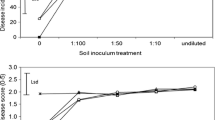
Abstract
Potato soft rot Pectobacteriaceae (SRP) cause large yield losses and are persistent in seed lots once established. In Norway, different Pectobacterium species are the predominant cause of soft rot and blackleg disease. This work aimed to evaluate the potential of real-time PCR for quantification of SRP in seed tubers, as well as investigating the status of potato seed health with respect to SRP in Norway. A total of 34 seed potato lots, including certified seeds, was grown and monitored over three consecutive years. All seed lots contained a quantifiable amount of SRP after enrichment, with very few subsamples being free of the pathogens. A high SRP prevalence based on a qPCR assay, as well as a high symptom incidence in certified seeds were observed, suggesting that current criteria for seed certification are insufficient to determine tuber health and predict field outcomes. Pectobacterium atrosepticum was the most abundant species in the examined seed lots and present in all lots. Consistently good performance of first generation seed lots with respect to blackleg and soft rot incidence, as well as low quantity of SRP in these seed lots demonstrated the importance of clean seed potatoes. Weather conditions during the growing season seemed to govern disease incidence and SRP prevalence more than seed grade. The impact of temperature, potato cultivar and Pectobacterium species on tuber soft rot development were further examined in tuber infection experiments, which showed that temperature was the most important factor in nearly all cultivars. Large-scale quantification of latent infection and predictive models that include contributing factors like weather, infecting bacterial species and cultivar are needed to reduce soft rot and blackleg.





Similar content being viewed by others
References
Boomsma, D., Velvis, H., Kristelijn, K., van Tent Becking, T., Kastelein, P., van der Zouwen, P. S., et al. (2013). Deltaplan Erwinia. Deel C-pootaardappelen. Eindrapport van het onderzoek 2009-2012. Nederlands Aardappel Organisatie (NAO), Productschap Akkerbouw (PA).
Burgess, P., Blakeman, J., & Perombelon, M. C. M. (1994). Contamination and subsequent multiplication of soft rot Erwinias on healthy potato leaves and debris after haulm destruction. Plant Pathology, 43(2), 286–299.
Burton, W. G., & Wigginton, M. J. (1970). The effect of a film of water upon the oxygen status of a potato tuber. Potato Research, 13(3), 180–186.
Chung, Y. S., Goeser, N. J., Cai, X., & Jansky, S. (2013). The effect of long term storage on bacterial soft rot resistance in potato. American Journal of Potato Research, 90(4), 351–356.
Czajkowski, R., de Boer, W. J., van Veen, J. A., & van der Wolf, J. M. (2010a). Downward vascular translocation of a green fluorescent protein-tagged strain of Dickeya sp. (biovar 3) from stem and leaf inoculation sites on potato. Phytopathology, 100(11), 1128–1137.
Czajkowski, R., de Boer, W. J., Velvis, H., & van der Wolf, J. M. (2010b). Systemic colonization of potato plants by a soilborne, green fluorescent protein-tagged strain of Dickeya sp. biovar 3. Phytopathology, 100(2), 134–142.
Czajkowski, R., Pérombelon, M. C. M., Jafra, S., Lojkowska, E., Potrykus, M., van der Wolf, J. M., et al. (2015). Detection, identification and differentiation of Pectobacterium and Dickeya species causing potato blackleg and tuber soft rot: A review. The Annals of Applied Biology, 166(1), 18–38.
Czajkowski, R., Perombelon, M. C. M., van Veen, J. A., & van der Wolf, J. M. (2011). Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathology, 60(6), 999–1013.
Dees, M. W., Lebecka, R., Perminow, J. I. S., Czajkowski, R., Grupa, A., Motyka, A., et al. (2017a). Characterization of Dickeya and Pectobacterium strains obtained from diseased potato plants in different climatic conditions of Norway and Poland. European Journal of Plant Pathology, 148(4), 839–851.
Dees, M. W., Lysøe, E., Rossmann, S., Perminow, J., & Brurberg, M. B. (2017b). Pectobacterium polaris sp. nov., isolated from potato (Solanum tuberosum). Int. J. Syst. Evol. Microbiol., 67(12), 5222–5229.
Degefu, Y., Potrykus, M., Golanowska, M., Virtanen, E., & Lojkowska, E. (2013). A new clade of Dickeya spp. plays a major role in potato blackleg outbreaks in North Finland. Ann. Appl. Biol., 162(2), 231–241.
du Raan, S., Coutinho, T. A., & van der Waals, J. E. (2016). Cardinal temperature differences, determined in vitro, between closely related species and subspecies of pectinolytic bacteria responsible for blackleg and soft rot on potatoes. European Journal of Plant Pathology, 144(2), 361–369.
Gill, E. D., Schaerer, S., & Dupuis, B. (2014). Factors impacting blackleg development caused by Dickeya spp. in the field. Eur. J. Plant Pathol., 140(2), 317–327.
Glasner, J. D., Marquez-Villavicencio, M., Kim, H. S., Jahn, C. E., Ma, B., Biehl, B. S., Rissman, A. I., Mole, B., Yi, X., Yang, C. H., Dangl, J. L., Grant, S. R., Perna, N. T., & Charkowski, A. O. (2008). Niche-specificity and the variable fraction of the Pectobacterium pan-genome. Molecular Plant-Microbe Interactions, 21(12), 1549–1560.
Hanssen-Bauer, I., Drange, H., Førland, E., Roald, L., Børsheim, K., Hisdal, H., et al. (2009). Climate in Norway 2100. Background information to NOU climate adaptation (in Norwegian: Klima i Norge 2100. Bakgrunnsmateriale til NOU Klimatilplassing). Oslo: Norsk klimasenter.
Kassambara, A. (2019a). Ggpubr: 'ggplot2' based publication ready plots. https://github.com/kassambara/ggpubr.
Kassambara, A. (2019b). Rstatix: Pipe-friendly framework for basic statistical tests in R. https://github.com/kassambara/rstatix.
Kõiv, V., Roosaare, M., Vedler, E., Ann Kivistik, P., Toppi, K., Schryer, D. W., et al. (2015). Microbial population dynamics in response to Pectobacterium atrosepticum infection in potato tubers. Scientific Reports, 5, 11606.
Laurila, J., Ahola, V., Lehtinen, A., Joutsjoki, T., Hannukkala, A., Rahkonen, A., et al. (2008). Characterization of Dickeya strains isolated from potato and river water samples in Finland. European Journal of Plant Pathology, 122(2), 213–225.
Lebecka, R., Flis, B., & Murawska, Z. (2018). Comparison of temperature effects on the in vitro growth and disease development in potato tubers inoculated with bacteria Pectobacterium atrosepticum, P. carotovorum subsp. carotovorum and Dickeya solani. J. Phytopathol., 654-662.
Lyon, G. (1989). The biochemical basis of resistance of potatoes to soft rot Erwinia spp.—A review. Plant Pathol., 38(3), 313–339.
Ma, B., Hibbing, M. E., Kim, H.-S., Reedy, R. M., Yedidia, I., Breuer, J., Breuer, J., Glasner, J. D., Perna, N. T., Kelman, A., & Charkowski, A. O. (2007). Host range and molecular phylogenies of the soft rot enterobacterial genera Pectobacterium and Dickeya. Phytopathology, 97(9), 1150–1163.
Ma, X., Schloop, A., Swingle, B., & Perry, K. L. (2018). Pectobacterium and Dickeya responsible for potato blackleg disease in New York state in 2016. Plant Disease, 1834–1840.
Ngadze, E., Coutinho, T. A., Icishahayo, D., & van der Waals, J. E. (2014). Effect of calcium soil amendments on phenolic compounds and soft rot resistance in potato tubers. Crop Protect., 62, 40–45.
Pérombelon, M. C. M. (2000). Blackleg risk potential of seed potatoes determined by quantification of tuber contamination by the causal agent and Erwinia carotovora subsp. atroseptica: A critical review. EPPO Bulletin, 30(3–4), 413–420.
Pérombelon, M. C. M. (2002). Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathology, 51(1), 1–12.
Pérombelon, M. C. M., & Kelman, A. (1987). Blackleg and other potato diseases caused by soft rot erwinias: Proposal for revision of terminology. Plant Disease, 71(3), 283–285.
Pérombelon, M. C. M., Lopez, M. M., Carbonell, J., & Hyman, L. J. (1988). Effects of contamination by Erwinia carotovora subsp. carotovora and E. carotovora subsp. atroseptica of potato seed tubers and of cultivar resistance on blanking or nonemergence and blackleg development in Valencia, Spain. Potato Res., 31(4), 591–599.
Pérombelon, M. C. M., & Lowe, R. (1975). Studies on the initiation of bacterial soft rot in potato tubers. Potato Research, 18(1), 64–82.
Pérombelon, M. C. M., Lumb, V. M., Zutra, D., Hyman, L. J., & Burnett, E. M. (1989). Factors affecting potato blackleg development. In E. C. Tjamos & C. H. Beckman (Eds.), Vascular wilt diseases of plants (pp. 421–431). Berlin, Heidelberg: Springer.
Potrykus, M., Golanowska, M., Sledz, W., Zoledowska, S., Motyka, A., Kolodziejska, A., et al. (2016). Biodiversity of Dickeya spp. isolated from potato plants and water sources in temperate climate. Plant Disease, 100(2), 408–417.
Pritchard, L., Humphris, S., Saddler, G. S., Parkinson, N. M., Bertrand, V., Elphinstone, J. G., et al. (2013). Detection of phytopathogens of the genus Dickeya using a PCR primer prediction pipeline for draft bacterial genome sequences. Plant Pathology, 62(3), 587–596.
Quinn, C. E., Sells, I. A., & Graham, D. C. (1980). Soft rot Erwinia bacteria in the atmospheric bacterial aerosol. The Journal of Applied Bacteriology, 49(1), 175–181.
Rossmann, S., Dees, M. W., Perminow, J., Meadow, R., & Brurberg, M. B. (2018). Soft rot Enterobacteriaceae are carried by a large range of insect species in potato fields. Appl Environ Microbiol (2018/04/08 ed., Vol. 84, pp. e00281-00218).
Rouffiange, J., Gerardin, D., Kellenberger, I., Schaerer, S., & Dupuis, B. (2014). Potato susceptibility to blackleg disease caused by Dickeya spp. Agrarforschung Schweiz, 5(3), 96–103.
Smadja, B., Latour, X., Trigui, S., Burini, J. F., Chevalier, S., & Orange, N. (2004). Thermodependence of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.). Can. J. Microbiol., 50(1), 19–27.
van der Wolf, J. M., de Haan, E. G., Kastelein, P., Krijger, M., Haas, B. H., Velvis, H., et al. (2017). Virulence of Pectobacterium carotovorum subsp. brasiliense on potato compared with that of other Pectobacterium and Dickeya species under climatic conditions prevailing in the Netherlands. Plant Pathol., 66, 571–583.
van der Wolf, J. M., Nijhuis, E. H., Kowalewska, M. J., Saddler, G. S., Parkinson, N., Elphinstone, J. G., et al. (2014). Dickeya solani sp. nov., a pectinolytic plant pathogenic bacterium isolated from potato (Solanum tuberosum). Int. J. Syst. Evol. Microbiol, 64(3), 768–774.
van Vuurde, J. W. L., & de Vries, P. M. (1994). Population dynamics of Erwinia carotovora subsp. atroseptica on the surface of intact and wounded seed potatoes during storage. J. Appl. Bacteriol., 76(6), 568–575.
Wastie, R. L., Jellis, G. J., Lapwood, D. H., Logan, C., Little, G., & Phillips, M. S. (1988). Assessing potato cultivars for resistance to tuber soft rot (Erwinia carotovora subsp. atroseptica) at four test centres in the UK. Potato Res., 31(1), 67–72.
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis: Springer-Verlag New York.
Zoledowska, S., Motyka, A., Zukowska, D., Sledz, W., & Lojkowska, E. (2018). Population structure and biodiversity of Pectobacterium parmentieri isolated from potato fields in temperate climate. Plant Disease, 102(1), 154–164.
Acknowledgments
This study was funded by a grant from Research Funding for Agriculture and the Food Industry - Matfondavtale (244207), including support from the following industry partners: Bama Gruppen AS, Gartnerhallen SA, Norgro AS, 7sense, Findus Norge AS, Totenpoteter AS, Orkla Confectionery & Snacks Norge, Strand Unikorn AS, Tromspotet AS, Lærdal Grønt AS, Bayer As, Overhalla Klonavlssenter. We thank the reviewers for their vital contributions to the improvement of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors have participated in the research and manuscript preparation, and all have reviewed and approved the manuscript. The manuscript has not been published before and has only been submitted to EJPP for evaluation.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Sudies
This research did not involve human participants or animals.
Rights and permissions
About this article
Cite this article
Rossmann, S., Dees, M.W., Torp, T. et al. Field-scale molecular testing of virulent potato soft rot Pectobacteriaceae in Norway. Eur J Plant Pathol 156, 501–517 (2020). https://doi.org/10.1007/s10658-019-01901-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01901-0