Abstract
Early blight, caused by Alternaria solani, is a common potato disease worldwide. Reduced field efficacy of the fungicide boscalid against this disease has been reported in several countries. Boscalid resistance has been mostly studied with in-vitro and/or greenhouse experiments. Field studies validating this phenomenon are largely missing. Here, for the first time in Scandinavia, we validated boscalid resistance in a Swedish population of A. solani both in the field and in the laboratory. Field trials between 2014 and 2017 in Nymö showed significant efficacy reduction by year. The target regions of the A. solani genes encoding the succinate dehydrogenase subunits (Sdh) B, C and D of samples collected from Nymö, and additional fields in south-eastern and central Sweden, were analysed for substitutions associated with loss of boscalid sensitivity. In 2014, the SdhC-H134R mutation was found at several sites at a low frequency, while, in 2017, the majority of the samples had either the SdhB-H278Y or the SdhC-H134R substitution. No mutations were detected in the gene encoding the SdhD subunit. Spore germination tests showed a high sensitivity (EC50 < 1 μg mL−1) of isolates lacking the substitutions. This was supported by a significant decrease in their radial growth rate, from 0.1 to 10 μg mL−1 boscalid. However, the mutated isolates had EC50 > 100 μg mL−1 and their growth rates hardly decreased at concentrations above 1–10 μg mL−1. These results add to the current knowledge of fungicide resistance development in field and indicate that early blight management in southeast Sweden should no longer rely on boscalid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early blight, caused by the phytopathogenic fungus Alternaria solani (Ellis & G. Martin) Sorauer, is an important potato disease and its management requires frequent fungicide use worldwide including European countries such as Sweden. Effective use of fungicides is frequently impeded by development of mutations in the population of fungal phytopathogens. This may result in increased frequency and amount of fungicide applications causing an inappropriate burden on farmers as well as adverse effects on ecosystem and human health. Reduced efficacy of boscalid against A. solani has been previously reported based on in-vitro and greenhouse experiments (Bauske et al., 2018b; Gudmestad et al., 2013). Nevertheless, data confirming these experiments in the field are largely lacking. Metz et al. (2019) studied boscalid efficacy against A. solani for the first time at the field level in 2016 and 2017 in Freising, Germany. However, their field trials were based on artificially inoculated A. solani isolates and field analyses of boscalid resistance based on natural populations of A. solani isolates carrying mutations that render them insensitive to boscalid, have, so far, not been reported.
During the last decade, early blight has become an increasing burden for farmers in the south-eastern part of Sweden, especially in starch potato crops. The disease occurs in all parts of Sweden where potato is grown but is much less of a problem in other regions of the country. The field incidence of early blight has been high in September most years, especially in south-eastern Sweden despite applications of succinate dehydrogenase inhibitor (SDHI) (boscalid) and Quinone outside Inhibitor (QoI) fungicides, such as azoxystrobin and pyraclostrobin (personal communication with growers and extension services). Boscalid resistance has not been previously shown in Scandinavia and the fungicide was, until recently, commonly used by farmers in Sweden.
The high incidence of early blight at the end of the season may be a consequence of a decline of the protective effect of the treatments. The active ingredients of SDHI fungicides suppress the process of cellular respiration in mitochondrial complex II, where SDHIs are able to bind to subunits SdhB, SdhC and SdhD. Five point-mutations on three Sdh genes (SdhB, SdhC and SdhD) have been characterized in A. solani (Mallik et al., 2014). Two of these mutations, H278Y and H278R, are found in the SdhB gene that lead to an exchange from histidine to tyrosine and histidine to arginine, respectively, at codon 278. Another mutation, H134R, in the SdhC gene, leads to an exchange from histidine to arginine at codon 134. The other two mutations, H133R and D123E, are in the SdhD gene and lead to an exchange from histidine to arginine at codon 133 and aspartic acid to glutamic acid at codon 123, respectively. Mallik et al. (2014) classified SdhB, C, and D mutated A. solani isolates into four boscalid resistance groups according to a varying range of 50% effective concentration (EC50) values: sensitive (EC50 ≤ 5 µg mL–1), moderately resistant (EC50 = 5.1 to 20 µg mL–1), highly resistant (EC50 = 20.1 to 100 µg mL–1), and very highly resistant (EC50 > 100 µg mL–1) phenotypes.
Resistance of A. solani to boscalid was first detected in 2009 and 2010 by Wharton et al. (2012) from Idaho, United States. All five mutations have been reported in the United States (Bauske et al., 2018a; Mallik et al., 2014; Miles et al., 2014). In Europe similar observations were also made recently. Landschoot et al. (2017) found the amino acid substitutions H278R and H278Y in subunit B, and H134R in subunit C, in Belgian A. solani populations. In German populations of the fungus, Metz et al. (2019) detected the H278Y and H278R in subunit B, H134R and H134Q in subunit C, and D123E in subunit D, all associated with reduced sensitivity to boscalid. A previous survey in Sweden revealed that the main cytochrome b genotype in A. solani was GII F129L, which is associated with the loss of sensitivity towards QoI fungicides (Edin et al., 2019). This showed that a rapid change in the Swedish A. solani populations occurred between 2011 and 2013, after which most of the isolates possessed the F129L substitution. In the current study we also examine the cytochrome b genotype to see if this mutation is still prevalent.
This study aims to: 1. investigate the inter-annual changes in the field efficacy of a fungicide strategy mainly based on boscalid against a naturally occurring population of A. solani, 2. identify and investigate the abundance of any amino acid substitution in the genes encoding the subunits SdhB, SdhC and SdhD in A. solani field populations that may affect the efficacy of boscalid as well as identify the cytochrome b genotype, and 3. assess the changes in sensitivity to boscalid induced by each mutation based on a set of spore germination and mycelial growth tests.
Materials and methods
Field trials
Field trials for testing the efficacy of fungicide treatments against early blight were performed every year between 2014 and 2017. The trials were carried out by the Swedish Rural Economy and Agricultural Societies at a farm in Nymö, about 15 km east of Kristianstad, in the county of Skåne Sweden (56.0 ºN, 14.3°E). Each year, the trials were carried out at the same farm, but in different, neighbouring fields, according to a standard crop rotation scheme. The fields were fertilized with nitrogen, phosphorous and potassium according to common practice for potato: 200, 80 and 215 kg ha−1 for N, P and K, respectively. Each trial had a randomized block design with four blocks and each plot had five 10 m long rows. Each year natural infection of A. solani occurred and thus inoculation with the fungus was not needed. The three middle rows were used for disease scoring. The field was treated with fungicides against late blight starting at treatment application 1 (T1 = treatment time point one, around June 20) and then followed weekly late blight treatments until T12 or T13 using Revus (Syngenta, a.i. mandipropamid 250 g L−1; dose rate 0.6 L ha−1) and Ranman Top (ISK Biosciences, a.i. cyazofamid 160 g L−1; dose rate 0.5 L ha−1) alternated with no or minor effect on early blight. Early blight treatments were applied as follows: Revus Top (Syngenta, a.i. difenoconazole 250 g L−1 against early blight and a.i. mandipropamid 250 g L−1 against late blight; dose rate 0.6 L ha−1) at T1 and T2, Signum (BASF, a.i. boscalid 267 g kg−1 and pyraclostrobin 67 g kg−1; dose rate 0.25 kg ha−1) at T3, T5, T7 and T9. In 2014 a treatment with only Signum at T3, T5, T7 and T9 was also included. Since we received reports about severe early blight infections in some farmers fields, despite repeated applications of Signum (a.i. boscalid), we included another fungicide regime 2016 and 2017 where Revus Top (a.i. difenoconazole) was applied later in the season: Revus Top (T4, T8, T12) alternated with Signum (T6, T10). These treatment strategies were compared with a control treatment where only late blight fungicides, as mentioned above, were applied. In 2014 two starch cultivars, Kuras and Kardal, were used whereas two ware potato cultivars, Bintje and Folva were grown in 2015. In 2016, starch potato cultivars Kuras and Stayer were grown and in 2017 only Kuras were used. The field trials were irrigated when needed. Weather data, daily temperature and precipitation, were obtained from a nearby weather station (Hönedal weather station: 56.1°N; 14.3°E) for each growing season (June–September) to investigate a possible link between weather factors and differences observed for the field trials in different years.
Disease scoring
The field trials were inspected weekly or biweekly from the beginning of June until mid-September and the development of early blight was visually assessed. Early blight disease severity was assessed by percent of infection, i.e. percent of remaining green leaf area that had necrotic early blight spots according to the EPPO scale (OEPP/EPPO 2004) as described by Odilbekov et al. (2019). No late blight occurred in the field trials. The relative area under the disease progress curve (rAUDPC) was calculated (Odilbekov et al., 2020) as a measurement of early blight disease severity over the season. The efficacy of the fungicide treatment was calculated as:
Sampling and isolation
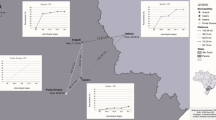
Potato leaflets with lesions resembling early blight were collected in the control treatment plots at the field trial at Nymö in 2014 at the end of August for identification of A. solani. Additional samplings of leaflets with lesions resembling early blight were made in four commercial starch potato fields at the end of August every year between 2014 and 2016. The leaflets were collected from an area of 20 m × 20 m in each potato field and one lesion each from eight leaflets was used for further analyses. The fields were randomly chosen within the starch potato growing area of south-eastern Sweden. Three of these fields were located near the city of Kristianstad (Kristianstad C-D and the field trial site in Nymö), one field south of Kalmar in the county of Småland (Kalmar B), 150 km northeast of Kristianstad, and one field on the island Öland (Öland A), 20 km east of the location south of Kalmar (Fig. 1). At the field trial site in Nymö, samplings were carried out on three occasions during 2017 to test for changes in the population during the growing season. Samples were taken in the beginning and end of August and in mid-September 2017 but the samples in August were pooled in the final analysis. In total, single spore isolates from 99 samples from that field in 2017 were analysed.
Map of Sweden representing the counties of collection and locations of the five farms where leaf collections were made in starch potato every year between 2014 and 2016. The field trials were located at the farm Nymö, where leaf collections for isolation of Alternaria solani occurred in 2011, 2014 and 2017. Additional samplings were performed in two ware potato fields in central Sweden (light blue dots)
To make a small analysis of the situation in other parts of Sweden, a few additional fields were sampled in 2016, including two ware potato fields in central Sweden (Västergötland and Östergötland) (Fig. 1) and two other starch potato fields south of Kristianstad (not shown in Fig. 1). All sampled fields during 2014–2016, except the one in Västergötland, had been treated with the fungicide Signum (a.i. boscalid and pyraclostrobin) during the season. Two samples, which did not contain mutations in the Sdh or cytochrome b genes, collected in 2011 from location Kristianstad C and one sampled collected at Nymö 2011 were used as references for the in vitro tests. The preparation of the leaflet samples was performed according to Edin et al. (2019) and the isolation according to Odilbekov et al. (2016).
Identification of SDHI and cytochrome b mutations
The gene sequences of the target regions Sdh subunits B, C, and D of the collected samples of A. solani were examined for potential substitutions. The samples collected during 2011, 2014 and 2015 were analysed for the target region in SdhC. The target regions in subunits SdhB, C and D were analysed in the samples collected in 2016 and 2017 by sequencing the products of the primers SdhB-F, SdhB-R, SdhC-F1, SdhC-R2, SdhD-F1 and SdhD-R2 (Mallik et al., 2014). Isolates without mutations in these regions and isolates mutated at the Sdh subunits B, C and D originating from Belgium, was used as references for the molecular analyses and sequencing. The PCR conditions and other methodological information were according to the protocols previously described by Edin et al. (2019). The sequences were compared to the FASTA sequences available at NCBI: KC517311.1, KC517312.1 and KC517310.1 for SdhB, KC517314 for C-H134R and KC517316.1 for SdhD. Isolates from the field trial at Nymö 2017 were also analysed for substitutions in SdhB by pyrosequencing at Bayer Crop Science, Monheim Germany.
The multiplex method for identification of the substitutions published by Mallik et al. (2014) was tested, but there were so many false positives when analysing SdhC we did not proceed with this method. New reverse primers without miss-matches did not improve the outcome of the analyses either, so we proceeded with separately sequencing individual target sites of the subunits.
All samples were analysed to assess the cytochrome b genotype (GI or GII) as well as for the F129L substitution according to Edin et al. (2019).
Assessment of A. solani isolates sensitivity to boscalid based on spore germination test
To specify the changes in sensitivity to boscalid induced by each mutation, a total of 16 isolates from the field trial of 2017 or collected in 2011 at Nymö were examined (Table 1). We selected both wildtype isolates and isolates with mutations for the experiment; five of the isolates showed no mutations at SDH (including the three isolates collected in 2011), five had the SdhC-H134R substitution and six had the SdhB-278Y substitution (Table 1). The wild-type isolates and the isolates with ID 151- 253 had not been treated with an SDHI fungicide prior to sampling. Due to the low solubility of pure boscalid in water in higher concentrations, the commercial product Cantus (BASF SE, a.i. 500 g boscalid kg−1) was used to prepare fungicide amended media according to Metz et al. (2019). To obtain a spore suspension of each A. solani isolate, pure cultures were grown on 20% strength potato dextrose agar medium (PDA) supplemented with 12 g L−1 Bacto Agar and incubated at 25 ºC for 7 days in darkness. Then, to increase sporulation, the cultures were incubated 7 days under UV-c light (model OSRAM HNS15G13 with dominant wavelength 254 nm) for 5–6 h per day. 1 mL of sterile, distilled water was added to each A. solani culture and conidia were dislodged using a sterile L-shape cell spreader. The final concentration of the conidial suspension was adjusted to 2 × 10 4 conidia mL−1 using a haemocytometer. Then, 50 μL of the conidial suspension of each isolate was spread across plates containing 1.5% bacto agar amended with 0, 0.01, 0.1, 1, 10, 20 and 100 µg mL−1 boscalid and salicylhydroxamic acid (SHAM) at 100 μg mL−1 as previously described by Gudmestad et al. (2013) but the concentration 20 µg mL−1 was added for increased accuracy of the in vitro-test. The plates were placed in Panasonic versatile environmental test chamber (model MLR-352H-PE) equipped with Panasonic FL40SS ENW/37 lights. The temperature of the incubator was set at 28 °C and three of the lights were on for 5 h. Then, germination of 100 conidia was scored using a binocular microscope. A conidium was considered germinated if the germ tube was at least half the length of the conidium (Metz et al., 2019). Fungicide sensitivity was determined based on EC50 value (effective concentration at which spore germination was inhibited by 50% relative to the untreated control). There were two replicate plates per isolate and concentration and the experiment was repeated once for each isolate and concentration.
Assessment of A. solani sensitivity to boscalid based on mycelial growth test
To assess the effect of boscalid on growth rate of the 16 isolates of A. solani, a 7 mm diameter agar disk from the margin of an actively growing culture of each isolate was placed at the middle of a series of petri dishes (9 cm diameter) containing 20% PDA amended with the same boscalid concentrations as in the spore germination test (above). The plates were incubated at 22 °C in darkness for 12 days. Two colony diameters were measured in perpendicular directions 3, 4, 5, 6, 7, 10, 11 and 12 days after incubation. The diameter of the mycelial plug was subtracted from the colony diameter before calculating the mean daily radial growth rate (mm day−1). There were three replicate plates per isolate and concentration. This test was performed three times in experiments 1, 2, and 3. Experiment 1 included all the 16 isolates while experiments 2 and 3 were based on a subset of isolates (Table 1). All isolates were tested at least twice.
Statistical analyses
Fischer´s exact test was used to test for changes in the pathogen populations of each field between years using data from all five fields sampled 2014–2016 using JMP 15.2 (SAS Institute, Cary, USA). Comparisons of mean values in radial growth rate tests were conducted using Tukey’s test (p-value < 0.05) for each concentration. This statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, USA).
Results
Field trials
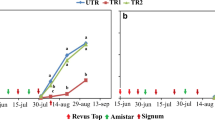
Early blight, caused by A. solani, occurred naturally every year in the field trials. The presence of A. solani in lesions resembling characteristic early blight lesions was confirmed each year after isolation and microscopy. In 2014, the infection started relatively early. The first characteristic symptoms of early blight were found around 10th July and on August 1st the infection had reached 5% in the untreated control plots. In 2015, the first symptoms were detected later, i.e., at the beginning of August, and the infection reached 5% in untreated control plots around August 20th in cv. Bintje and August 25th in cv. Folva. Similarly, in 2016 and 2017 the infection was first observed in the beginning of August and reached 5% infection in untreated control plots on August 20th in 2016 for both cvs. Kuras and Stayer and on August 25th in 2017 for cv. Kuras (Fig. 2).
Disease progress curves from the field trials 2014–2017 in untreated plots. Means of 4 replicated plots. The coefficients of variation for rAUDPC were in 2014: 5% and 11% for Kardal and Kuras, repectively; in 2015: 30% and 3% for Bintje and Folva, respectively; in 2016: 12% and 19% for Kuras and Stayer, respectively; in 2017: 7% for Kuras
We used rAUDPC as a measure of the severity of the early blight disease over the season and thus calculated the efficacy of the fungicide treatment for the whole season. Our results show that in 2014 the efficacy was around 70–75% and in 2015 above 80% in one of the cultivars but after 2015, there was a clear drop in the efficacy down to 30–40% (Fig. 3). There was a significant negative correlation between year and efficacy according to Pearson correlation coefficient (R = 0.84, p-value < 0.02). In 2014 the efficacy of using only Signum without the two early applications of Revus Top was almost the same as for the treatment including the Revus Top applications. There was no significant difference in rAUDPC between those treatments. In 2016 and 2017 a fungicide strategy based on Revus Top applied three times with one month interval alternated with Signum resulted in much better efficacy than the strategy with early applications of Revus Top followed by four times Signum (Fig. 3). The onset of infection and the disease severity varied between years. To exclude that the observed drop in efficacy was due to differences in disease pressure over the years or was related to the cultivar that was grown, we plotted the efficacy against disease pressure (rAUDPC for untreated control) for all the seven trials (Fig. 4). However, we did not find any relationship between disease pressure and efficacy or cultivar used.
Decrease in field efficacy of a fungicide strategy in starch potato on an experiment farm outside Kristianstad, Sweden, between 2014 and 2017. Every year a field trial was carried out at the same farm but on different fields. The field was treated with late blight fungicides starting at T1 (around June 20) and then followed weekly late blight treatments until T12. Revus and Ranman Top was alternated with no or minor effect on early blight. Early blight treatments were applied as follows: Revus Top (a.i. difenoconazole) at T1 and T2, Signum (a.i. boscalid and pyraclostrobin) at T3, T5, T7 and T9. Data is shown for each of the cultivars used: Kuras and Kardal, 2014; Bintje and Folva, 2015; Kuras and Stayer, 2016 and Kuras, 2017. In 2014 we also had an early blight treatment with only Signum at T3, T5, T7 and T9 (cultivar Kuras, open squares). In 2016 and 2017 there was also a treatment with Revus Top at T4, T8 and T12 alternated with Signum at T6 and T10 included (cultivar Kuras, open triangles). RT: Revus Top, S: Signum
Field efficacy of early blight fungicides in relation to disease pressure different years (expressed as infection rate in untreated control). Data from the same trials as in Fig. 3
The weather conditions varied between the years regarding average temperature and precipitation (Fig. 5a-d). The temperature was similar throughout the season of 2016 and 2017, while the temperature was higher in July than in August 2014 and vice versa in 2015. The accumulated precipitation during the period from 1 June to 30 September was 321 mm in 2014, 179 mm in 2015, 219 in 2016 and 259 mm in 2017.
Identification of substitutions in Sdh subunits B, C and D from the field trial site 2014 and 2017
The sequencing results from the field trial site at Nymö in 2014 revealed that all samples were wildtype at SdhC (Fig. 6). On the contrary, 90 out of a total of 99 samples from the field trial in 2017 had either the SdhB-H278Y (25 samples) or SdhC-H134R (65 samples) substitution (Table 2). The proportion of samples possessing the SdhB-H278Y substitution increased from August to September, while the proportion of wildtype samples and samples possessing the SdhC-H134R substitution decreased. All samples were wildtype in SdhD.
Identification of substitutions in Sdh subunits B, C and D in samples collected 2014–2016
In contrast to the result from the field trial site Nymö in 2014, the amino acid substitution C-H134R in SdhC was observed in the other four fields analysed in 2014, although at a relative low frequency (Fig. 6). The following two years, the SdhC-H134R substitution was found in all fields and in most of the samples from four of the fields both years, including Nymö as outlined above. Eight samples were used at each location but sometimes the sequencing failed, and therefore a lower number of analysed samples are presented in Fig. 6. The shift from dominance of wildtype samples in the populations to dominance of samples possessing the SdhC-H134R substitution occurred between 2014 and 2015 for all locations (Fischer´s exact test, p-value < 0.05 at each field) except Öland A, where no shift occurred. The four samples collected in 2016 from the two additional fields in Skåne that were successfully sequenced also had the SdhC-H134R substitution. Furthermore, one of the two samples successfully sequenced from the field in the county of Östergötland in central Sweden possessed the SdhC-H134R substitution, while the two samples from the other field in central Sweden (county of Västergötland) did not show any mutations in these genes (data not shown).
Mutations in the target regions of SdhB and SdhD were not present in any of the samples collected in 2016.
Identification of the cytochrome b genotype among the samples collected in 2014–2016
Approximately 90% of the population of A. solani (N = 99) in the fields sampled in 2014–2016 also possessed the amino acid substitution GII F129L (data not shown). The four samples collected from central Sweden in 2016 were not mutated in the cytochrome b gene.
Assessment of A. solani isolates sensitivity to boscalid based on spore germination tests
In assessing boscalid sensitivity based on EC50 value, all wildtype isolates were highly sensitive to boscalid with an EC50 value lower than 1 μg mL−1. In contrast, all mutated isolates had very low sensitivity to boscalid with EC50 values higher than 100 μg mL−1 (Table 3).
Assessment of A. solani sensitivity to boscalid based on mycelial growth test
The differences in growth rate between the wildtype and mutated isolates were small and not statistically significant up to the concentration 0.1 μg mL−1 (Fig. 7, Exp. 1,). At 1 μg mL−1, the growth rate of the wildtype isolates was only statistically different (p-value < 0.05) for one of the mutated isolates (SdhB-H278Y), which generally had the highest growth rate for all concentrations except 0. While the growth rates of the mutated isolates only slightly decreased with further increase of boscalid concentration, a considerable reduction in growth rate for the wildtype isolates not displaying mutations appeared between 0.1 and 10 μg mL−1 boscalid. Statistically significant (p-value < 0.05) differences in growth rate between wildtype isolates and isolates harbouring either SdhC-H134R or SdhB-H278Y were observed in the range of 10–100 μg mL−1 boscalid (Fig. 7). The difference in growth rate between isolates with the SdhC-H134R mutation and isolates with the SdhB-H278Y mutation was not significant at any of the concentrations.
Average radial growth rate of different groups of isolates of Alternaria solani in relation to the boscalid concentration in three separate experiments (Exp. 1, 2, and 3). Different letters (a or b) on the plot for the experiment 1 show statistically significant differences between isolate groups according to Tukey's test (p-value < 0.05) for each concentration. Vertical bars in the experiment 1 show standard deviation. The error bars and significance letters are not depicted for experiments 2 and 3 because the number of isolates per group were low (see in Table 1). WT: wildtype (isolates without mutations), H134R: isolates with C-H134R substitution, H278Y: isolates with B-H278Ysubstitution
The results of the repeated experiments (2 and 3) were similar to experiment 1 (Fig. 7).
Discussion
Since the registration of boscalid in 2003 in the United States, the substance has been frequently used against different diseases in a wide variety of crops (Reilly et al., 2012), including early blight of potato. The fungus A. solani is the main pathogen causing potato early blight and is widespread in potato fields (Metz et al., 2019). Due to its high genetic variability, polycyclic nature, reproductive capacity-producing high amounts of secondary inoculum, and given that multiple fungicide applications are required to effectively control the disease, the fungus represents a high-risk pathogen in terms of resistance development (Leiminger et al., 2014; Rosenzweig et al., 2008). The present study addresses boscalid resistance in A. solani populations in potato fields by analysing samples from several sites in central and south-eastern Sweden during 2014–2017. The infection pressure varied from year to year for the growing season (June–September). Higher precipitation during July in 2014 together with somewhat higher temperatures could explain the earlier and more severe early blight infections of that year. The lowest infection pressure was observed for 2015, the year that had the lowest precipitation (almost half the value for 2014). Moreover, the more or less similar weather conditions of 2016 and 2017 coincided with almost similar infection pressures. We report here, a major drop in field efficacy of a fungicide strategy, mainly based on boscalid, against potato early blight (caused by A. solani in Sweden) from 2014 to 2017. This coincides with an increased frequency of substitutions in the A. solani Sdh subunits B and C. Isolates harbouring either of these substitutions showed much lower sensitivity to boscalid in laboratory experiments. Frequent application of fungicides often leads to fungicide resistance (Jørgensen et al., 2017). Most of the evidence for fungicide resistance is based on observed loss of sensitivity in laboratory and greenhouse experiments. Our study adds to the few previous studies demonstrating reduced sensitivity from field experiments (e.g., Campbell et al., 2020; Metz et al., 2019). Therefore, we need to apply strategies to prevent fungicide resistance by implementation of integrated pest management (IPM), such as crop rotation and weed management to reduce the amount of inoculum in the field and the strong directional selection on the pathogen (Karlsson Green et al., 2020) and by maintaining good nutritional and water status to improve crop resilience.
We investigated the frequency of amino acid substitutions leading to reduced sensitivity to boscalid in naturally occurring populations of A. solani in Sweden. Already in 2014 samples with the substitution SdhC-H134R were found at low frequency in several fields. The following year, samples with SdhC-H134R dominated in four out of five investigated fields indicating a quick shift in the pathogen population. Such a shift in the population of mutated isolates of A. solani based on inter-annual study of boscalid resistance had not been reported before in the literature. In addition, the substitution SdhB-H278Y was found at quite high frequency at the field trial site sampled in 2017. However, we did not find any isolate with both mutations. One shortcoming was that the analyses for the SdhB target region often failed among the isolates, hence the pyrosequencing of the isolates from the field trial in 2017. Other substitutions associated with reduced sensitivity to boscalid reported in A. solani populations from Germany and Belgium, such as SdhC-H134Q, SdhB-H278R and SdhD-D123E (Metz et al., 2019), Landschoot et al., 2017), were not observed in our study.
The substitution SdhB-H278Y was first found in 2017. However, there was a tendency that the frequency of isolates with this substitution increased in the field within the season (Table 2). The general pattern also indicates that a shift in the population can occur very fast probably due to the very strong selection pressure when the fungicide is applied, and the mutation already exists in the population. This pattern coincides with the report of general genetic changes in the A. solani pathogen population within one growing season as described by (Odilbekov et al., 2019).
The main part of the population of A. solani in south-eastern Sweden also possess the amino acid substitution F129L in genotype II of cytochrome b (Edin et al., 2019). This mutation is associated with reduced sensitivity to QoI fungicides, such as azoxystrobin and pyraclostrobin. Edin et al. (2019) reported that the Swedish A. solani populations were dominated by the F129L mutation already in 2013, which means that the practical control of early blight became restricted. The use of strobilurins as single active ingredients is very limited in southern Sweden nowadays. The interpretation is that the population has acquired multiple resistance mutations towards fungicides since almost all samples possessing either SdhB-H278Y or SdhC-H134R also possessed the F127L substitution in cytochrome b. The situation with double substitutions corresponds to the situation in Belgium (Landschoot et al., 2017), Germany (Metz et al., 2019; Nottensteiner et al., 2019) and USA (Gudmestad et al., 2013), where all samples had substitutions in both the Sdh target region and cytochrome b.
The fungicide we used in the field efficacy trials was Signum (BASF), which has both boscalid and pyraclostrobin as active ingredients. The A. solani populations in 2014 and onwards were dominated by the F129L mutation. So, we conclude that the heavy drop in field efficacy of Signum from 2014 to 2017 was due to the observed increase in substitutions associated with reduced sensitivity to boscalid. The observed good efficacy of Signum in 2014 could not be explained by the early Revus Top treatments. Furthermore, the observation that applying Revus Top (difenoconazole) later in the season in 2016 and 2017 resulted in much better efficacy confirms the drop in efficacy of boscalid between 2015 and 2016.
The substitution SdhC-H134R was present in four of the five analysed fields in south-eastern Sweden already in 2014. The only exception was the field trial at Nymö, as mentioned above. It is noticeable that the distance between the fields in Skåne is about 20 km as well as between the fields of Kalmar B and Öland A, whereas the distance between regions of Skåne and Kalmar/Öland is approximately 150 km. Likewise, the distance to the SdhC-H134R substitution found in Östergötland was > 250 km to both Kalmar and Kristianstad. The long distance between fields may indicate that the substitutions causing resistance against fungicides based on SDHI may have occurred independently in the three regions, since that pattern was also observed for QoI resistance emergence in European populations of the fungus Zymoseptoria tritici (Torriani et al., 2009). The genetic structure in the population of A. solani in the region of Skåne and Kalmar/Öland was diverse both within and between regions in the collections of 2011 (Odilbekov et al., 2016). The finding of the isolate possessing SdhC-H134R + wildtype cytochrome b in Östergötland is worth following up with further monitoring and genetic analysis of the population in all potato producing areas of Sweden in order to disentangle the origin of the substitutions and the dispersal pattern of the A. solani populations.
All isolates of A. solani in this study without mutations in the Sdh target region were highly sensitive to boscalid with an EC50 value lower than 1 μg mL1, including isolates collected both before and after the substitution was first detected in Sweden. On the other hand, all isolates with mutations had very low sensitivity to boscalid indicated by EC50 values higher than 100 μg mL−1. This is generally in agreement with Metz et al., (2019), who showed that among the German isolates of A. solani, all wildtype isolates were sensitive to boscalid (EC50 value less than 5 μg mL−1). Also, the majority of the German isolates possessing the SdhC-H134R or SdhB-H278Y substitutions were classified as highly resistant to boscalid (EC50 values higher than 20 μg mL−1).
Although boscalid mainly inhibits spore germination, it is also effective against other stages of fungal development, such as mycelial growth (Stammler et al., 2008). For example, Myresiotis et al. (2008) showed that the effective concentrations of boscalid to reduce spore germination and mycelial growth of Botrytis cinerea by 50% were 2.14 and 2.09 μg mL−1, respectively. Therefore, in our study sensitivity to boscalid based on mycelial growth rate was studied as well. While isolates harbouring either of the substitutions continued to grow at high boscalid concentrations, there was a drastic decrease in growth rate of wildtype isolates between 0.1 and 10 μg boscalid mL1. Thus, as expected, wildtype isolates were much more sensitive to boscalid as compared to mutated isolates.
It seemed that the differences in sensitivity to boscalid between wildtype isolates and isolates with a substitution at the Sdh target region appeared larger when analysed using a low concentration for the spore germination test (Table 3) compared to the growth rate test on agar media (Fig. 7). A significant (p-value < 0.05) difference in growth rate between wildtype isolates and isolates harbouring SdhB-H278Y and SdhC-H134R started from the concentration 1 and 10 μg boscalid mL−1, respectively. In the spore germination test, the significant difference in EC50 value between wildtype and mutated isolates started at concentrations lower than 1 μg mL−1. This might be related to the duration the fungus was in contact with boscalid: for the growth rate test, the isolates were on the surface of the media for 12 days but for the EC50 test, we studied spore germination five hours after spreading the spores on media containing boscalid. After this time, some spores had started germinating but we did not consider them as germinated spores since the germination tube was too short.
In conclusion, the results from this study suggest that early blight caused by A. solani is difficult to control with fungicides based on QoIs or boscalid. The field efficacy of the SDHIs is also an important aspect for practical farming and the use of boscalid has declined in Sweden after 2017. Recently, a new fungicide (Propulse, Bayer Crop Science) with another SHDI fungicide (fluopyram) as active ingredient, besides the triazole prothiokonazol, was registered for potato in Sweden. This product still has a good effect. However, there is an apparent risk of development of resistance to these fungicides also. Likewise, we need to continue the monitoring of the A. solani populations in terms of fungicide sensitivity against all registered modes of action, as well as genetic studies, to maintain a resilient potato cultivation.
Data availability
Not applicable.
Code availability
Not applicable.
References
Bauske, M. J., Mallik, I., Yellareddygari, S., & Gudmestad, N. C. (2018a). Spatial and temporal distribution of mutations conferring QoI and SDHI resistance in Alternariasolani across the United States. Plant Disease, 102(2), 349–358. https://doi.org/10.1094/PDIS-06-17-0852-RE
Bauske, M. J., Yellareddygari, S., & Gudmestad, N. C. (2018b). Potential impact of fluopyram on the frequency of the D123E mutation in Alternariasolani. Plant Disease, 102(3), 656–665. https://doi.org/10.1094/PDIS-06-17-0853-RE
Campbell, S. E., Brannen, P. M., Scherm, H., Eason, N., & MacAllister, C. (2020). Efficacy of fungicide treatments for Plasmoparaviticola control and occurrence of strobilurin field resistance in vineyards in Georgia, USA. Crop Protection, 139, 105371. https://doi.org/10.1016/j.cropro.2020.105371
Edin, E., Liljeroth, E., & Andersson, B. (2019). Long term field sampling in Sweden reveals a shift in occurrence of cytochrome b genotype and amino acid substitution F129L in Alternariasolani, together with a high incidence of the G143A substitution in Alternariaalternata. European Journal of Plant Pathology, 155(2), 627–641. https://doi.org/10.1007/s10658-019-01798-9
Gudmestad, N. C., Arabiat, S., Miller, J. S., & Pasche, J. S. (2013). Prevalence and impact of SDHI fungicide resistance in Alternariasolani. Plant Disease, 97(7), 952–960. https://doi.org/10.1094/PDIS-12-12-1176-RE
Jørgensen, L. N., van den Bosch, F., Oliver, R. P., Heick, T. M., & Paveley, N. D. (2017). Targeting fungicide inputs according to need. Annual Review of Phytopathology, 55(1), 181–203. https://doi.org/10.1146/annurev-phyto-080516-035357
Karlsson Green, K., Stenberg, J. A., & Lankinen, Å. (2020). Making sense of integrated pest management (IPM) in the light of evolution. Evolutionary Applications, 13(8), 1791–1805. https://doi.org/10.1111/eva.13067
Landschoot, S., Carrette, J., Vandecasteele, M., De Baets, B., Höfte, M., Audenaert, K., et al. (2017). Boscalid-resistance in Alternariaalternata and Alternariasolani populations: An emerging problem in Europe. Crop Protection, 92, 49–59. https://doi.org/10.1016/j.cropro.2016.10.011
Leiminger, J., Adolf, B., & Hausladen, H. (2014). Occurrence of the F129L mutation in Alternariasolani populations in Germany in response to QoI application, and its effect on sensitivity. Plant Pathology, 63, 640–650. https://doi.org/10.1111/ppa.12120
Mallik, I., Arabiat, S., Pasche, J. S., Bolton, M. D., Patel, J. S., & Gudmestad, N. C. (2014). Molecular characterization and detection of mutations associated with resistance to succinate dehydrogenase-inhibiting fungicides in Alternariasolani. Phytopathology, 104(1), 40–49. https://doi.org/10.1094/PHYTO-02-13-0041-R
Metz, N., Adolf, B., Chaluppa, N., Hückelhoven, R., & Hausladen, H. (2019). Occurrence of Sdh mutations in german Alternariasolani isolates and potential impact on boscalid sensitivity in vitro, in the greenhouse, and in the field. Plant Disease, 103(12), 3065–3071. https://doi.org/10.1094/PDIS-03-19-0617-RE
Miles, T. D., Miles, L. A., Fairchild, K. L., & Wharton, P. S. (2014). Screening and characterization of resistance to succinate dehydrogenase inhibitors in Alternariasolani. Plant Pathology, 63(1), 155–164. https://doi.org/10.1111/ppa.12077
Myresiotis, C., Bardas, G., & Karaoglanidis, G. (2008). Baseline sensitivity of Botrytis cinerea to pyraclostrobin and boscalid and control of anilinopyrimidine-and benzimidazole-resistant strains by these fungicides. Plant Disease, 92(10), 1427–1431. https://doi.org/10.1094/PDIS-92-10-1427
Nottensteiner, M., Absmeier, C., & Zellner, M. (2019). QoI fungicide resistance mutations in Alternariasolani and Alternariaalternata are fully established in potato growing areas in Bavaria and dual resistance against SDHI fungicides is upcoming. Gesunde Pflanzen, 71(3), 155–164. https://doi.org/10.1007/s10343-019-00475-5
Odilbekov, F., Edin, E., Garkava-Gustavsson, L., Hovmalm, H. P., & Liljeroth, E. (2016). Genetic diversity and occurrence of the F129L substitutions among isolates of Alternariasolani in south-eastern Sweden. Hereditas, 153(1), 1–10. https://doi.org/10.1186/s41065-016-0014-0
Odilbekov, F., Edin, E., Mostafanezhad, H., Coolman, H., Grenville-Briggs, L. J., & Liljeroth, E. (2019). Within-season changes in Alternariasolani populations in potato in response to fungicide application strategies. European Journal of Plant Pathology, 155(3), 953–965. https://doi.org/10.1007/s10658-019-01826-8
Odilbekov, F., Selga, C., Ortiz, R., Chawade, A., & Liljeroth, E. (2020). QTL mapping for resistance to early blight in a tetraploid potato population. Agronomy, 10(5), 728. https://doi.org/10.3390/agronomy10050728
OEPP/EPPO. (2004). EPPO standards. efficacy evaluation of plant protection products. In Fungicides & bactericides (vol. 2). European and Mediterranean Plant Protection Organization
Reilly, T. J., Smalling, K. L., Orlando, J. L., & Kuivila, K. M. (2012). Occurrence of boscalid and other selected fungicides in surface water and groundwater in three targeted use areas in the United States. Chemosphere, 89(3), 228–234. https://doi.org/10.1016/j.chemosphere.2012.04.023
Rosenzweig, N., Atallah, Z. K., Olaya, G., & Stevenson, W. R. (2008). Evaluation of QoI fungicide application strategies for managing fungicide resistance and potato early blight epidemics in Wisconsin. Plant Disease, 92(4), 561–568. https://doi.org/10.1094/PDIS-92-4-0561
Stammler, G., Brix, H., Nave, B., Gold, R., Schoefl, U. (2008). Studies on the biological performance of boscalid and its mode of action. In Dehne, H.W., Deising, H.B., Gisi, U., Kuck, K. H., Russell, P.E., Lyr, H. (Eds.), Modern fungicides and antifungal compounds V (pp. 45–51). 5th International reinhardsbrunn symposium, Germany
Torriani, S. F., Brunner, P. C., McDonald, B. A., & Sierotzki, H. (2009). QoI resistance emerged independently at least 4 times in European populations of Mycosphaerellagraminicola. Pest Management Science, 65(2), 155–162. https://doi.org/10.1002/ps.1662
Wharton, P., Fairchild, K., Belcher, A., & Wood, E. (2012). First report of in-vitro boscalid-resistant isolates of Alternariasolani causing early blight of potato in Idaho. Plant Disease, 96(3), 454–454. https://doi.org/10.1094/PDIS-07-11-0544
Acknowledgements
The project was financed by The Swedish Farmers' Foundation for Agricultural Research, SLF (grant numbers H0842015 and H1342054) and Swedish Board of Agriculture (grant numbers Dnr. 4.1.18-11323/13 and Dnr.4.1.18-9959/16). LGB also acknowledges additional support from Formas (grant numbers 2015-00430 and 2019-00881). Mycelium and DNA from pure isolates collected in 2011, 2014 and 2017 used for the time series as well as sensitivity data from in vitro tests were kindly provided by Firuz Odilbekov and Hilde Coolman, SLU Alnarp. We thank Sofie Landshoot for supplying DNA from mutated reference isolates. We are very grateful to Jürgen Derpmann and Simone Leonard at Bayer AG, Division Crop Science, in Monheim, who performed the pyrosequencing for us. Likewise, we would like to thank Jan-Eric Englund, The Centre for statistics SLU Alnarp, for kind assistance with the statistical calculations.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. All forms of financial support are acknowledged in the acknowledgements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/competing interests
The authors declare that they have no conflicts of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mostafanezhad, H., Edin, E., Grenville-Briggs, L.J. et al. Rapid emergence of boscalid resistance in Swedish populations of Alternaria solani revealed by a combination of field and laboratory experiments. Eur J Plant Pathol 162, 289–303 (2022). https://doi.org/10.1007/s10658-021-02403-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02403-8