting suspensions were applied to Str & Rif-LB medium at 100 μL per plate. Plates were incubated for 48 h at 37 °C, and the phenotype of bacterial colonies identical to strain RSS-1 were determined.
Light microscopy and scanning electron microscopy
Hyphae were scraped from the positive control and the interaction zone between pathogens (including S. sclerotiorum) and RSS-1 on PDA (or CM) plates at 96 hpi, and fixed on a microscope slide with a coverslip. Samples were observed with an Olympus BX51 microscope (Olympus, Japan). Photographs were taken with an Olympus DP71 camera coupled to Image-Pro Express C software.
Scanning electron microscopy (SEM) was carried out by a modification of a procedure described previously (Heller and Witt-Geiges, 2013). Bacterial cells and 5-mm-diameter discs were taken from the edges of lesions on the diseased rapeseed leaves treated with RSS-1 suspension and inoculated with S. sclerotiorum, and inoculated with S. sclerotiorum alone, respectively. The leaf-discs were washed three times in 100 mmol L−1 phosphate buffer (pH 7.0), and then samples were fixed in 2.5% glutaraldehyde in 100 mmol L−1 phosphate buffer (pH 7.0) for 16 h. The samples were washed three times with the same buffer, and dehydrated in a series of ethanol (30%, 50%, 70%, 85%, 95% and 100%). The fixed samples were dried for 2 h in a K850 critical point drier (Emitech, UK), and gold-coated in an E-1010 Polaron SEM coating system device (Hitachi, Japan). The samples were photographed under an S-4800 scanning electron microscopy (Hitachi, Japan).
Statistical analysis
All statistical analyses were performed using Data Processing System (DPS v7.05; Reifeng Information Technology, Hangzhou, China) software. Differences in the control efficacy among different treatments, activity of PGs and cellulase and oxalic acid accumulation among different treatments, and colonization of RSS-1 on leaves and in soil within different treatments were analyzed using analysis of variance (ANOVA, with F and P values for treatment effects) with mean separation using Duncan’s new multiple-range test at P < 0.05 and P < 0.01, respectively. All of the data analyzed using ANOVA were square-root transformed prior to analysis.
Results
Isolation of antagonistic bacteria and identification of RSS-1
Sixteen antagonistic bacterial strains were obtained from the soil samples, and of them, RSS-1 had the highest antagonistic activity against S. sclerotiorum, in a dual-culture assay. RSS-1 was initially identified by phenotypic characteristics. The results indicated that RSS-1 was a Gram-positive, rod shaped, endo-spore forming bacterium with positive reaction for catalase activity and negative for oxidase activity. It produced amylase, hydrogen sulfide (H2S) and hydrolyzes gelatin and could grow in the temperature range from 10 to 45 °C. It was positive for V-P reaction, nitrate reduction and citrate utilization but negative for indole test and tyrosine hydrolysis. All these phenotypic characteristics are uniform with members of Bacillus. In addition to the phenotypic identification, BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) analysis of gyrA (GenBank accession number: OP889260), gyrB (MW286209), rpoB (OP883602), and rpoC (OP889261) gene sequences revealed significant identity (> 99%) with the four standard B. subtilis strains (Supplementary Table S1). Furthermore, the phylogenetic tree constructed with the concatenated sequences of gyrA, gyrB, rpoB, and rpoC genes of diverse Bacillus members indicated that RSS-1 belonged to B. subtilis (Fig. 1).
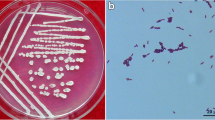
Phylogenetic tree based on Neighbor-Joining algorithm using Maximum likelihood analyses with the concatenated sequences of the gyrA, gyrB, rpoB, and rpoC genes of Bacillus strains, showing the phylogenetic relationship between strain RSS-1 and other members of the genus Bacillus from NCBI database. The Pseudomonas syringae pv. actinidiae strain ICMP 18,884 was served as an out-group. Bootstrap support values (calculated from 1000 replicate runs) are given at the nodes (Maximum likelihood bootstrap values of only > 50 are shown). Bar: the estimated nucleotide substitutions per site are 0.01. Stars indicate Bacillus type strains. a Single colony morphological characteristic of RSS-1 on LB. b Scanning electron micrograph of RSS-1 cells (Scale bar = 1 μm)
Antagonistic activity of RSS-1 in vitro
Both RSS-1 cell fermentation broth and cell-free filtrate significantly inhibited mycelial growth and sclerotial production after 14 days of dual culturing with S. sclerotiorum. Compared with the untreated plates, RSS-1 cell fermentation broth reduced mycelial growth of S. sclerotiorum by 93.4% (Fig. 2a, b), and RSS-1 cell-free filtrate decreased the average diameter of colonies up to 26.0 mm (Fig. 2c, d). Both RSS-1 cell fermentation broth and cell-free filtrate suppressed sclerotial production by 97–100% after 14 days of dual culturing with S. sclerotiorum (Fig. 2e, f).
Antagonistic activity agaist Sclerotinia sclerotiorum and biocontrol effects of Bacillus subtilis strain RSS-1 on detached leaves of rapeseed. (a-d) Antagonistic activity of strain RSS-1 using dual culturing assay on CM (a, b) and well diffusion assay on PDA (c, d). 100 μl of cell-free filtrate in each well was used for inhibitory effect on mycelial growth of S. sclerotiorum (d). (e, f) Effects of cell fermentation broth (108 cfu mL−1) of B. subtilis strain RSS-1 on sclerotial production of S. sclerotiorum. (g, h) Biocontrol effects of strain RSS-1 agaist sclerotinia stem rot on detached leaves of rapeseed. RSS-1: leaves were treated first with RSS-1 at the concentration of 108 cfu mL.−1 and then inoculated immediately with S. sclerotiorum; NGA4: leaves were inoculated with S. sclerotiorum alone. hpi: hours post inoculation. Bars represent standard errors of the mean (n = 10). Asterisks on top of the bars indicate that the differences between RSS-1 treatment and the control (NGA4) in lesion diameters are very significant (P < 0.01)
Biocontrol efficacies of RSS-1 against sclerotinia stem rot on detached leaves
All three concentrations, 106, 107 and 108 cfu mL−1 of cell fermentation broth, and culture filtrate of RSS-1 significantly inhibited (P < 0.05) the expansion of lesions on detached leaves of rapeseed (Supplementary Fig. S1). In the control group, the lesion diameters expanded rapidly, and the average lesion diameters were 27.5 mm to 75.2 mm over 72 h (Fig. 2g, h). In contrast, although the lesions were also formed in treatment with 108 cfu mL−1 of cell fermentation broth, the lesions expanded more slowly than those of the control group. Furthermore, the lesions were limited to the inoculation site, with the average range from 3.7 mm to 14.0 mm over 72 h (Fig. 2g, h). Time interval between application of cell fermentation broth (108 cfu mL−1) of RSS-1 and inoculation of S. sclerotiorum greatly influenced the lesion expansion on detached leaves (Supplementary Fig. S1). Both treatment with RSS-1 at 24 h before inoculation (BI) and treatment with RSS-1 at 24 h post inoculation (PI) significantly lowered (P < 0.05) the rate of lesion expansion compared with the control group. Nevertheless, the lesion diameters in the BI group expanded more slowly than those in the PI group (Supplementary Fig. S1).
Biocontrol efficacy of RSS-1 on sclerotinia stem rot
All three concentrations, 106, 107 and 108 cfu mL−1 of cell fermentation broth, and culture filtrate of RSS-1 showed significant (P < 0.05) suppression to sclerotinia stem rot of oilseed rape development on plants at 1 dpi with S. sclerotiorum, compared with the untreated plants (CK) (Fig. 3). Significant effect differences (P < 0.05) of the three concentrations of suspension and culture filtrate on the disease index were observed from 1 to 15 dpi. At 15 dpi, most of the plants in the untreated control were dying, with an average disease index of 91.0, whereas the average disease index in the treated plants remained lower than 18.2 (Fig. 3). At 15 dpi, all the concentration treatments, 106, 107 and 108 cfu mL−1 of suspension or culture filtrate significantly reduced sclerotinia stem rot of oilseed rape, with the disease index of 17.9, 18.2, 9.5 and 11, respectively. Accordingly, the biocontrol efficacy of the four treatments against the disease was 80.3%, 80.0%, 89.6% and 87.9%, respectively.
Control effectiveness of different treatments of Bacillus subtilis RSS-1 agaist sclerotinia stem rot on rapeseed. dpi: days post inoculation. CK: plants inoculated with S. sclerotiorum alone and untreated with RSS-1. PI: plants were treated with RSS-1 at 24 h after inoculation with S. sclerotiorum. BI: plants were treated with RSS-1 at 24 h before inoculation with S. sclerotiorum. T6, T7 and T8: plants were treated first with RSS-1 at the concentration of 106, 107 and 108 cfu mL.−1, respectively, and then inoculated immediately with S. sclerotiorum. CF: plants were treated first with cell-free culture filtrate and then inoculated immediately with S. sclerotiorum. Concentration treatments with different letters in rows were significantly different from each other (P < 0.05)
The time interval between application of cell fermentation broth (108 cfu mL−1) of B. subtilis RSS-1 and inoculation of S. sclerotiorum greatly influenced the disease development under control conditions (Fig. 3). The two time interval treatments, both treatment with RSS-1 at 24 h before inoculation (BI) and treatment with RSS-1 at 24 h post inoculation (PI), significantly reduced (P < 0.05) the disease severity, with the disease index range from 19.0 to 23.8 (BI) and 27.0 to 30.0 (PI) over 15 dpi, respectively, while the disease indices of the control plants (CK) ranged from 19.0 to 91.0 over 15 dpi. Furthermore, the disease indices in treatment with RSS-1 at 24 h before inoculation (BI) were significantly (P < 0.05) lower than those in treatment with RSS-1 at 24 h post inoculation (PI) (Fig. 3), i.e. the control effectiveness of the former was significantly superior than that of the latter.
Enzyme activities and oxalic acid accumulation during infection
The activities of polygalacturonases (PGs) and cellulase and oxalic acid accumulation were measured during the infection course of the fungus. Obvious necrotic symptoms were observed at 6 h after S. sclerotiorum inoculation (hpi) on rapeseed (B. napus) leaves. There was no significant difference (P < 0.05) in cellulase activity among different treatments within 6 hpi (Fig. 4a, b). Both PGs and cellulose activity increased promptly from 12 to 96 hpi (Fig. 4a, b) in the untreated group. In contrast, although PGs and cellulase activity increased in the RSS-1 treated group, the accumulation rates of the two enzymes activity were slower than those in the untreated group. Compared to the untreated group, PGs and cellulase activities in the treated group decreased by 37.9% and 42.1% at 72 hpi, 44.2% and 37.8% at 96 hpi, respectively, which shown that RSS-1 significantly suppresses (P < 0.01) the PGs and cellulase production during the course of infection (Fig. 4a, b).
Polygalacturonases, cellulose activities and oxalic acid accumulation produced by Sclerotinia sclerotiorum during the course of infection. RSS-1: leaves were treated first with RSS-1 at the concentration of 108 cfu mL.−1 and then inoculated immediately with S. sclerotiorum; NGA4: leaves inoculated with S. sclerotiorum alone and untreated with RSS-1; CK: healthy leaves of rapeseed (B. napus). Error bars represent standard deviations. Asterisks indicate significant differences among different treatments (*: P < 0.05; **: P < 0.01; ns: no significant difference)
Oxalic acid accumulation was also detected during the course of infection. Significant differences (P < 0.05) of oxalic acid accumulation among different treatments were detected at 24 hpi (Fig. 4c). In the untreated group, the concentration of oxalate increased from 9.38 mg g−1 FW at 12 hpi to 12.03 mg g−1 FW at 96 hpi. However, in the treated group, the concentration of oxalate increased from 8.41 mg g−1 FW at 12 hpi to 9.43 mg g−1 FW at 96 hpi (Fig. 4c). The above results indicated that RSS-1 significantly inhibited (P < 0.05) the accumulation of oxalic acid during S. sclerotiorum infection (Fig. 4c).
Colonization of RSS-1 population on rapeseed leaves and in rhizospheric soil
The ability of RSS-1 to survive on rapeseed leaves and in rhizospheric soil under greenhouse conditions was investigated with the double-resistant mutant (resistant to streptomycin and rifampicin) of RSS-1 as the tested organism. The results showed that the RSS-1 population on rapeseed leaves significantly decreased over time under greenhouse conditions (Fig. 5a). During the first 6 days, the RSS-1 population size significantly (P < 0.05) decreased, and was reduced by 3.45 log units at day 6 (Fig. 5a). RSS-1 was not detected on rapeseed leaves at 10 days after being applied on leaves (Fig. 5a). Conversely, the RSS-1 population size significantly (P < 0.05) increased in rhizospheric soil during the first 4 days after being bacterized on leaves (Fig. 5a). From day 4, the population size in rhizospheric soil had no significant (P < 0.05) changes until day 10 (Fig. 5a). Subsequently, the population size in rhizospheric soil declined, and reduced by 2.00 log units at day 20, and then maintained no significant (P < 0.05) variation until day 30 (Fig. 5a).
Dynamics of the Bacillus subtilis strain RSS-1 population on rapeseed leaves and in rhizospheric soil in greenhouse. a Dynamics of strain RSS-1 population on rapeseed leaves (


The dynamics of the RSS-1 population in rhizospheric soil with the irrigating root treatment was also tested further. The results indicated that the RSS-1 population size significantly (P < 0.05) increased in rhizospheric soil during the first 4 days after being bacterized in rhizospheric soil (Fig. 5b). From day 4, the RSS-1 population size in rhizospheric soil increased by 0.75 log units, and then leveled off until day 30 (Fig. 5b).
Discussion
Previously published research has suggested that the Bacillus genus exhibits potential effectiveness for control of plant pathogens on important crops. Balthazar et al. (2022) reported that four Bacillus strains displayed strong inhibitory activity against 10 culturable plant pathogens, inluding Botrytis cinerea, S. sclerotiorum, Fusarium culmorum, F. sporotrichoides, F. oxysporum, Nigrospora sphaerica, N. oryzae, Alternaria alternata, Phoma sp., and Cercospora sp., significantly suppressing gray mold development on cannabis leaves, resulting in at least half disease severity reduction compared with water-treated controls. Hu et al. (2005) found that B. subtilis Tu-100 at approximately 8.1 × 108 cfu mL−1 of inoculation could effectively decrease disease incidence at harvest and significantly inhibit S. sclerotiorum in field trials based on two continuous years. Fernando et al. (2007) reported that Bacillus genus could be used to control sclerotinia stem rot on canola over a period of a 2-year field trial, and disease control was comparable to that achieved with the fungicide Rovral Flo (iprodione). In this study, we determined that a highly antagonistic B. subtilis strain RSS-1, recovered from rhizospheric soils of rapeseed in Anhui Province of China and identified by applying morphological, physiological, biochemical and molecular analyses, significantly inhibited the mycelial growth in vitro and weakened the severity of sclerotinia stem rot on rapeseed caused by S. sclerotiorum under greenhouse conditions. In vitro antagonistic assays, RSS-1 resulted in as high as 93.4% of mycelial growth inhibition. In greenhouse conditions, foliar application of three concentrations (106, 107 and 108 cfu mL−1 of RSS-1) of cell fermentation broth achieved a significant effectiveness against sclerotinia stem rot on rapeseed, with the concentration of 108 cfu mL−1 of cell fermentation broth obtaining the best suppression to sclerotinia stem rot of rapeseed, despite RSS-1 origination from the soil and a low control efficiency obtaining from root irrigation (data not shown). These results suggested that RSS-1 has a potential for management of sclerotinia stem rot caused by S. sclerotiorum on rapeseed. Hence, a further study should be performed to assess the control efficacy of RSS-1 under field conditions.
In rapeseed-growing regions, sclerotia are constantly hiding in soils to overcome adverse environmental conditions, which guarantees an initial infection source of the pathogen in future production seasons. Ascospores released by mature apothecia which were produced by germinated sclerotia, can infect senescent tissues and petals and destroy a zone of adjacent healthy plant tissue into which the pathogen can grow (Rogers et al., 2009). A previous study demonstrated that disease incidence of sclerotinia stem rot was significantly correlated with the percentage of petals infected by ascospores of S. sclerotiorum at the early flowering stage (McCartney et al., 2001). This challenge increases the difficulty in management of sclerotinia stem rot on rapeseed (Sabatéa et al., 2018). The frequently-used chemical fungicides for controlling sclerotinia stem rot (e.g., fluazinam) have been rendered ineffective owing to circumstances associated with sclerotial production in the fields (de Aguiar et al., 2014; Sabatéa et al. 2018), despite fungicides still playing a crucial effect in the control of sclerotinia stem rot caused by S. sclerotiorum (McCreary et al., 2016). In this study, both RSS-1 cell fermentation broth and cell-free filtrate markedly suppressed sclerotial production in vitro and reduced disease severity on plants, implying that RSS-1 may be considered as an alternative for biocontrol of sclerotial production in the S. sclerotiorum-colonized fields. Since ascospores produced from germinating sclerotia are the initial infection sources for sclerotinia stem rot on rapeseed, the inhibition of sclerotial production by application of RSS-1 into rapeseed fields during disease epidemics might decrease the amount of sclerotia in the fields and thus lessen primary infection in future crop seasons. Nevertheless, the effectiveness of RSS-1 against sclerotial formation in the field is still unknown, which should be further studied.
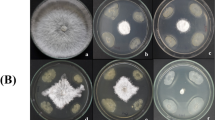
The major mechanisms associated with the defense provided by microbial biocontrol agents against plant pathogens are generally classified as parasitism, antibiosis, and plant growth-promoting effects (Alabouvette et al., 2006; Lo, 1998; Nithyapriya et al., 2021). Members of the Bacillus family frequently produce various metabolic compounds, such as cyclic lipopeptides (LPs) of the surfactin, iturin and fengycin (or plipastatin) families (Harwood et al., 2018; Kaspar et al., 2019; Kiesewalter et al., 2021; Ongena & Jacques, 2007; Stein, 2005). These metabolic substances have often been reported as the most beneficial and broad-spectrum antibiotic compounds for antagonist against plant pathogens. A previously study indicated that S. sclerotiorum suppression by Bacillus species is mainly owing to the production of pumilacidin, bacillibactin, bacilysin, and other antifungal compounds (Ribeiro et al., 2021). Sabatéa et al. (2018) demonstrated the ability of Bacillus species to synthesize kurstakins, surfactins, iturins, polimyxin and fengycins against S. sclerotiorum when the bacteria interact with the pathogen. In the present study, we also found that RSS-1 cell-free filtrate significantly suppressed mycelial growth and sclerotial production in vitro and reduced disease severity on plants. In addition, micro observation and SEM analysis showed crucial morphological and microstructural destruction of S. sclerotiorum hyphae treated with RSS-1 compared with untreated groups (Fig. 6), suggesting that secondary metabolites produced by RSS-1 might play a major role in disease suppression. Therefore, further study is necessary to determine the category of secondary metabolites produced by RSS-1 and the role they play in the antagonism against the fungus.
Microscopic observation of the interaction between Sclerotinia sclerotiorum and Bacillus subtilis strain RSS-1 on rapeseed leaves. In the figure, a, b, c, d, e and f are scanning electron micrographs; leaves of rapeseed were inoculated with S. sclerotiorum alone (a-c); leaves were treated with RSS-1 at the concentration of 108 cfu mL−1 and then inoculated immediately with S. sclerotiorum (d-f). a: hyphae of S. sclerotiorum colonized on rapeseed leaves; b: single hypha of S. sclerotiorum infected into rapeseed leaf; c: hyphae of S. sclerotiorum infected into rapeseed leaf by forming infection cushion; d: hypha of S. sclerotiorum was degraded on the leaf surface of rapeseed; e, f: biocontrol strain RSS-1 colonized on the rapeseed leaf at 72 hpi. Bars = 10 μm (a-d); Bars = 2 μm (e–f). g: normal hyphae of S. sclerotiorum on PDA under light microscopy; h: hyphae of S. sclerotiorum were degraded by anti-pathogen strain RSS-1 with dual culturing on PDA at 4 dpi under light microscopy
Oxalic acid and cell wall degrading enzymes, in particular polygalacturonases (PGs) and cellulases, are well known to be the main pathogenetic determinants of S. sclerotiorum (Cessna et al., 2000; Riou et al., 1991). PGs and cellulases secreted by S. sclerotiorum are functionally activated by low-pH conditions (pH 3.0 to 4.5) (Kim et al., 2008; Liang et al., 2015). To result in such acidic conditions, S. sclerotiorum can secrete oxalic acid during the early stage of infection (Kim et al., 2008; Liang et al., 2015). In our study, both PGs and cellulase activities increased rapidly from 12 to 96 h post inoculation with S. sclerotiorum alone. In contrast, although PGs and cellulase activities increased in plants treated with RSS-1 and inoculated with S. sclerotiorum, the growth rates of two enzyme activities were slower than those in the untreated group. Oxalic acid accumulation was also detected during infection. The results indicated that RSS-1 significantly suppressed the accumulation of oxalic acid during S. sclerotiorum infection.
It is critical that factitiously introduced biocontrol agents are able or unable to colonize, establish, compete and survive in the intricate natural environment. Failure of biocontrol agents to survive and afford long term disease control is a major obstacle to the application of biocontrol strategies in the management of plant diseases. Conversely, native populations of plant pathogens are often well established and accommodated, and may survive in the soil for long periods, from where they breed and cause disease. In practice, repeated application of antagonists is often necessary to prolong the biocontrol effect. However, that undoubtedly increases production costs. Chen et al. (1995) reported that Bacillus strain JM-1128 population, after an initial decrease in number by day 7 in cotton plants, leveled off and changed slightly from 7 to 21 days. Hu et al. (2005) reported that B. subtilis populations could survive at least 30 days in the rapeseed plant ectorhizosphere under control conditions, with population size decreasing from 8.5 × 108 cfu per seed to approximately 102 cfu within 30 days of sowing in autoclaved soil. When used for biocontrol of sclerotinia stem rot of soybean, B. subtilis SB24 population density on soybean leaves was reduced by 0.8 log units over 5 weeks under control conditions and by 1.5 to 2.5 log units over 15 days under field conditions (Zhang & Xue, 2010). The present study revealed that the population size of B. subtilis RSS-1 on rapeseed leaves decreased by 3.45 log units over 6 days under control conditions, but the RSS-1 population size in rhizospheric soil of rapeseed leveled off and changed little from 4 to 30 days, indicating that the RSS-1 population declined more slowly in rhizospheric soil than on rapeseed leaves under control conditions. A further study should be conducted to clarify whether active growing RSS-1 in soil can suppress sclerotial production at the late stage of disease or inhibit infection by ascospores at the early stage of releasing.
In conclusion, the results obtained in this study showed that a highly pathogenic B. subtilis strain RSS-1 isolated from rhizospheric soil of oilseed rape resulted in microstructural and morphological detriment to the destructive pathogen S. sclerotiorum, which was observed to damage its hyphal structure, causing unnatural growth and complete failure of sclerotia production. In addition, we first report the ability of RSS-1 to restrict the levels of polygalacturonase and cellulase activities and oxalic acid production to a threshold during the S. sclerotiorum infection whereby it could not maintain complete virulence on the leaves of oilseed rape. Further studies are necessary to assess the biocontrol effectiveness of RSS-1 in the field and the role RSS-1 plays in inhibiting sclerotial production under natural conditions. Furthermore, studies using a suite of preparative and purified technologies for purification of antimicrobial substances produced by RSS-1 would provide direct evidence of the mechanism of biological control.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request
References
Alabouvette, C., Olivain, C., & Steinberg, C. (2006). Biological control of plant diseases: The European situation. European Journal of Plant Pathology, 114, 329–341.
Baharlouei, A., Sharifi-Sirchi, G. R., & Bonjar, G. H. S. (2011). Biological control of Sclerotinia sclerotiorum (oilseed rape isolate) by an effective antagonist Streptomyces. African Journal of Biotechnology, 10, 5785–5794.
Baker, C. J. L. (1952). The determination of oxalates in fresh plant material. The Analyst, 77, 340–344.
Balthazar, C., Novinscak, A., Cantin, G., Joly, D. L., & Filion, M. (2022). Biocontrol activity of Bacillus spp. and Pseudomonas spp. against Botrytis cinerea and other cannabis fungal pathogens. Phytopathology, 112, 549–560.
Boland, G. J., & Hall, R. (1994). Index of plant hosts of Sclerotinia sclerotiorum. Canadian Journal of Plant Pathology, 16, 93–108.
Bolton, M. D., Thomma, B. P. H. J., & Nelson, B. D. (2006). Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Molecular Plant Pathology, 7, 1–16.
Cessna, S. G., Sears, V. E., Dickman, M. B., & Low, P. S. (2000). Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. The Plant Cell, 12, 2191–2200.
Chen, C., Bauske, E. M., Musson, G., Rodriguezkabana, R., & Kloepper, J. W. (1995). Biological control of Fusarium wilt on cotton by use of endophytic bacteria. Biological Control, 5, 83–91.
Chen, D. M., Yang, H. J., Huang, J. G., & Yuan, L. (2020). Lysobacter enzymogenes LE16 autolysates have potential as biocontrol agents—Lysobacter sp. autolysates as biofungicide. Journal of Applied Microbiology, 129, 1684–1692.
Chenna, R., Sugawara, H., Koike, T., Lopez, R., Gibson, T. J., Higgins, D. G., & Thompson, J. D. (2003). Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Research, 31, 3497–3500.
Choudhary, D. K., & Johri, B. N. (2009). Interactions of Bacillus spp. and plants – with special reference to induced systemic resistance (ISR). Microbiological Research, 164, 493–513.
de Aguiar, R. A., da Cunha, M. G., & Junior, M. L. (2014). Management of white mold in processing tomatoes by Trichoderma spp. and chemical fungicides applied by drip irrigation. Biological Control, 74, 1–5.
Dong, X. Z., & Cai, M. Y. (2001). Manual for Systematic Identification Common Bacteria. Science Press.
Fernando, W. G. D., Nakkeeran, S., Zhang, Y., & Savchuk, S. (2007). Biological control of Sclerotinia sclerotiorum (Lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop Protection, 26, 100–107.
Gerlagh, M., Goossen, H. M., Fokkema, N. J., & Vereijken, P. F. G. (1999). Long-term biosanitation by application of Coniothyrium minitans on Sclerotinia sclerotiorum-infected crops. Phytopathology, 89, 141–147.
Harwood, C. R., Mouillon, J. M., Pohl, S., & Arnau, J. (2018). Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiology Reviews, 42, 721–738.
Heller, A., & Witt-Geiges, T. (2013). Oxalic acid has an additional, detoxifying function in Sclerotinia sclerotiorum pathogenesis. PLoS ONE, 8, e72292.
Hu, X. J., Roberts, D. P., Jiang, M. L., & Zhang, Y. B. (2005). Decreased incidence of disease caused by Sclerotinia sclerotiorum and improved plant vigor of oilseed rape with Bacillus subtilis Tu-100. Applied Microbiology and Biotechnology, 68, 802–807.
Hu, X. J., Roberts, D. P., Xie, L. H., Maul, J. E., Yu, C. B., Li, Y. S., Jiang, M. L., Liao, X. S., Che, Z., & Liao, Z. (2014). Formulations of Bacillus subtilis BY-2 suppress Sclerotinia sclerotiorum on oilseed rape in the field. Biological Control, 70, 54–64.
Kaspar, F., Neubauer, P., & Gimpel, M. (2019). Bioactive secondary metabolites from Bacillus subtilis: A comprehensive review. Journal of Natural Products, 82, 2038–2053.
Kiesewalter, H. T., Lozano-Andrade, C. N., Wibowo, M., Strube, M. L., Maróti, G., Snyder, D., Jørgensen, T. S., Larsen, T. O., Cooper, V. S., Weber, T., & Kovács, Á. T. (2021). Genomic and chemical diversity of Bacillus subtilis secondary metabolites against plant pathogenic fungi. mSystems, 6, 00770–20.
Kim, K. S., Min, J.-Y., & Dickman, M. B. (2008). Oxalic acid is an elicitor of plant programmed cell death during Sclerotinia sclerotiorum disease development. Molecular Plant-Microbe Interactions, 21, 605–612.
Kloepper, J. W., Ryu, C.-M., & Zhang, S. (2004). Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology, 94, 1259–1266.
Kumar, S., Dudley, J., Nei, M., & Tamura, K. (2008). MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in Bioinformatics, 9, 299–306.
Le Tourneau, D. (1979). Morphology, cytology and physiology of Sclerotinia species in culture. Phytopathology, 69, 887–890.
Li, C. X., Liu, S. Y., Sivasithamparam, K., & Barbetti, M. J. (2008). New sources of resistance to sclerotinia stem rot caused by Sclerotinia sclerotiorum in Chinese and Australian Brassica napus and Brassica juncea germplasm screened under western Australian conditions. Australasian Plant Pathology, 38, 149–152.
Li, G. Q., Huang, H. C., Acharya, S. N., & Erickson, R. S. (2005). Effectiveness of Coniothyrium minitans and Trichoderma atroviride in suppression of sclerotinia blossom blight of alfalfa. Plant Pathology, 54, 204–211.
Li, G. Q., Huang, H. C., Miao, H. J., Erickson, R. S., Jiang, D. H., & Xiao, Y. N. (2006). Biological control of Sclerotinia diseases of rapeseed by aerial applications of the mycoparasite Coniothyrium minitans. European Journal of Plant Pathology, 114, 345–355.
Li, Y., Qin, L., Roberts, D. P., Hu, X., Xie, L., Gu, C., Liao, X., Han, P., & Liao, X. (2020). Biological fertilizer containing Bacillus subtilis BY-2 for control of Sclerotinia sclerotiorum on oilseed rape. Crop Protection, 138, 105340.
Liang, X., Liberti, D., Li, M., Kim, Y.-T., Hutchens, A., Wilson, R., & Rollins, J. A. (2015). Oxaloacetate acetylhydrolase gene mutants of Sclerotinia sclerotiorum do not accumulate oxalic acid, but do produce limited lesions on host plants. Molecular Plant Pathology, 16, 559–571.
Liang, X., & Rollins, J. A. (2018). Mechanisms of broad host range necrotrophic pathogenesis in Sclerotinia sclerotiorum. Phytopathology, 108, 1128–1140.
Lo, C. T. (1998). General mechanisms of action of microbial biocontrol agents. Plant Pathology Bulletin, 7, 155–166.
McCartney, H. A., Heran, A., & Li, Q. (2001). Infection of oilseed rape (Brassica napus) by petals containing ascospores of Sclerotinia sclerotiorum. In C. S. Young & K. J. D. Hughes (Eds.), Proceedings of Sclerotinia 2001, the XI International Sclerotinia Workshop. pp 183–184. York UK: British Society for Plant Pathology.
McCreary, C. M., Depuydt, D., Vyn, R. J., & Gillard, C. L. (2016). Fungicide efficacy of dry bean white mold [Sclerotinia sclerotiorum (Lib.) de Bary, causal organism] and economic analysis at moderate to high disease pressure. Crop Protection, 82, 75–81.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Biochemistry, 31, 426–428.
Monteiro, F. P., Ferreira, L. C., Pacheco, L. P., & Souza, P. E. (2013). Antagonism of Bacillus subtilis against Sclerotinia sclerotiorum on Lactuca sativa. Journal of Agricultural Science, 5, 214–223.
Moyo, S., Gashe, B. A., Collison, E. K., & Mpuchane, S. (2003). Optimising growth conditions for the pectinolytic activity of Kluyveromyces wickerhamii by using response surface methodology. International Journal of Food Microbiology, 85, 87–100.
Nithyapriya, S., Lalitha, S., Sayyed, R. Z., Reddy, M. S., Dailin, D. J., El Enshasy, H. A., Suriani, N. L., & Herlambang, S. (2021). Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability, 13, 5394.
Ongena, M., & Jacques, P. (2007). Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends in Microbiology, 16, 115–125.
Ribeiro, I. D. A., Bach, E., Moreira, F. D. S., Müller, A. R., Rangel, C. P., Wilhelm, C. M., Barth, A. L., & Passaglia, L. M. P. (2021). Antifungal potential against Sclerotinia sclerotiorum (Lib.) de Bary and plant growth promoting abilities of Bacillus isolates from canola (Brassica napus L.) roots. Microbiological Research, 248, 126754.
Riou, C., Freyssinet, G., & Fevre, M. (1991). Production of cell wall-degrading enzymes by the phytopathogenic fungus Sclerotinia sclerotiorum. Applied and Environmental Microbiology, 57, 1478–1484.
Rogers, S. L., Atkins, S. D., & West, J. S. (2009). Detection and quantification of airborne inoculum of Sclerotinia sclerotiorum using quantitative PCR. Plant Pathology, 58, 324–331.
Sabatéa, D. C., Brandan, C. P., Petroselli, G., Erra-Balsells, R., & Audisioa, M. C. (2018). Biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary on common bean by native lipopeptide-producer Bacillus strains. Microbiological Research, 211, 21–30.
Silva, L. R. D., Mello, S. C. M. D., Valadares-Inglis, M. C., Costa, M. M. D. C., Saraiva, M. A. D. P., Rêgo, E. C. S., Zacaroni, A. B., Muniz, P. H. P. C., & Pappas, M. D. C. R. (2022). Transcriptional responses and reduction in carpogenic germination of Sclerotinia sclerotiorum exposed to volatile organic compounds of Trichoderma azevedoi. Biological Control, 169, 104897.
Stein, T. (2005). Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Molecular Microbiology, 56, 845–857.
Talbot, N. J., Ebbole, D. J., & Hamer, J. E. (1993). Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. The Plant Cell, 5, 1575–1590.
Vinodkumar, S., Nakkeeran, S., Renukadevi, P., & Malathi, V. G. (2017). Biocontrol potentials of antimicrobial peptide producing Bacillus species: Multifaceted antagonists for the management of stem rot of carnation caused by Sclerotinia sclerotiorum. Frontiers in Microbiology, 8, 446.
Wang, M. L., Geng, L. L., Sun, X. X., Shu, C. L., Song, F. P., & Zhang, J. (2020). Screening of Bacillus thuringiensis strains to identify new potential biocontrol agents against Sclerotinia sclerotiorum and Plutella xylostella in Brassica campestris L. Biological Control, 145, 104262.
Willetts, H. J., & Wong, J. A. L. (1980). The biology of Sclerotinia sclerotiorum, S. trifoliorum and S. minor with emphasis on specific nomenclature. The Botanical Review, 46, 101–165.
Yamamoto, S., & Harayama, S. (1995). PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Applied and Environmental Microbiology, 61, 1104–1109.
Yang, D. J., Wang, B., Wang, J. X., Chen, Y., & Zhou, M. G. (2009). Activity and efficacy of Bacillus subtilis strain NJ-18 against rice sheath blight and Sclerotinia stem rot of rape. Biological Control, 51, 61–65.
Yang, M., Zhang, W., Lv, Z., Shi, L., Zhang, K., & Ge, B. (2022). Evaluation of the inhibitory effects of wuyiencin, a secondary metabolite of Streptomyces albulus CK-15, against Sclerotinia sclerotiorum in vitro. Plant Disease, 106, 156–164.
Yu, X., Li, B., Jiang, D. H., Ghabrial, S. A., Li, G. Q., Peng, Y. L., Xie, J. T., Cheng, J. S., Huang, J. B., & Yi, X. H. (2010). A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proceedings of the National Academy of Sciences, 107, 8387–8392.
Zhang, H., Xie, J., Fu, Y., Cheng, J., Qu, Z., Zhao, Z., Cheng, S., Chen, T., Li, B., Wang, Q., Liu, X., Tian, B., Collinge, D. B., & Jiang, D. (2020). A 2-kb mycovirus converts a pathogenic fungus into a beneficial endophyte for Brassica protection and yield enhancement. Molecular Plant, 13, 1420–1433.
Zhang, J. X., & Xue, A. G. (2010). Biocontrol of sclerotinia stem rot (Sclerotinia sclerotiorum) of soybean using novel Bacillus subtilis strain SB24 under control conditions. Plant Pathology, 59, 382–391.
Acknowledgements
This work was supported by (a) the National Key Research and Development Program of China (Grant No. 2018YFD0200900), (b) the National Key Research and Development Program of China (2021YFC2600402), and (c) “5511” Collaborative Innovation Project of High-quality Agricultural Development and Surpassment in Fujian Province (XTCXGC2021011, XTCXGC2021017). And we are thankful to Senior Agronomists Changwen Zhu and Zhangxiang Ko for their work in the collection of the soil samples.
Author information
Authors and Affiliations
Contributions
Conceptualization, Shun Cao, Yuli Dai and Zhimou Gao; methodology and software, Yuli Dai and Shun Cao; validation, Bingxin Jiang and Yuemin Pan; formal analysis, Yuli Dai, Shun Cao and Bingxin Jiang; investigation, Yuli Dai, Shun Cao, Guogen Yang, Guangxue Pan and Fangxin Chen; resources and data curation, Yuli Dai, Shun Cao and Yuemin Pan; writing—original draft preparation, Yuli Dai and Shun Cao; writing—review and editing, Yuli Dai and Zhimou Gao; supervision and project administration, Zhimou Gao. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors state that there are no conflicts of interest deriving from the publication of this work.
Research involving human participants and/or animals
Not applicable. The research involved no human participants or animals.
Informed consent
The research involved no human participants, and no animals so that the statement on the welfare of animals is not required.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, S., Jiang, B., Yang, G. et al. Isolation and evaluation of Bacillus subtilis RSS-1 as a potential biocontrol agent against Sclerotinia sclerotiorum on oilseed rape. Eur J Plant Pathol 166, 9–25 (2023). https://doi.org/10.1007/s10658-023-02642-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02642-x