Abstract
Leaf compounds may contribute to plant defense against Cronartium rusts. Secondary compounds are either natural or induced in leaves. We studied the variation of compounds in leaves of six alternate hosts of Cronartium pini and two of C. ribicola that represented either susceptible or resistant species to these rusts. Extracts from the plant leaves were analyzed using LC-MSMS (liquid chromatography tandem mass spectrometry) and compounds were compared between susceptible and resistant species of the same plant genera to identify significant differences between resistant and susceptible species. Also, LC–MS (liquid chromatography mass spectrometry) with external calibration was used to quantify 12 candidate compounds known from the literature. Among these compounds, the most abundant significant ones in C. pini -resistant Melampyrum pratense were chlorogenic acid and quercitrin, in Veronica chamaedrys ferulic acid, quercitrin and luteolin and in Impatiens glandulifera quercitrin, ferulic acid, kaempferol, rutin and hyperoside. In C. ribicola -resistant Ribes rubrum the most abundant significant compounds were caffeic acid, p-coumaric acid and quercitrin. Among all extracted leaf compounds, concentrations of three compounds were over 1000 times greater in rust-resistant M. pratense, three compounds in V. chamaedrys, eight compounds in I. glandulifera, and one compound in R. rubrum than in rust-susceptible species. Among the compounds, the most promising possibly linked to rust resistance were chlorogenic acid and quercitrin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tree rusts of Cronartium are important pathogens of Pinus spp. in the northern hemisphere (Gäumann, 1959; Ziller, 1974). Cronartium pini (Willd.) Jørst. is a significant rust disease that kills Pinus spp. in Europe and Asia (CABI, 2020), while the rust is a quarantine species in North America (Kim et al., 2022). Cronartium pini causes severe damage especially on Pinus sylvestris L. in northern Fennoscandia (Kaitera, 2000; Samils et al., 2021; Wulff et al., 2012). In the 2000s, C. pini has caused severe losses especially on young Scots pine plantations in nutrient-rich soils (Wulff et al., 2012). The chemical compounds enriched in the wood after Cronartium infection have been investigated (Bullington et al., 2018; Kaitera et al., 2021). The results suggested that terpenes and resin acids are produced by the host to protect it from Cronartium rust. The rust spreads via alternate hosts, and over 50 susceptible species are known from 13 plant families (Kaitera et al., 2015; Kim et al., 2022). Important species belong especially to Orobanchaceae, Paeoniaceae and Balsaminaceae (Kaitera et al., 2015).
Another Cronartium, C. ribicola Fisch., is a serious pathogen of five-needle pines in North America (Zambino, 2010). It spreads via Ribes (Grossulariaceae), of which R. nigrum L. is a highly susceptible species, while R. rubrum L. is a resistant species. In Finland, nearly all R. nigrum cultivars are susceptible and R. rubrum cultivars are resistant to C. ribicola (Kaitera & Nuorteva, 2006).
Among plant genera, Melampyrum is one of the most susceptible ones to C. pini (Kaitera, 1999; Kaitera et al., 1999, 2012, 2015, 2017, 2018). M. sylvaticum L., M. nemorosum L., M. arvense L. and M. cristatum L. are highly susceptible species, while M. pratense L. is a resistant species (Kaitera, 1999; Kaitera & Nuorteva, 2003a, b; Kaitera et al., 1999, 2012). Other important alternate host genera are Pedicularis, Euphrasia, Impatiens and Veronica.
Reasons for the differences in susceptibility and resistance are mostly unknown, but certain intrinsic compounds are likely to play a role. Terpenes are among such compounds (Bullington et al., 2018). They may be normally present or induced by pathogens and other factors. Variation in rust resistance among closely related species may be due to variation in leaf chemistry as was proposed for M. sylvaticum and M. pratense (Kaitera & Witzell, 2016). Especially chlorogenic acid was abundant in the resistant M. pratense, while the compound was lacking in M. sylvaticum. Chemical variation on M. pratense and M. sylvaticum in leaves of different ages, time of collection and among locations have not been studied.
Secondary compounds such as phenolics may be involved in plant resistance. In Melampyrum scardicum Wettst, luteolin and apigenin flavonoids were found to be rich (Naumov et al., 1998). These compounds have an antimicrobial impact on bacterial, viral and fungal pathogens (Cushnie & Lamb, 2005). Phenolics are also potential defensive compounds on Pinus against Cronartium rusts (Boyer, 1964; Hanover & Hoff, 1966; Hudgins et al., 2005; Sniezko et al., 2014).
Based on literature concerning plant resistance 12 compounds were selected to study concentration differences in the plant species: chlorogenic acid, caffeic acid, ferulic acid, p-coumaric acid, syringic acid, luteolin, kaempferol, myricetin, quercitrin, rutin, apigenin and hyperoside. Chlorogenic acid is a polyphenol and the ester of caffeic acid and quinic acid. In humans, it has been reported to have antioxidant, antibacterial, chemopreventive, antiviral and neuroprotective characteristics (Clifford et al., 2017; Magana et al., 2021). In the human diet coffee, fruits and vegetables are its major sources (Upadhyay & Rao, 2013). Chlorogenic acid has been shown to have bioactivity against various plant pathogens playing a defensive role against biotic and abiotic stresses (Kundu & Vadassery, 2019; Soviguidi et al., 2022). Caffeic acid is a hydroxycinnamic acid derivative and polyphenol. Like chlorogenic acid it also has many beneficial effects on human health. Caffeic acid is found at high levels in some herbs and fruits, and its bioactivity has been shown (Kiokias et al., 2020). Ferulic acid is a phenolic acid that can be found in the seeds of coffee, apple, artichoke, peanut and orange (Kiokias et al., 2020). p-Coumaric acid is found in many natural plants and organisms like fungi, peanuts, beans, tomatoes, carrots, basil and garlic (Kiokias et al., 2020). In addition, most fruits contain p-coumaric acid. Evidence for its high bioactivity has been reported. Syringic acid is a derivative of gallic acid. Its bioactivity for suppression of chronic diseases like human leukemia (HL)-60 and DV-145 human prostate carcinoma cells has been shown (Shahidi & Yeo, 2018). Luteolin, kaempferol, myricetin, quercitrin, rutin, apigenin and hyperoside are hydroxyflavones having potential bioactivity (Adamczak et al., 2020; Cirak et al., 2007; Dall’Agnol et al., 2003; Elansary et al., 2020; Gharibi et al., 2019).
In addition, an unbiased liquid chromatography-tandem mass spectrometry (LC-MSMS) approach was chosen to characterize differences in the compound spectrum of the different plant species. Here all detectable compounds were characterized by their accurate mass and chromatographic retention time and differences in signal intensity (integrated ion counts of chromatographic peaks of the SICs) were compared between susceptible and resistant species. Data were collected in data dependent acquisition mode, in which the instrument control software recognizes signals and switches automatically to MSMS mode to select and fragmentate the corresponding ions. Accurate mass and MSMS spectra were then used to search different compound databases to identify compounds of interest.
The aim of this study was 1) to investigate the variation of compounds in leaves of alternate hosts species susceptible and resistant to C. pini and C. ribicola, 2) to compare compounds of resistant and susceptible species groups to one another, and 3) to characterize compounds that may be linked to rust resistance. These compounds may be important in developing control of rust diseases and might be utilized against other pathogens in the future.
Material and methods
Plant material
Circa 10–20 young leaves of eight species were harvested from 20 randomly selected wild or cultivated plants per species from the city area of Oulu. Resistant wild species of C. pini were M. pratense, Impatiens glandulifera Royle and Veronica chamaedrys L., and a resistant cultivated species of C. ribicola was Ribes rubrum. Susceptible wild species of C. pini were M. sylvaticum and V. longifolia L., while a susceptible cultivated species was I. balsamina L. Susceptible cultivated species of C. ribicola was R. nigrum. The cultivated plants were located in the Botanical Gardens of the University of Oulu (65°3,86 N, 25°27,79E). All plants of Melampyrum, Impatiens and Veronica were collected first into paper bags and transported to the laboratory prior to leaf collection. Ten leaves of Ribes spp. per species were collected directly into paper bags and transported similarly to the laboratory. The collection locations of the plants in Oulu were: M. sylvaticum and M. pratense (65°2,69 N, 25°28,04E), V. longifolia (65°2,27 N, 25°29,16E), V. chamaedrys (65°1,30 N, 25°25,96E), I. glandulifera (65°3,03 N, 25°25,20E), I. balsamina (garden plants grown from seed in the botanical Garden), R. nigrum and R. rubrum (cultivated plants in the botanical garden). The plants were collected in late June 2021. The habitats of the wild species were determined by the personnel of the Botanical Gardens. The leaves were collected mainly during flowering of the plants to ensure correct identification of the plants described in Hämet-Ahti et al. (1998).
Standards and reagents
The standard compounds used in the quantitative LC–MS analysis were as follows: caffeic acid (TCI, Tokyo Chemical Industry, C002), p-coumaric acid (TCI, C0393), syringic acid (TCI, G0014), luteolin (TCI, T2682), myricetin (TCI, M2131), rutin (TCI, R0035), kaempferol (TCI, K0018), apigenin (TCI, A1514), quercitrin (Cayman Chemical Company, CAYM19866), hyperoside (PanReac Appli Chem, A1791,0100), chlorogenic acid (Acros Organics, 109,240,010), ferulic acid (Sigma, PHR1791) and ampicillin sodium crystalline (Sigma, A9518). Methanol was HPLC grade (Merck, 1.06007.2500).
Water for the chromatography was produced in house with a Synergy UV instrument (Millipore, cat.no SYNSV0000), equipped with a LC-PAK Polisher (Cat.No. LCPAK 0001) cartridge for the final purification step. Acetonitrile and formic acid were OPTIMA LCMS grade (Fisher Chemical, code A955-212 and A117-50, respectively).
Pretreatment and extraction of leaves
In the laboratory, healthy green leaves of the plants without any sign of fungal or insect damage, were separated from the rest of the plant material in a laminar cabin with sterile tweezers. Then the leaves were air-dried for ca. a week in a laminar cabin in open paper bags and stored at -20 °C prior to analysis. The leaf samples were crushed manually inside a paper bag until a powdery consistency was achieved. About 15 mg of each plant material was weighed into Eppendorf vials. Methanol, containing 5 mg/l of internal standard (ampicillin), was used as an extraction solvent. The solvent was cooled to + 4 °C before usage. 1 ml of the solvent was added to vials which were kept at + 4 °C for one hour. The samples were shaken using Eppendorf MixMate (5 min, 1400 rpm) after which they were centrifuged at + 4 °C (5 min, 12,000 rpm, Hettich Mikro 200). The supernatant was transferred to another vial and the residue was extracted with 0.5 ml of pure methanol (without internal standard). The supernatants were combined and filtered through a disposable syringe filter (pore size 0.2 µm, Pall Corporation). The extracts were stored at -20 °C.
Chemical analysis
20 biological repeats i.e. all collected leaves from individual plants per species, 160 samples in total, were analyzed. In the LC–MS approach compounds were characterized by retention time, accurate mass and peak area. This dataset was used to obtain maximal data points for quantitation and quantify the 12 candidate compounds with external calibration. Additionally, the same samples were analyzed with an LC-MSMS approach to obtain data for the identification of unknown compounds. 5 µl sample aliquots were eluted from a Waters Aquity Premier HSS T3 column (2.1 × 100 mm, 1.8 µm, Part No. 186009468)) with a gradient made with 0.1% formic acid in water and acetonitrile from 3 to 70% over 14 min, column temperature 40 °C (Waters Aquity UPLC-system comprising column oven (186015010), binary, high pressure mixing pump module (186016007), and autosampler (186015001)). The detector was a Q-Exactive plus orbitrap mass spectrometer in biopharma configuration (Thermos Fisher Scientific) operated in negative polarity at resolution set to 70,000 A in m/z range from 115 to 1200. For the MSMS acquisition, the same conditions were used with the addition of DDA- controlled fragmentation with stepped collision energy (nce) of 25 and 35 and fragment acquisition with m/z range 200 to 2000. The DDA data were processed with Compound Discoverer (Thermo) using standard settings for natural compound analysis. Mz vault, mz cloud, ChemSpider and mass lists were applied in compound identification.
For the quantitation of the candidate compounds a calibration curve with 8 levels from 1 µg/ml to 1 ng/ml was established, data processing was done with the X-calibur and its Quanbrowser option (Thermo). As quality controls two pools comprising 30 µl aliquots of 50 different samples were applied. The compounds, known to occur in leaves of M. pratense and M. sylvaticum (Kaitera and Witzell 2017), were: chlorogenic acid, caffeic acid, syringic acid, p-coumaric acid, rutin, hyperoside, ferulic acid, quercitrin, myricetin, luteolin, apigenin and kaempferol.
Statistical analysis
The data set for negative ionization, filtered for features (compounds) with intensity counts over 106 in any of the individual samples, was used in the statistical analyses. About 40 features (compounds) with the highest ratios at minimum 25 (e.g. M. pratense:M.sylvaticum > 25) distinguishing the susceptible and resistant plant groups at p < 0.001 (Compound Discoverer) were listed with the identification suggested by the data base search. Identification leading to compounds non-existing in plant leaves (e.g. fluorine compounds) were left unnamed like compounds without identification. The concentrations of the 12 compounds selected prior to the chemical analysis were compared between resistant and susceptible species with Welch two-sample t-test with unequal variances using the R program (R Core Team 2019, Version 3.6).
Results
Variation of compounds in plants
In the C. pini -susceptible-resistant species pair M. sylvaticum-M. pratense among the 40 compounds with the highest loadings differentiating the groups, 26 compounds were listed as significant ones (Table 1). The compounds that significantly distinguished M. pratense and M. sylvaticum were: afzelechin 3–0-alpha-L-rhamno-pyranoside, apigenin 7,4’-diglucuronide, acacetin 7-glucuronosyl-(1 = > 2)-glucaronide, 6-hydroxyapigenin 7-glucuronosyl (1 = > 2)-glucuronide and chrysoeriol 7,4’-diglucuronide. Thirteen compounds remained unidentified (Table 1).
In another C. pini -susceptible-resistant species pair V. longifolia – V. chamaedrys, 30 compounds were listed as significant ones (Table 2). The significant compounds that distinguished V. chamaedrys and V. longifolia were: methyl 3-O-({1-[(1S)-1-carboxy-2-(1H-indol-2-yl)ethyl]-1H-1,2,3-triazol-4-yl}methyl)-alpha-D-galactopyranoside, phloretin 2'-O- (6"-O-acetylglucoside), okanin 3,4,3'-trimethyl ether 4'-glucoside and luteolin 4'-methyl ether 7- (4G-rhamnosylneohesperidoside). Fourteen compounds remained unidentified (Table 2).
In the third C. pini -susceptible-resistant species pair I. balsamina – I. glandulifera, 27 compounds were listed as significant ones (Table 3). The significant compounds that distinguished I. glandulifera and I. balsamina were: peniisocoumarin I, eriodictyol, (2S,3S)-2-{[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]oxy}-3,4-dihydroxy-2-methylbutanoic acid, sinensin, 6-O-[(2E)-3-(4-Hydroxyphenyl)-2-propenoyl]-D-glucopyranose, 1-O-feruloyl-beta-D-glucose, astragalin, quercetin 3- (3"-acetylrhamnoside) and luteolin 7-O- (6"-malonylglucoside). Sixteen compounds remained unidentified (Table 3).
In the fourth C. ribicola -susceptible-resistant species pair R. nigrum – R. rubrum, 37 compounds were listed as significant ones (Table 4). The significant compounds that distinguished R. rubrum and R. nigrum were: ( +) -maackiain 3-O-glucoside, 1-({[3,5-bis(hydroxymethyl)-2,4,6-trioxo-1,3,5-triazinan-1-yl]methoxy}methyl)-3-(hydroxymethyl)urea, gliricidol, quercetin 3- (2"-p-coumarylglucoside) and quercetin 3- (2Gal-apiosylrobinobioside). Five compounds remained unidentified (Table 4).
Concentrations of pre-selected compounds in the samples
Concentrations of chlorogenic acid were significantly 570 times higher in samples of M. pratense compared to those in M. sylvaticum (p < 0.0001, Table 5). They were also insignificantly two times higher in samples of V. chamaedrys compared to samples of V. longifolia (Table 6), 17 times higher in samples of I. glandulifera compared to samples of I. balsamina (Table 7), and 5 times higher in samples of R. rubrum compared to samples of R. nigrum (Table 8).
The mean concentration of caffeic acid in samples of M. sylvaticum was 0.04 ng/mg, whereas samples of M. pratense did not contain measurable amounts (NF, not found; Table 5). Concentrations of caffeic acid were insignificantly 1.1 times higher in samples of V. longifolia compared to those in V. chamaedrys and 1.8 times higher in samples of I. balsamina compared to those in I. glandulifera (Tables 6 and 7). However, concentrations of caffeic acid were significantly 21 times higher in samples of R. rubrum than in samples of R. nigrum (p < 0.0001, Table 8).
Concentrations of syringic acid were insignificantly 3.5 times higher in samples of M. pratense compared to those in M. sylvaticum (Table 5). The mean concentrations in samples of V. chamaedrys and I. balsamina were 0.28 ng/mg and 0.12 ng/mg (Tables 6 and 7). Samples of V. longifolia, I. glandulifera, R. rubrum and R. nigrum did not contain any syringic acid (Tables 6, 7 and 8).
Concentrations of p-coumaric acid were insignificantly 1.1 times higher in samples of M. pratense compared to those in M. sylvaticum, and 1.5 times higher in samples of I. balsamina than those in I. longifolia (Tables 5 and 7). However, concentrations were significantly 3.6 times higher in samples of V. longifolia than in samples of V. chamaedrys (p < 0.0001, Table 6), and 44 times higher in samples of R. rubrum than those in R. nigrum (p < 0.0001, Table 8).
Concentrations of rutin were significantly 13 times higher in samples of M. sylvaticum compared to those in M. pratense (p < 0.0001), 6 times higher in samples of I. glandulifera than those in I. balsamina (p < 0.0001), and 7 times higher in samples of R. nigrum than those in R. rubrum (p < 0.0001; Tables 5,7 and 8). Samples of V. chamaedrys and V. longifolia did not contain any rutin (Table 6).
Concentrations of hyperoside were significantly 1.8 times higher in samples of M. sylvaticum compared to those in M. pratense (p < 0.001), and 35 times higher in samples of I. glandulifera compared to those in I. balsamina (p < 0.0001; Tables 5 and 7). Concentrations were insignificantly 1.4 times higher in samples of R. rubrum than those in R. nigrum (Table 8). The mean concentration of hyperoside in samples of V. chamaedrys was 1.17 ng/mg, whereas samples of V. longifolia did not contain measurable amount (Table 6).
Concentrations of ferulic acid were significantly 20 times higher in samples of M. sylvaticum compared to those in M. pratense (p < 0.0001; Table 5). Concentrations in samples of V. chamaedrys and I. balsamina were also significantly higher compared to those in V. longifolia and I. glandulifera (p < 0.0001, Tables 6 and 7). Concentrations were insignificantly 1.7 times higher in samples of R. nigrum than those in R. rubrum (Table 8).
Concentrations of quercitrin were significantly 18 times higher in samples of M. pratense compared to those in M. sylvaticum (p < 0.0001; Table 5), 3.5 times higher in samples of I. glandulifera compared to those in I. balsamina (p < 0.0001; Table 7), and 4.7 times higher in samples of R. rubrum compared to those in R. nigrum (p < 0.01; Table 8). The mean concentration of quercitrin in samples of V. chamaedrys was 0.50 ng/mg compared to NF for V. longifolia (p < 0.0001; Table 6).
Concentrations of myricetin were insignificantly 1.9 times higher in samples of M. sylvaticum compared to those in M. pratense, and 1.7 times higher in samples of V. longifolia than those in V. chamaedrys (Tables 5 and 6). Samples of I. glandulifera, I. balsamina, R. rubrum and R. nigrum did not contain any myricetin (Tables 7 and 8).
Concentrations of luteolin were significantly 15 times higher in samples of M. sylvaticum compared to those in M. pratense (p < 0.001; Table 5), and 4 times higher in samples of V. chamaedrys compared to those in V. longifolia (p < 0.001; Table 6). The mean concentration of luteolin in samples of I. glandulifera was 0.35 ng/mg (Table 7). Samples of I. balsamina, R. rubrum and R. nigrum did not contain any luteolin (Table 7 and 8).
Concentrations of apigenin were insignificantly 1.4 times higher in samples of M. sylvaticum compared to those in M. pratense, and 14 times higher in samples of V. chamaedrys compared to those in V. longifolia (Tables 5 and 6). The mean concentration in samples of R. nigrum was 20.25 ng/mg being significantly higher compared to R. rubrum (p < 0.0001, Table 8). Samples of I. glandulifera, I. balsamina and R. rubrum did not contain any apigenin (Tables 7 and 8).
Concentrations of kaempferol were significantly three times higher in samples of I. glandulifera than those in I. balsamina (p < 0.0001; Table 7). The mean concentration of kaempferol in samples of R. nigrum was 0.53 ng/mg being significantly higher compared to R. rubrum (p < 0.01; Table 8). Concentrations were insignificantly 1130 times higher in samples of V. chamaedrys than those in V. longifolia, and 1.1 times higher in samples of M. pratense compared to those in M. sylvaticum (Tables 5 and 6).
Discussion
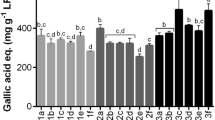
The highest concentration of chlorogenic acid (5-O-caffeoylquinic acid) was found in M. pratense (182.48 ng/mg, Table 5). R. rubrum (58.78 ng/mg) and R. nigrum (11.98 ng/mg) contained also significant amounts of chlorogenic acid (Table 8). In the other studied plant species the concentrations were low (Tables 6 and 7). It is well known that chlorogenic acid plays a defensive role against biotic and abiotic stresses in plants. Petkovsek et al. (2009) noticed seasonal changes in phenolic compound concentrations in the leaves of scab-resistant and susceptible apple cultivars. The mean chlorogenic acid concentrations found from the resistant and susceptible species were 365–3262 ng/mg and 184–500 ng/mg, respectively. They discovered that concentrations of total phenolics as well as single phenolic compounds, like chlorogenic acid, were statistically significantly higher in resistant than in susceptible apple varieties during the growing season. The concentration of chlorogenic acid was also higher in leaves of rust-resistant M. pratense compared to those of rust-susceptible M. sylvaticum in a recent study (Kaitera & Witzell, 2016). The role of chlorogenic acid in plant response to abiotic stresses (heavy metal, UV light, heat, cold, salinity and drought) has also been extensively studied (Soviguidi et al., 2022). Our results clearly support the significant role of chlorogenic acid in plant defense mechanism against biotic stresses.
In this study, the highest concentrations of quercitrin were measured from I. glandulifera (5493.58 ng/mg) and I. balsamina (1567.80 ng/mg) (Table 7). The concentration in the resistant species I. glandulifera was significantly higher than in the susceptible species I. balsamina. Also, high concentrations were found in R. rubrum (451.34 ng/mg) and R. nigrum (96.02 ng/mg; Table 8). The concentrations of quercitrin in other tested plant species were low, but statistically significantly higher in the resistant species, M. pratense and V. chamaedrys, compared to the susceptible species M. sylvaticum and V. longifolia (Tables 5 and 6). Elansary et al. (2020) studied polyphenols of Frangula alnus Mill. and Peganum harmala L. leaves and their bioactivity. They studied the concentrations of several polyphenolic compounds and reported that quercitrin was the main flavonoid in F. alnus (11,323 mg/kg). Leaf extracts of both species showed cytotoxic effects against Jurkat, MCF-7, HeLa and HT-29 cancer cells. They concluded that the polyphenolic composition of leaves including quercitrin, trifolin and cymaroside play a significant role in the bioactivity of these plants.
Also, other statistically significant compounds that distinguish the selected resistant vs. susceptible plant species pairs were searched from the LC-MSMS data in this study. In the M. pratense and M. sylvaticum species pair, the most significant compounds were afzelechin 3–0-alpha-L-rhamno-pyranoside, apigenin 7,4’-diglucuronide, acacetin 7-glucuronosyl-(1 = > 2)-glucaronide and chrysoeriol 7,4’-diglucuronide (Table 1). Afzelechin 3–0-alpha-L-rhamno-pyranoside is a flavonoid glycoside. Based on earlier studies, this compound has been isolated from e.g. Artocarpus sepicanus Diels leaves (Radwan et al., 2009), Cassipourea malosana (Baker) Alston bark (Drewes et al., 1992) and Averrhoa bilimbi L. leaves (Ahmed et al., 2018). Averrhoa bilimbi is widely used in traditional medicine. Ahmed et al. (2018) found that the n-butanol fraction of A. bilimbi crude methanol leaf extract showed significant antioxidant properties. They concluded that afzelechin 3–0-alpha-L-rhamno-pyranoside and cucumerin A likely cause this bioactivity in the methanol leaf extract of A. bilimbi. Radwan et al. (2009) studied the compounds antimicrobial activity against the fungi Candida albicans (C.-P. Robin) Berkhout, Aspergillus fumigatus Fresen, Cryptococcus neoformans (San Felice) Vuill., and the bacteria Escherichia coli (Migula) Castellani & Chalmers, Pseudomonas aeruginosa (Schroeter) Migula, Mycobacterium intracellulare Runyon and MRSA (methicillin-resistant Staphylococcus aureus Rosenbach). Afzelechin 3–0-alpha-L-rhamno-pyranoside was inactive against all microbes tested. Apigenin 7,4’-diglucuronide and chrysoeriol 7,4’-diglucuronide are members of flavonoids and a glucosiduronic acid. Ichimura et al. (2021) investigated the effects of temperature and light intensity on anthosyanin biosynthesis in snapdragons (Antirrhinum majus L.). In this study they also measured the apigenin 7,4’-diglucuronide content of the flowers of the plants. They concluded that the high temperature affects anthocyanin synthesis more than flavone biosynthesis in snapdragon petals. Acacetin, chrysoeriol and their respective glycosides are common flavones in Citrus fruits and juices with good pharmacological effects (Barreca et al., 2020), but for acacetin 7-glucuronosyl-(1 = > 2)-glucaronide and chrysoeriol 7,4’-diglucuronide, we couldn’t find any literature. The apigenin derivate acacetin was reported to be richer in leaves of rust-resistant M. pratense compared to those of rust-susceptible M. sylvaticum and luteolin derivate chrysoeriol vice versa in a previous study (Kaitera & Witzell, 2016).
For the V. chamaedrys and V. longifolia species pair the most significant compounds were phloretin 2'-O- (6"-O-acetylglucoside), okanin 3,4,3'-trimethyl ether 4'-glucoside and luteolin 4'-methyl ether 7- (4G-rhamnosylneohesperidoside) (Table 2). Methylated okanin derivatives can be found from Bidens torta Sherff (McCormick et al., 1984). McCormick et al. (1984) determined the structures of four methylated chalcones including okanin 3,4,3'-trimethyl ether 4'-glucoside. Rao et al. (2020) studied the response of phenolic compounds in rice to different growing conditions. Luteolin 4'-methyl ether 7- (4G-rhamnosylneohesperidoside) was one of the compounds determined from different rice varieties and growing locations. They discovered that the effect of cultivation environment on the concentration and antioxidant activity of this compound varied between rice varieties indicating the influence of both genetics and environment on the compound. Earlier, luteolin was reported to be richer in leaves of rust-susceptible M. sylvaticum compared to those of rust-resistant M. pratense (Kaitera & Witzell, 2016). Phloretin can be found in apple tree leaves. Antifungal activity of phloretin against several plant pathogenic fungi has been reported (Shim et al., 2010). Phlorizin, a glucoside of phloretin, is also present in the apple tree (root bark, shoots, leaves) and experimental evidence suggests that it plays a significant role in apple tree physiology (Ehrenkranz et al., 2005). For the bioactivity of phloretin 2'-O- (6"-O-acetylglucoside) we couldn’t find any literature.
For the I. glandulifera and I. balsamina species pair the most significant compounds were peniisocoumarin I, eriodictyol, (2S,3S)-2-{[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]oxy}-3,4-dihydroxy-2-methylbutanoic acid, sinensin, 1-O-feruloyl-beta-D-glucose, astragalin and luteolin 7-O- (6"-malonylglucoside (Table 3). Peniisocoumarin 1 is a natural product in Penicillium commune Charles Thom. Eriodictyol is a tetrahydroxyflavanone that can be found from wide range of medicinal plants, citrus fruits and vegetables. The medicinal properties of eriodictyol have been extensively studied (Deng et al., 2020; Islam et al., 2020; Khan et al., 2014). (2S,3S)-2-{[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoyl]oxy}-3,4-dihydroxy-2-methylbutanoic acid is a hydroxycinnamic acid. Sinensin is a flavonoid and a glycoside. Baek et al. (2015) identified kaempferol, astragalin, quercetin, isoquercitrin, sexangularetin and sinensin from the calyx of Fragaria ananassa Duchesne ex Rozier. Quercetin showed the highest radical-scavenging activity whereas sinensin showed the lowest activity. Astragalin (kaempferol 3-glucoside) is a bioactive compound found in several medicinal plants such as Cuscuta chinensis Lam. (Riaz et al. 2018). Astragalin is well known for its pharmacological properties. Kaempferol was reported to be richer in leaves of rust-susceptible M. sylvaticum compared to those of rust-resistant M. pratense recently (Kaitera & Witzell, 2016). 1-O-feruloyl-beta-D-glucose is a natural product having a role as an antioxidant and a plant metabolite (Arnaldos et al., 2001; Delazar et al., 2017; Du et al., 2006; Jia et al., 2017). Luteolin 7-O- (6"-malonylglucoside) is a trihydroxyflavone. Luteolin was reported to be richer in leaves of rust-susceptible M. sylvaticum compared to those of rust-resistant M. pratense recently (Kaitera & Witzell, 2016).
For the R. rubrum and R. nigrum species pair the most interesting compounds were ( +) -maackiain 3-O-glucoside, gliricidol, quercetin 3- (2"-p-coumarylglucoside) and quercetin 3- (2Gal-apiosylrobinobioside) (Table 4). ( +) -Maackiain 3-O-glucoside, also called sophojaponicin, belongs to the pterocarpans group of compounds. It has been isolated from the roots of Cicer judaicum Baksier, which is an annual herb from the Middle East (Stevenson & Veitch, 1996). Gliricidol is a flavonoid found from the methanolic extract of Gliricidia sepium (Jacq.) Steud. bark (Rastrelli et al., 1999). It has shown bioactivity against Artemia salina L. larvae. For the two quercetin derivatives we couldn’t find any literature about their bioactivity in plants, but generally, the quercetin compounds are known to have many possible health effects on humans. Quercetin compounds were also rich in leaves of Melampyrum spp. in a recent study (Kaitera & Witzell, 2016). In conclusion, our quantitative results of the pre-selected compounds revealed two compounds, chlorogenic acid and quercitrin, whose concentrations differ significantly between rust-resistant and susceptible plant species. The literature also supported the probable bioactivity of these compounds against rust diseases. From the discovery approach, we could find additional compounds with a putative role in the plant defense against rust disease. It is also known that in infected wood of mature P. sylvestris, C. pini induced a 1.3–108 fold increase in concentrations of monoterpenes, resin acids and several sesquiterpenes compared to control wood (Kaitera et al., 2021). In P. albicaulis Engelm. seedlings, terpene concentrations were higher in C. ribicola -resistant trees compared to susceptible ones (Bullington et al., 2018). Also C. quercuum f.sp. fusiforme Burds. & G.A.Snow -susceptible P. elliotii Engelm. trees contained lower amounts of some monoterpenes than resistant ones (Michelozzi et al., 1991). Therefore, monoterpenes are important compounds in Cronartium resistance to Pinus spp. Further research is needed to describe the temporal and spatial variation of the compounds in alternate host plants of Cronartium. Inoculation tests in controlled environment should be done to study the induced chemical changes in alternate hosts due to rust infections. Also the effect of leaf extracts and individual compounds of extracts of rust-resistant species should be tested against Cronartium rusts in controlled experiments.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ahmed, Q. U., Alhassan, A. M., Khatib, A., Shah, S. A. A., Hasan, M. M., & Sarian, M. N. (2018). Antiradical and xanthine oxidase inhibitory activity evaluations of Averrhoa bilimbi L. leaves and tentative identification of bioactive constituents through LC-QTOF-MS/MS and molecular docking approach. Antioxidants, 7(10), 137–153.
Adamczak, A., Ozarowski, M., & Karpinski, T. M. (2020). Antibacterial activity of some flavonoids and organic acids widely distributed in plants. Journal of Clinical Medicine, 9, 109–126.
Arnaldos, T. L., Munoz, R., Ferrer, M. A., & Calderon, A. A. (2001). Changes in phenol content during strawberry (Fragaria ananassa, cv. Chandler) callus culture. Physiologia Plantarum, 113, 315–322.
Barreca, D., Mandalari, G., Calderaro, A., Smeriglio, A., Trombetta, D., Felice, M. R., & Gattuso, G. (2020). Citrus flavones: An update on sources, biological functions, and health promoting properties. Plants, 9(3), 288–311.
Boyer, M. G. (1964). Studies on white pine phenols in relation to blister rust. Canadian Journal of Botany, 42, 979–987.
Bullington, L. S., Lekberg, Y., Sniezko, R., & Larkin, B. (2018). The influence of genetics, defensive chemistry and the fungal microbiome on disease outcome in whitebark pine trees. Molecular Plant Pathology, 19(8), 1847–1858.
CABI. (2020). Cronartium flaccidum (Scots pine blister rust) in: Invasive Species Compendium. Wallingford, UK: CAB International. Retrieved 21 May 2021 from https://www.cabi.org/isc/datasheet/16148.
Cirak, C., Radusiene, J., Janulis, V., Ivanauskas, L., & Arslan, B. (2007). Chemical constituents of some Hypericum species growing in Turkey. Journal of Plant Biology, 50(6), 632–635.
Clifford, M. N., Jaganath, I. B., Ludwig, I. A., & Crozier, A. (2017). Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Natural Product Reports, 34, 1391–1421.
Cushnie, T. P. T., & Lamb, A. J. (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26, 343–356.
Dall’Agnol, R., Ferraz, A., Bernardi, A. P., Albring, D., Nör, C., Sarmento, L., Lamb, L., Hass, M., von Poser, G., & Schapoval, E. E. S. (2003). Antimicrobial activity of some Hypericum species. Phytomedicine, 10, 511–516.
Delazar, A., Nazemiyeh, H., Afshar, F. H., Barghi, N., Esnaashari, S., & Asgharian, P. (2017). Chemical compositions and biological activities of Scutellaria pinnatifida A. Hamilt aerial parts. Research in Pharmaceutical Sciences, 12(3), 187–195.
Deng, Z., Hassan, S., Rafiq, M., Li, H., He, Y, Cai, Y., Kang, X., Liu, Z., & Yan, T. (2020). Pharmacological activity of eriodictyol: The major natural polyphenolic flavanone. Evidence-based complementary and alternative medicine, 11 p. Article id 6681352. https://doi.org/10.1155/2020/668/352
Drewes, S. E., Taylor, C. W., & Cunningham, A. B. (1992). (+)-Afzelechin 3-rhamnoside from Cassipourea gerrardii. Phytochemistry, 31(3), 1073–1075.
Du, Q., Xu, Y., Li, L., Zhao, Y., Jerz, G., & Winterhalter, P. (2006). Antioxidant constituents in the fruits of Luffa cylindrica (L.) Roem. Journal of Agricultural and Food Chemistry, 54, 4186–4190.
Ehrenkranz, J. R. I., Lewis, N. G., Kahn, C. R., & Roth, J. (2005). Phlorizin: A review. Diabetes/metabolism Research and Reviews, 21(1), 31–38.
Elansary, H. O., Szopa, A., Kubica, P., Ekiert, H., Al-Mana, F. A., & El-Shafei, A. A. (2020). Polyphenols of Frangula alnus and Peganum harmala leaves and associated biological activities. Plants, 9, 1086–1101.
Gäumann, E. (1959). Die Rostpilze Mitteleuropas. Beiträge Zur Kryptogamenflora Der Schweiz, 12, 1–1407.
Gharibi, S., Tabatabaei, B. E. S., Saeidi, G., Talebi, M., & Matkowski, A. (2019). The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry, 162, 90–98.
Hämet-Ahti, L., Suominen, J., Ulvinen, T., & Uotila, P. (1998). Retkeilykasvio Suomen Luonnontieteellinen keskusmuseo. Kasvimuseo.
Hanover, J. W., & Hoff, R. J. (1966). A comparison of phenolic constituents of Pinus monticola resistant and susceptible to Cronartium ribicola. Physiologia Plantarum, 19, 554–562.
Hudgins, J. W., McDonald, G. I., Zambino, P. J., Klopfenstein, N. B., & Franceschi, V. R. (2005). Anatomical and cellular responses of Pinus monticola stem tissues to invasion by Cronartium ribicola. Forest Pathology, 35(6), 423–443.
Ichimura, K., Niki, T., Matoh, M., & Nakayama, M. (2021). High temperature under low light conditions suppresses anthocyanin biosynthesis in snapdragon petals associated with decreased sugar levels. Scientia Horticulturae, 290, 1–9.
Islam, A., Islam, M. S., Rahman, M. K., Uddin, M. N., & Akanda, M. R. (2020). The pharmacological and biological roles of eriodictyol. Archives of Pharmacal Research, 43, 582–592.
Jia, X., Yang, D., Xie, H., Jiang, Y., & Wei, X. (2017). Non-flavonoid phenolics from Averrhoa carambola fresh fruit. Journal of Functional Foods, 32, 419–425.
Kaitera, J. (1999). Cronartium flaccidum fruitbody production on Melampyrum spp. and some important alternate hosts to pine. European Journal of Forest Pathology, 29, 391–398.
Kaitera, J. (2000). Analysis of Cronartium flaccidum lesion development on pole-stage Scots pines. Silva Fennica, 34, 21–27.
Kaitera, J., & Nuorteva, H. (2003a). Cronartium flaccidum produces uredinia and telia on Melampyrum nemorosum and on Finnish Vincetoxicum hirundinaria. Forest Pathology, 33, 205–213.
Kaitera, J., & Nuorteva, H. (2003b). Relative susceptibility of four Melampyrum species to Cronartium flaccidum. Scandinavian Journal of Forest Research, 18, 499–504.
Kaitera, J., & Nuorteva, H. (2006). Susceptibility of Ribes spp. to pine stem rusts in Finland. Forest Pathology, 36, 225–246.
Kaitera, J., & Witzell, J. (2016). Phenolic profiles of two Melampyrum species differing in susceptibility to Cronartium rust. European Journal of Plant Pathology, 144(1), 133–140.
Kaitera, J., Seitamäki, L., Hantula, J., Jalkanen, R., & Kurkela, T. (1999). Inoculation of known and potential alternate hosts with Peridermium pini and Cronartium flaccidum aeciospores. Mycological Research, 103, 235–241.
Kaitera, J., Hiltunen, R., & Samils, B. (2012). Alternate host ranges of Cronartium flaccidum and C. ribicola in northern Europe. Botany, 90, 694–703.
Kaitera, J., Hiltunen, R., & Hantula, J. (2015). Cronartium rust sporulation on hemiparasitic plants. Plant Pathology, 64, 738–747.
Kaitera, J., Kalleinen, L., Mikkilä, J., & Hantula, J. (2017). Cronartium flaccidum sporulates on new Euphrasia species in natural habitats in Finland. Forest Pathology, 47(5), e12349.
Kaitera, J., Kauppila, T., & Hantula, J. (2018). New alternate hosts for Cronartium spp.: Odontites, Euphrasia. Rhinanthus and Papaver. Forest Pathology, 48, e12466.
Kaitera, J., Piispanen, J., & Bergmann, U. (2021). Terpene and resin acid contents in Scots pine stem lesions colonized by the rust fungus Cronartium pini. Forest Pathology, 51(4), e12700.
Khan, M. K., Zill-E-Huma, & Dangles, O. (2014). A comprehensive review on flavanones, the major citrus polyphenols. Journal of Food Composition and Analysis, 33, 85–104.
Kim, M.-S., Hantula, J., Kaitera, J., Zambino, P. J., Woodward, S., Richardson, B. A., Stewart, J. E., Spaine, P., Shaw, D. C., Takeuchi, Y., & Klopfenstein, N. B. (2022). Recovery plan for Scots pine blister rust caused by Cronartium pini. Plant Health Progress, 23(1), 105–130.
Kiokias, S., Proestos, C., & Oreopoulou, V. (2020). Phenolic Acids of Plant Origin - A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their In Vivo Health Biochemical Properties. Foods, 9, 534–556.
Kundu, A., & Vadassery, J. (2019). Chlorogenic acid-mediated chemical defence of plants against insect herbivores. Plant Biology, 21, 185–189.
Magana, A. A., Kamimura, N., Soumyanath, A., Stevens, J. F., & Maier, C. S. (2021). Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. The Plant Journal, 107, 1299–1319.
McCormick, S. P., Bohm, B. A., & Ganders, F. R. (1984). Methylated chalcones from Bidens torta. Phytochemistry, 23(10), 2400–2401.
Michelozzi, M., Squillace, A. E., & White, T. L. (1991). Monoterpene composition and fusiforme rust resistance in Slash pine. Forest Science, 36, 470–475.
Naumov, P., Kuzmanovski, I., & Stefova, M. (1998). Flavonoids of Verbascum scardicolum and Melampyrum scardicum. Bulletin of the Chemists and Technologists of Macedonia, 17(1), 41–44.
Petkovsek, M. M., Stampar, F., & Veberic, R. (2009). Seasonal changes in phenolic compounds in the leaves of scab-resistant and susceptible apple cultivars. Canadian Journal of Plant Science, 89(4), 745–753.
Radwan, M. M., Rodriguez-Guzman, R., Manly, S. P., Jacob, M., & Ross, S. A. (2009). Sepicanin A – A new geranyl flavanone from Artocarpus sepicanus with activity against methicillin-resistant Staphylococcus aureus (MRSA). Phytochemistry Letters, 2(4), 141–143.
Rao, S., Santhakumar, A. B., Chinkwo, K., Snell, P., Oli, P., & Blanchard, C. L. (2020). Rice phenolic compounds and their response to variability in growing conditions. Cereal Chemistry, 97, 1045–1055.
Rastrelli, L., Berger, I., Kubelka, W., Caceres, A., De Tommasi, N., & De Simone, F. (1999). New 12a-hydroxyrotenoids from Gliricidia sepium bark. Journal of Natural Products, 62(1), 188–190.
Samils, B., Kaitera, J., Persson, T., Stenlid, J., & Barklund, P. (2021). Relationship and genetic structure among autoecious and heteroecious populations of Cronartium pini in northern Fennoscandia. Fungal Ecology, 50(2021), 101032.
Shahidi, F., & Yeo, J. D. (2018). Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. International Journal of Molecular Sciences, 19, 1573–1589.
Shim, S.-H., Jo, S.-J., Kim, J.-C., & Choi, G. J. (2010). Control efficacy of phloretin isolated from apple fruits against several plant diseases. Plant Pathology Journal, 26(3), 280–285.
Sniezko, R. A., Smith, J., Liu, J.-J., & Hamelin, R. C. (2014). Genetic resistance to fusiform rust in southern pines and white pine blister rust in white pines – a contrasting tale of two rust pathosystems - current status and future prospects. Forests, 5(9), 2050–2083.
Soviguidi, D. R. J., Pan, R., Liu, Y., Rao, L., Zhang, W., & Yang, X. (2022). Chlorogenic acid metabolism: The evolution and roles in plant response to abiotic stress. Phyton, 91(2), 239–255.
Stevenson, P. C., & Veitch, N. C. (1996). Isoflavenes from the roots of Cicer judaicum. Phytochemistry, 43(3), 695–700.
Upadhyay, R., & Rao, L. J. M. (2013). An Outlook on Chlorogenic Acids - Occurrence, Chemistry, Technology, and Biological Activities. Critical Reviews in Food Science and Nutrition, 53(9), 968–984.
Wulff, S., Lindelöw, Å., Lundin, L., Hansson, P., Axelsson, A.-L., Barklund, P., Wijk, S., & Ståhl, G. (2012). Adapting forest health assessments to changing perspectives on threats – a case example from Sweden. Environmental Monitoring Assessments, 184, 2453–2464.
Zambino, P. J. (2010). Biology and pathology of Ribes and their implications for management of white pine blister rust. Forest Pathology, 40, 264–291.
Ziller, W. G. (1974). The tree rusts of western Canada. Canadian Forest Service Publications, 1329, 1–272.
Acknowledgements
We thank Timo Mikkonen for helping in the laboratory and greenhouse work, and Hannele Härkman and Anu Myllymäki for carrying out the mass spectrometry. The study was financed by the Finnish Academy of Science (grant no. 332811).
Funding
Open access funding provided by Natural Resources Institute Finland (LUKE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors bear all the ethical responsibilities of this manuscript. They declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest and that it does not include any animal and/or human trials.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piispanen, J., Bergmann, U., Karhu, J. et al. Variation of compounds in leaves of susceptible and resistant alternate hosts of Cronartium pini and C. ribicola. Eur J Plant Pathol 165, 677–692 (2023). https://doi.org/10.1007/s10658-022-02636-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02636-1