Abstract
Plant-parasitic nematodes (PPNs) in particular those belonging to the genera Meloidogyne and Tylenchulus are a limiting factor in the production of many plants. In this research, we investigate a strategy for the control of PPNs in vivo and in vitro. The purpose of this research is to evaluate different concentrations of Tilapia fish powder (TFP) and of (in the form of BECTO Grow Roots®) against egg hatching and second-stage juveniles of Meloiodogyne incognita (Tylenchida: Heteroderidae) under laboratory condition. We also assessed the effect of TFP and on M. incognita and Tylenchulus semipenterans (Tylenchida: Tylenchulidae) reproduction. Our data showed that the percentage of egg hatching inhibition ranged from 8.03 to 53.21% and 42.25 to 75.12% after five days of treatment at different concentrations of TFP and PGPR, respectively compared with the control. The percentage of M. incognita J2 mortality increase significantly (p ≤ 0.05) from 52.1 to 86.7 and 44.6 to 92.3% after seven days of treatment at different concentrations of TFP and PGPR, respectively compared with control. Under greenhouse conditions, a remarkable (p ≤ 0.05) increase in plant growth parameters was observed in cucumber plants that received TFP and In the field experiment, the highest reduction of T. semipeneterans density was TFP + PGPR This holds both for healthy and infected trees. Healthy navel orange (HNO) + PGPR + TFP achieved maximum enhancement in orange weight/tree compared to other treatments. Our study recommended TFP and PGPR not only because of their potential against nematodes, but also because of their safety for humans, mammals and non-target organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 4000 species of plant-parasitic nematodes (PPNs) have been identified, and they may be found in all major biomes (Press & Phoenix, 2005). Plant-parasitic nematodes are parasitic worms that damage food crops including maize (Zea mays), potato (Solanum tuberosum), and tomato (Solanum lycopersicum) (Nicol et al., 2011). The global agriculture industry spends $173 billion a year on the control of PPNs (Elling, 2013). Meloidogyne incognita (Tylenchida: Heteroderidae) and Tylenchulus semipenetrans (Tylenchida: Tylenchulidae) are the most economically significant nematodes due to the high levels of damage and infection they cause, their large host range, and interaction with the host plant (Abd-Elgawad et al., 2016; Aioub et al., 2022). Plants infected with M. incognita show symptoms both above and below ground. Aboveground, infected plants show poor growth, fewer, smaller, pale green leaves, and leaves that wilt at high temperatures. Large galls on the roots, obstruct the plant's capacity to absorb and move dissolved nutrients, are the first signs of the condition. Nematodes feed on roots, harming them and opening the door for other soil-borne diseases (Agrios, 2005). Above ground T. semipenetrans symptoms are stunting, poor development, yellowing, diminished foliage and smaller fruits. The feeder roots infected with the citrus nematode are slightly thicker than healthy ones and have a dirty appearance because soil particles adhere to the gelatinous matrix deposited by the female nematode on the root surface. Infected roots reduce the tree's ability to absorb water and nutrients necessary for normal growth (Abd-Elgawad et al., 2016). Consequently, nematodes are considered one of the most dangerous pests that cause damage to crops and reduce economic production.
Indiscriminate usage of chemical nematicides bear the risk of harm to humans, animals, plants, and the ecosystem as a whole because of their a non-target impacts. Therefore, biological control of nematodes has drawn interest from researchers (Ali et al., 2022; Feng et al., 2022).
Amongst the alternative methods in the management of nematodes are the use of Tilapia fish powder (TFP) and plant growth-promoting rhizobacteria (PGPR). Using TFP is beneficial because it can reduce M. incognita population size and improve eggplant (Solanum melongena L.) growth (Kesba et al., 2021). TFP can reduc the number of M. incognita and Rotylenchulus reniformis on different strains of cowpea (Vigna unguiculata L.) under greenhouse conditions (Kesba et al., 2013). Little work has been done on the use of TFP as a material against nematodes. Plant growth-promoting rhizobacteria (PGPR) promote plant growth are host-specific biological agents that may have a negative influence on the growth and survival of PPNs (Alberton et al., 2020). Plant growth-promoting rhizobacteria are alsoknown as plant health-promoting rhizobacteria or nodule-promoting rhizobacteria (Hayat et al., 2010) and can be divided into two groups depend on their habitat, viz., iPGPR (i.e., symbiotic bacteria), which live inside plant cells, produce nodules, and are restricted to particular structures, and ePGPR (i.e., free living rhizobacteria), which exist outside plant cells, do not produce nodules, but nevertheless promote plant development (Gray & Smith, 2005). The use of PGPR in conjunction with animal manures have been suggested as aneffective, environmentally friendly method to control a variety of nematode communities and improve cucumber (Cucumis sativus) and tomato (S. lycopersicum) development might be considered as an alternative to the synthetic nematicide oxamyl (Ali et al. (2022).
The main hypothesis of this research is that TFP and PGPR might be alternatives for nematicides for the control of nematodes We evaluate TFP and PGPRefficacy against eggs and J2 of M. incognita under laboratory condition. Moreover, we assess the impact of TFP and PGPRon M. incognita reproduction on Cucumis sativus under greenhouse conditions and the reduction of T. semipenterans population density under field trials.
Material and methods
Preparation of Tilapia fish powder (TFP)
The Nile tilapia were collected from markets and rinsed with tap water, then dried in the oven at (60 ± 5 °C) for 3 days with removing the digestive gland and gills from the whole body before grinding with a Microhammer-mill and then sieved (2 mm mesh) (Kofoid & White) Prepared fish powders were stored at 10 °C in a sealed plastic bags until use.
PGPR source
Plant growth-promoting rhizobacteria (in the form of BECTO Grow Roots ®, involved Pseudomonas putida/and fluorescens Serratia marcescens) was obtained from the Central Laboratory of Pesticides, Dokki, Giza.
Source and inoculum of root-knot nematode, Meloidogyne incognita
The nematode population of M. incognita was established from a single egg-mass and the culture was maintained in the greenhouse on atomato Solanum lycopersicum L., susceptible cultivar Super Strain B as a source of egg-masses, free eggs and second stage juveniles according to El-Ashry (2021). Species identification was based on measurements on second stage juvenile and examination of the perineal pattern system of adult females according to Eisenback (1985) and Jepson (1987)
In vitro assay
Preparation of free eggs and second juvenile of Meloidogyne incognita
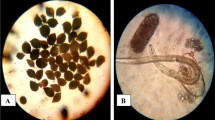
The infected roots of tomato were cut into pieces of 2 cm long and placed in a flask of volume 600 mL, containing 200 mL of 0.5% sodium hypochlorite. The flask was capped tightly and shaken for 3 min to dissolve the gelatinous matrix partially freeing eggs from the egg masses (Hussey, 1973).The liquid suspension of eggs was poured through a 200 mesh sieve nested upon a 500 mesh sieve. Eggs collected on the 500-mesh sieve were immediately washed free of residual sodium hypochlorite solution under a slow stream of tap water and incubated in Petri dishes at 25 ± 1 °C until hatching. Newly hatched juveniles were collected using a micropipette.
Egg masses of equal size needed to study the effect of the tested extracts on egg hatching of M. incognita were hand-picked with fine forceps from small galls on the infected tomato roots obtained from previously maintained pure culture. The collected egg masses were surface sterilized in 1:500 (v/v) aqueous solution of sodium hypochlorite (Clorox) for 5 min (Akhtar et al., 2005).
-
a-
Free eggs
Free eggs of M. incognita were extracted from infected tomato roots according to Hussey (1973) and transferred to 10-cm diameter Petri dishes containing 10 mL of PGPR at different concentrations (108 cfu/mL, 0.5 × 108 cfu /mL, 1 × 107 cfu /mL, 1 × 106 cfu /mL and 1 × 105 cfu /mL) and TFP at different concentrations (1.0, 2.5, 5.0, 10.0 and 15 g).
Extracted eggs were suspended by distilled water and counted by using a counting slide under a research microscope (100X magnification). The concentration of eggs was adjusted to about 1000 eggs per ml by diluting the suspension. Approximately 200 free eggs in 0.1 mL water were exposed to the aforementioned concentrations of Rhizobacteria or Tilapia fish powder (TFP).
Control treatment Petri dishes contained only free eggs mixed with 10 mL of distilled water only. Treatments were left under ambient temperature of 25 ± 3 °C to determine the bacterial biocontrol efficiency and TFP. All treatments were left under laboratory temperature 25 ± 3˚C and all treatments were replicated five times. Numbers of hatched juveniles were counted using a research microscope (100X magnification). The cumulative number of hatched juveniles in each Petri dish was counted after 1, 3, 5-, 7-, 10- and 12-days post treatment. The percentage of hatching inhibition was calculated in comparison with the control treatment, according to the following equation:
-
b-
Second juvenile mortality
The concentration of emerged juveniles was adjusted to 100 juveniles per 0.1 mL. Ten mL of PGPR or Tilapia fish powder were screened in-vitro for their biocontrol efficiency against of M. incognita at different time periods. The control treatment contained only nematodes in distilled water, and all treatments were replicated five times. Treatments were left under ambient temperature of 25 ± 3 °C to determine the bacterial biocontrol efficiency.
Tested materials were observed daily for juvenile mortality but tables only contain data of 1, 2, 3, 5, 7, 10 and 12 days as mentioned before. Juveniles showing inactive straight posture or did not show any movement after prodding were considered dead (De Nardo & Grewal, 2003). The mortality percentages were calculated as the following equation:
In vivo assay
Greenhouse experiment setup and procedures
Under greenhouse conditions, a pot experiment was conducted at the faculty of agriculture, Zagazig University, Egypt. The experiment was conducted under natural light conditions. The greenhouse temperature and relative humidity were 25–27 °C and 66–69%, respectively. This experiment aimed to test the efficiency of Tilapia fish powder and PGPR on cucumber (C. sativus L.) plant growth of cultivar Raian (CB898) as well as galling, and reproduction of M. incognita. Plants of C. sativus L. were transplanted into 20-cm-diameter plastic pots including 1950 g mixture of sterilized sandy soil (70.1% sand, 12.3% clay, 8.1% silt) and 120 g peat moss. The experimental pots were arranged according to a random design that consisted of eight treatments: (1) Healthy C. sativus without PGPR or Tilapia fish powder (HCS), (2) Healthy C. sativus amended with a mixture of 3 g urea and 40 mL PGPR (HCS + PGPR), (3) Healthy C. sativus amended with 10 g of Tilapia fish powder (HCS + TFP), (4) Healthy C. sativus amended with a mixture of 3 g urea and 40 mL PGPR with 10 g Tilapia fish powder (HCS + PGPR + TFP), (5) C. sativus infected with J2 of M. incognita without PGPR or TFP (CSJ), (6) C. sativus infected with J2 of M. incognita amended with a mixture of 3 g urea and 40 mL PGPR (CSJ + PGPR), (7) C. sativus infected with J2 of M. incognita amended with 10 g of Tilapia fish powder (CSJ + TFP), (8) C. sativus infected with J2 of M. incognita amended with a mixture of 3 g urea and 40 mL PGPR with 10 g Tilapia fish powder (CSJ + BECTO + TFP).
12 days after planting, (5–8) treatments were inoculated with 1000 M. incognita second stage juveniles (J2) in 2 mL water suspension, using a micropipette, at the base of the seedling via two holes 4 cm deep made using a pencil.
Two months after inoculation, plants were removed carefully from the pots and data on plant growth as indicated by length and fresh weight of shoots were recorded. Nematodes were extracted from samples of 100 g soil using a combination of sieving and Baermann trays technique (Hooper et al., 2005). Roots were soaked in tape water for one hour to remove adhering soil and to keep egg -masses on the root surface. Roots were wrapped in tissue paper to prevent drying out and the number of galls and egg masses was counted per root system. The recorded plant growth parameters included fresh root weight (g), root length (cm) and shoot weight (g). Moreover, galling and nematode development parameters including the number of egg masses/plant were assessed according to Eldeeb et al. (2022). The numbers of galls per root were enumerated and the RGI per plant root was determined using the 1–5 gall index (1 = 1–2 galls, 2 = 3–10 galls, 3 = 11–30 galls, 4 = 31–100 galls, 5 ≥ 100 galls) (Taylor & Sasser, 1978). The nematode extracts were removed, allowed to settle for 5 h, and the amount was reduced to 30 mL by getting rid of the excess (Caveness, 1975). Under the microscope, the nematode density was determined and the total number of soil nematodes per pot was computed. The reproduction factor (RF) per pot was calculated according to Sasser et al. (1984). RF = Final population/ Initial population. Host category was determined according to Canto-Saenz (1983) as follows: (RGI ≤ 2 & R ≤ 1) resistant (R); (RGI ≤ 2 & R > 1) tolerant (T); (RGI > 2 & R ≤ 1) hypersusceptible (HS) and (RGI > 2 & R > 1) susceptible (S). The percentage of changes in parameters was calculated from:
Field experiment setup and procedures
The experimental area is located in Belbes District, Sharqia Governorate, Egypt (30° 25′ 8"N, 31° 33′ 53"E). A drip irrigation system was used, and the experimental site received traditional horticulture treatments. The field experiment was conducted in navel orange treesnaturally infested by the citrus nematode, Tylenchulus semipenetrans during 2021 to test the efficiency of TFP and PGPR (35.7 L/ha of 108 colony-forming units/mL include S. marcescens and P. putida/P. fuorescens) against T. semipenetrans. After harvest 250 g soil and 10 g roots were collected to determine the nematode speciesWe monitored the population fluctuations of T. semipenetrans in soil samples after one, two and three months. with TFP and PGPR treatments. The experimental trees were arranged according to the following designed: (1) Navel orange infected with T. semipenetrans (Positive control) with TFP and PGPR (NOI), (2) Healthy navel orange without PGPR and TFP (Negative control) (HNO), (3) Healthy navel orange amended with a mixture of 9 mL urea and 100 mL PGPR (HNO + PGPR), (4) Healthy navel orange amended with 30 g of TFP (HNO + TFP), (5) Healthy navel orange amended with a mixture of 9 mL urea and 100 mL PGPR + and 30 g TFP (HNO + PGPR + TFP), (6) Navel orange infected with T. semipenetrans amended with a mixture of 9 mL urea + 100 mL PGPR(NOI + PGPR), (7) Navel orange infected with T. semipenetrans amended with 30 g TFP (NOI + TFP), (8) Navel orange infected with T. semipenetrans amended with a mixture of 9 mL urea and 100 mL PGPR plus 30 g TFP (NOI + PGPR + TFP). Each treatment has ten replicates. The T. semipenetrans population densities in healthy navel orange trees is around 300 individuals which below the economic threshold under field conditions. Fruit yield/trees (total weight of fruit/ trees) were evaluated at harvest time from each tested tree.
Statistical analysis
A fully randomized design was used for the laboratory experiments, whereas a randomized full block design was used for the greenhouse and field experiments. Dataanalysis was performed using MSTAT version 4, and Duncan's multiple range test was used to compare the means (p ≤ 0.05).
Results
In Vitro nematicidal effect of Tilapia fish powder and PGPR on egg hatching and juvenile mortality of M. incognita
The efficacy of rhizobacteria formulated as PGPR and Tilapia fish powder (TFP) on M. incognita eggs were tested under laboratory conditions (Tables 1 and 2). Data analysis of both tested materials showed significant differences (p ≤ 0.05) at different exposure times (days) and concentration in reduced egg viability by reducing emerged juveniles (IJs). Analysis of exposure times (days) by different concentrations of TFP and PGPR shows a high and negative effect on the number of hatched M. incognita eggs mainly. Within the first week, the PGPR and TFP significantly inhibited M. incognita J2s emergence with egg hatching inhibition (EHI) percentages 98.38 and 65.53% at concentrations 108 cfu/mL of PGPR and 15.0 g of TFP, respectively. After 12 days, PGPR had the highest effect (~ 100% EHI) with all tested concentrations.whilst with TFP EHI % reached 70.38% In general, PGPR exhibited higher inhibition effect by all treatments against M. incognita eggs compared with TFP.
Tables 3 and 4 showed that the effectiveness of PGPR and TFP on M. incognita J2s mortality compared with distilled water. PGPR induced higher J2 mortality (98.38%) compared toTFP 86.7% after 7 days. After 10 days of treatment with PGPR and TFP the mortality in J2 varied from 50.55% to reach 100% with PGPR concentrations of 1 × 105 cfu /mL and 108 cfu /mL compared to 60 and 96.4% with TFP concentrations of 1.0 g and 15.0 g, respectively.
Comparing Treatment 12 with Treatment 23 we see that PGPR is more effective that TFP in reducing egg hatching and TFP is more effective that PGPR in increasing J2 death rates.
Efficacy of Tilapia fish powder and PGPR on galling and reproduction of M. incognita under greenhouse conditions
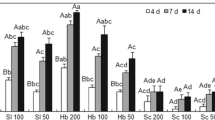
Table 5 showed that in health plants the maximum increase in fresh root weight, root long and shoot weight were observed in the HCS+PGPR+ TFP treatment followed by HCS+PGPR and theHCS+TFP. The same trend was observed with infected plants amended and non-amended with PGPR and TFP. Regarding to the impact of PGPR and TFP on the M. incognita parameters (number of galls/root, number of egg masses /root, number IJs /100 g soil and number of eggs /100 g soil.The effect of PGPR+TFP is always largesr than that of BETCO alone or TFP alone. The effect of PGPR alone on the M. incognita parameters is generally larger than that of TFP alone, with the exception of the number of egg masses per root. These trends in the individual parateters is clearly reflected in the population level parameters (Table 5). RGI and EMI values (5.0) were the highest in the CSJ treatment, whilst, the smallest values (3.0 and 3.8) were found in the CSJ+PGPR+ TFP treatment (Fig. 1). Through RGI and RF values, all infected treatments were susceptible since RGI value > 2 and RF > 1.
Impact of Tilapia fish powder and PGPR on Tylenchulus semipeneterans population densities under field conditions
Table 6 showed that all treatments significantly decreased the number of T. semipeneterans compared with control (NOI treatment). The highest reduction of T. semipeneterans number in both health and infected trees withPGPR and TFP Moreover, all treatments remarkably increased orange fruits weight PGPR was more effective in reducing T. semipenetrans population density and increasing the percentage of navel orange weight than with TFP.
Discussions
Alternative materials are widely used as eco-friendly methods for biological control of parasite pests like root-knot nematodes (Sarker et al., 2021). The result of this study showed that TFP and PGPR show a significant reduction (p ≤ 0.05) in egg hatchability and egg viability by reducing infective juveniles (IJs) of M. incognita. Also, adding PGPR and Tilapia fish powder (TFP) to the soil not only controlled M. incognita but also raised soil fertility, improved soil physical qualities to preserve production,. This is due to TFP containing ammonia, phosphorus, and orthophosphate. Anhydrous and aqueous ammonia, urea, and other ammonium compounds have all been employed directly to control nematodes, therefore ammonia plays a significant part in this process (Farahat et al., 2012). These findings agree with those of Ferial et al. (2011) who reported that phospho-fertilizer was most successful in lowering egg mass production, root galling, and egg hatching and survival of J2. Additionally, phosphate fertiliser indirectly affects nematode control by causing plants to develop systemic acquired resistance to RKN (Habash & Al-Banna, 2011). Furthermore, the effect of TFP may be attributed to nutrient content which improve plant growth and induce plant resistance to J2s infection to minimize juvenile numbers able to penetrate roots (Castro et al., 2006; Mazur & Radziemska, 2014). PGPR directly and indirectly inhibit nematodes. PGPR functions directly as biofertilizers that create organic compounds that encourage plant development by enhancing soil nutrient absorption. Whilst, indirect effects include the creation of antibiotics, Fe chelators (also known as siderophores), and external cell wall-degrading enzymes (such as chitinase and glucanase) that may hydrolyze the cell wall of nematodes (Mhatre et al., 2019). These results are agree with (Kumar et al., 2020) who reported that one of the indirect processes is the production of 1-aminocyclopropane-1-carboxylic acid deaminase, which lowers the quantity of ethylene in plants and increases plant tolerance. HCN is a significant inhibitor of M. incognita and Agrobacterium tumefaciens,and is produced by the majority of PGPR isolates. HCN-producing rhizobacteria increased all growth parameters of tomatoes (El-Rahman et al., 2019). Therefore, extracellular secretion explained the effectiveness of the culture filtrate method for the management of RKN (Sharma & Sharma, 2017), and the quantity and type of extracellular secretion molecules determine how different PGPR isolates may be distinguished. P. putida, P. fluorescens PF19, and Bacillus subtilis isolates all have high potency (Ludwig et al., 2013; Nikoo et al., 2014; Sohrabi et al., 2018) which may depend on secondary metabolites (Viljoen et al., 2019; Zhang et al., 2022).
Under Field condition, the combination of PGPR and TFP decrease T. semipenetrans population density and increased orange yield. This may be due to TFP not only functioning as a source of inorganic nutrients, but also as a substrate for PGPR. Moreover, potential effects of TFP may be ascribed to nutritional content that enhances plant development and increases plant resistance to J2s infection (Mazur & Radziemska, 2014). Fish emulsion has previously been used as a fertilizer for plant growth (Aung and Flick 1980; Emino and ER 1981). This result is consistent with the previous report by Garcıa et al. (2005) who demonstrated that fish waste is an important source of minerals and important sources of crude protein. Biodegradable fish waste is known to containhigh levels of dry matter, crude protein, ether extract, crude fiber, nitrogen free extract and ash as well as having a high mineral content. Additionally, fish powder alters soil pH to alkaline and it may be responsible for the reduction of plant-parasitic nematodes (Yang et al., 2016). the decomposition process of organic amendments like fish powder or fish waste converte nitrogen to ammonia that kills PPNs (Oka, 2010; Thoden et al., 2011). Akhtar & Mahmood 1995) also report that TFP decreases PPNs populations. Overall, these findings offer compelling proof that TFP and PGPR are usefullmaterials for nematode control and protect the environment from pollution.
Conclusions
This research discussed using alternatives methods to control PPNs. PGPR and Tilapia fish powder contributed significantly to the suppression of M. incognita and T. semipenetrans in vivo and in vitro. The use of PGPR and Tilapia fish powder are more environmentally friendly for controlling PPNs populations than nematicides. The combined use of PGPRand Tilapia fish powder may constitute a new biocontrol strategy for integrated pest management programs to manage PPNs. This paves the way for using environmentally safe materials (TFP and PGPR) in the management nematodes.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PPNs:
-
Plant-parasitic nematodes
- TFP:
-
Tilapia Fish powder
- BECTO:
-
BECTO Grow Roots®
- M. incognita :
-
Meloiodogyne incognita
- T. semipenterans :
-
Tylenchulus semipenterans
- PGPR:
-
plant growth-promoting rhizobacteria
- P. fluorescens :
-
Pseudomonas fluorescens
- S. marcescens :
-
Serratia marcescens
- S. lycopersicum :
-
Solanum lycopersicum
- C. sativus :
-
Cucumis sativus
- HCS:
-
Healthy C. sativus
- CSJ:
-
Infected cucumber
- HNO:
-
healthy navel orange
- J2:
-
second stage juveniles
- RGI:
-
Root galls index
- RF:
-
reproduction factor
- HS:
-
hypersusceptible
- NOI:
-
Navel orange infected
- IJs:
-
infective juveniles
- EHI:
-
egg hatching inhibition
References
Abd-Elgawad, M. M., Koura, F. F., Montasser, S. A., & Hammam, M. M. (2016). Distribution and losses of Tylenchulus semipenetrans in citrus orchards on reclaimed land in Egypt. Nematology, 18, 1141–1150.
Agrios GN. (2005). Plant pathology: Elsevier.
Aioub, A. A. , Elesawy, A. E., Ammar, E. E. (2022). Plant growth promoting rhizobacteria (PGPR) and their role in plant-parasitic nematodes control: a fresh look at an old issue. Journal of Plant Diseases and Protection.1–17.
Akhtar, H., Anita, S., & Prabhat, K. S. (2005). Studies on the management of root-knot nematode, Meloidogyne incognita-wilt fungus, Fusarium oxysporum disease complex of green gram, Vigna radiata cv ML-1108. Journal of Zhejiang University Science B., 6, 736–742.
Alberton, D., Valdameri, G., Moure, V. R., Monteiro, R. A., Pedrosa, Fd. O., Müller-Santos, M., & de Souza, E. M. (2020). What did we learn from plant growth-promoting rhizobacteria (PGPR)-grass associations studies through proteomic and metabolomic approaches? Frontiers in Sustainable Food Systems., 4, 607343.
Ali, A. A., El-Ashry, R. M., & Aioub, A. A. (2022). Animal manure rhizobacteria co-fertilization suppresses phytonematodes and enhances plant production: Evidence from field and greenhouse. Journal of Plant Diseases and Protection., 129, 155–169.
Aung, L., & Flick, G., Jr. (1980). The influence of fish solubles on growth and fruiting of tomato. HortScience, 15, 32–33.
Canto-Saenz, M. (1983). The nature of resistance to Meloidogyne incognita (Kofoid and White 1919) Chitwood 1949.
Castro RS, Azevedo CMB, Bezerra-Neto F. 2006. Increasing cherry tomato yield using fish effluent as irrigation water in Northeast Brazil. Scientia horticulturae.110:44–50.
Caveness F. 1975. Other Contributions: A Simple Siphon Method for Separating Nematodes from Excess Water. Nematropica.30–32.
De Nardo, E. A., & Grewal, P. S. (2003). Compatibility of Steinernema feltiae (Nematoda: Steinernematidae) with pesticides and plant growth regulators used in glasshouse plant production. Biocontrol Science and Technology., 13, 441–448.
Eisenback, J. (1985). Detailed morphology and anatomy of second-stage juveniles, males, and females of the genus Meloidogyne (root-knot nematodes). An Advanced Treatise on Meloidogyne., 1, 47–77.
El-Ashry, R. M. (2021). Application of animal manure and plant growth-promoting rhizobacteria as effective tools to control soil nematode population and increase crop yield in grapevine orchards. Egyptian Journal of Agronematology., 20, 34–52.
El-Rahman, A., Shaheen, H. A., El-Aziz, A., Rabab, M., & Ibrahim, D. S. (2019). Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egyptian Journal of Biological Pest Control., 29, 1–11.
Eldeeb, A. M., Farag, A. A. G., Al-Harbi, M. S., Kesba, H., Sayed, S., Elesawy, A. E., Hendawi, M. A., Mostafa, E. M., & Aioub, A. A. (2022). Controlling of Meloidgyne incognita (Tylenchida: Heteroderidae) using nematicides, Linum usitatissimum extract and certain organic acids on four peppers cultivars under greenhouse conditions. Saudi Journal of Biological Sciences., 29, 3107–3113.
Elling, A. A. (2013). Major emerging problems with minor Meloidogyne species. Phytopathology, 103, 1092–1102.
Emino E, ER E. (1981). Effectiveness of fish soluble nutrients as fertilizers on container-grown plants.
Farahat AA, Alsayed AA, EL-BELTAGI HS, Mahfoud NM. 2012. Impact of organic and inorganic fertilizers on nematode reproduction and biochemical alterations on tomato. Notulae Scientia Biologicae.4:48-55.
Feng, Y., Rui, L., Wang, X., & Wu, X. (2022). Adaptation of pine wood nematode, Bursaphelenchus xylophilus, early in its interaction with two Pinus species that differ in resistance. Journal of Forestry Research., 33, 1391–1400.
Ferial, M. R., Hosny, H. K., Waleed, D. S., & Mohamed, A. M. (2011). Impact of rice straw composts on microbial population, plant growth, nutrient uptake and root-knot nematode under greenhouse conditions. African Journal of Agricultural Research., 6, 1188–1203.
Garcıa, A., Esteban, M., Marquez, M., & Ramos, P. (2005). Biodegradable municipal solid waste: Characterization and potential use as animal feedstuffs. Waste Management., 25, 780–787.
Gray E, Smith D. 2005. Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil biology and biochemistry.37:395–412.
Habash S, Al-Banna L. 2011. Phosphonate fertilizers suppressed root knot nematodes Meloidogyne javanica and M. incognita. Journal of Nematology.43:95.
Hayat R, Ali S, Amara U, Khalid R, Ahmed I. 2010. Soil beneficial bacteria and their role in plant growth promotion: a review. Annals of microbiology.60:579–598.
Hooper DJ, Hallmann J, Subbotin SA. 2005. Methods for extraction, processing and detection of plant and soil nematodes. In: Plant parasitic nematodes in subtropical and tropical agriculture. CABI Publishing Wallingford UK. p. 53–86.
Hussey, R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Report, 57, 1025–1028.
Jepson SB. 1987. Identification of root-knot nematodes (Meloidogyne species).
Kesba, H., El-Helaly, M., Abdel Ghanny, S., & Suloma, A. (2013). Potentials of aquaculture effluents on nematode management: 1-effect of tilapia effluents on two nematode species and cowpea growth. Journal of Animal and Plant Sciences., 23, 281–289.
Kesba H, Suloma A, Sayed S, Abdel-Rahman A, Diab S. 2021. Effect of Water of Tilapia Pond on Reproduction of Meloidogyne incognita and Growth of Eggplant in Relation to Soil Type.
Kumar, A., Singh, S., Gaurav, A. K., Srivastava, S., & Verma, J. P. (2020). Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Frontiers in Microbiology., 11, 1216.
Ludwig, J., Moura, A. B., & Gomes, C. B. (2013). Potential of microbiolization of rice seeds with rhizobacteria for root-knot nematode biocontrol. Tropical Plant Pathology., 38, 264–268.
Mazur, Z., & Radziemska, M. (2014). Influence of compost from fish by-products on nutrient supply in radish. Ecological Chemistry and Engineering a., 21, 231–240.
Mhatre PH, Karthik C, Kadirvelu K, Divya K, Venkatasalam E, Srinivasan S, Ramkumar G, Saranya C, Shanmuganathan R. 2019. Plant growth promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocatalysis and agricultural biotechnology.17:119–128.
Nicol J, Turner S, Coyne DL, Nijs Ld, Hockland S, Maafi ZT. 2011. Current nematode threats to world agriculture. In: Genomics and molecular genetics of plant-nematode interactions. Springer. p. 21–43.
Nikoo FS, Sahebani N, Aminian H, Mokhtarnejad L, Ghaderi R. 2014. Induction of systemic resistance and defense-related enzymes in tomato plants using Pseudomonas fluorescens CHAO and salicylic acid against root-knot nematode Meloidogyne javanica. Journal of Plant Protection Research.54.
Oka, Y. (2010). Mechanisms of nematode suppression by organic soil amendments—a review. Applied Soil Ecology., 44, 101–115.
Press MC, Phoenix GK. 2005. Impacts of parasitic plants on natural communities. New phytologist.166:737–751.
Sarker M, Ali M, Islam M, Huda M, Pun I, Podder R, Tomczak A, Hossain A. 2021. Use of different eco-friendly management approaches for controlling root-knot nematode (Meloidogyne incognita L.) in tomato (Solanum lycopersicum L.). Thai Journal of Agricultural Science.54:89− 103–189− 103.
Sasser JN, Carter CC, Hartman KM. 1984. Standardization of host suitability studies and reporting of resistance to root-knot nematodes.
Sharma, I. P., & Sharma, A. (2017). Effective control of root-knot nematode disease with Pseudomonad rhizobacteria filtrate. Rhizosphere., 3, 123–125.
Sohrabi, F., Sheikholeslami, M., Heydari, R., Rezaee, S., & Sharifi, R. (2018). Evaluation of four rhizobacteria on tomato growth and suppression of root-knot nematode, Meloidogyne javanica under greenhouse conditions, a pilot study. Egyptian Journal of Biological Pest Control., 28, 1–5.
Taylor A, Sasser J. 1978. Biology, identification and control of root-knot nematodes. North Carolina State University Graphics.111.
Thoden, T. C., Korthals, G. W., & Termorshuizen, A. J. (2011). Organic amendments and their influences on plant-parasitic and free-living nematodes: A promising method for nematode management? Nematology, 13, 133–153.
Viljoen, J. J., Labuschagne, N., Fourie, H., & Sikora, R. A. (2019). Biological control of the root-knot nematode Meloidogyne incognita on tomatoes and carrots by plant growth-promoting rhizobacteria. Tropical Plant Pathology., 44, 284–291.
Yang Y-R, Li X-G, Zhou Z-G, Zhang T-L, Wang X-X. 2016. Differential responses of soil nematode community to pig manure application levels in Ferric Acrisols. Scientific reports.6:1–10.
Zhang, Y., Fang, S., Tian, Y., Wang, L., & Lv, Y. (2022). Responses of radial growth, wood density and fiber traits to planting space in poplar plantations at a lowland site. Journal of Forestry Research., 33, 963–976.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A. Aioub and R. El-Ashry planned the experiments, collected the data, and performed data analysis and S. Awad prepared tables and figures. A. Aioub and R. El-Ashry wrote the draft. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Ashry, R.M., Aioub, A.A.A. & Awad, S.E. Suppression of Meloiodogyne incognita (Tylenchida: Heteroderidae) and Tylenchulus semipenterans (Tylenchida: Tylenchulidae) using Tilapia fish powder and plant growth promoting rhizobacteria in vivo and in vitro. Eur J Plant Pathol 165, 665–676 (2023). https://doi.org/10.1007/s10658-023-02637-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02637-8