Abstract
Glasshouse experiments were conducted to study the colonization of seedpods (siliques) and seeds of rapid cycling Brassica oleracea plants after spraying inoculum on clusters of recently opened flowers with Xanthomonas campestris pv. campestris (Xcc) at densities of 107–108 cfu ml−1. A green fluorescent protein (GFP) tagged Xcc strain was used to allow visualization of the bacteria by epifluorescence stereo microscopy (ESM) and confocal laser scanning microscopy (CLSM). The GFP-tagged strain showed reduced virulence compared to the untagged parental strain, but was still able to cause black rot symptoms. Two to three days after spray-inoculation, sepals, stamen and petals were colonized by Xcc, as observed by ESM. In green siliques a GFP-signal was observed on valves, septa and seeds, despite the fact that a high percentage of Xcc cells had lost their ability to express GFP as found by dilution-plating. Densities of Xcc in infected silique tissues were up to 109 cfu g−1. A fluorescent signal using ESM was found in seeds harvested from symptomatic siliques after incubation of seeds on blotting paper wetted with broth to enhance the multiplication of Xcc. Xcc was found in association with the seed coat and in a single seed, also in the endosperm and embryo, indicating deep-seated seed infection. The estimated incidence of contaminated seeds in both years was ca. 7%. The estimated incidence of deep-seated infections, still detectable after warm water treatment of seeds, was also high (2–3.8%). It is concluded that spray-inoculation of flower clusters with Xcc can result in the infection of sepals and reproductive organs, and in deep-seated seed infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Brassicaceae is a large botanical family that includes 372 genera, under which several plant species of great economic and agronomic importance, such as oil seed rape and vegetables, but also the model plant in botanical research Arabidopsis thaliana. (Bailey et al. 2006). Black rot, caused by the seed- and soil borne bacterial pathogen Xanthomonas campestris pv. campestris (Xcc), is considered to be the most important bacterial disease of Brassica crops (Williams 1980). Black rot occurs worldwide and affects a wide number of cultivated and wild brassicas (Williams 1980; Bradbury 1986; Schaad and Alvarez 1993). Black rot control is based on the use of Xcc-free seed, sanitation and management practices which include crop rotation and weed control (Vicente and Holub 2013). Besides crop residues in soil (Köhl et al. 2011) and cruciferous weeds (Dane and Shaw 1996; Schaad and Dianese 1981), contaminated seed is a major source of primary inoculum for black rot (Randhawa and Schaad 1984). Despite considerable efforts to produce Xcc-free seed, seed infections still occur (Williams 1980; Gitaitis and Walcott 2007). Physical treatment such as with hot water is useful to eliminate Xcc from seeds (Walker 1923; Nega et al. 2003). However, it is not always effective in the eradication of deep-seated seed infections, present in seed coat, endosperm or embryo (Schaad 1983; Van der Wolf et al. 2013). Low rates of seed infection already may cause serious epidemics in transplants, wherein production yield favorable conditions for black rot to develop (Roberts et al. 1999).
How seeds become infected is not fully known, but essentially there are two possible routes. The first is through systemic colonization of vascular tissue. This may occur after root infection from inoculum present in soil or after leaf infection through air-borne inoculum carried by wind, aerosols, irrigation water, rain, and pollinating insects, farm equipment and workers (Vicente and Holub 2013). Xcc can move upwards in xylem sap and may reach the seeds via translocation through peduncles. However, scientific evidence is lacking.
The other potential route is via infection of reproductive organs. If flowers or developing siliques become infected via airborne inoculum, it may result in infection and translocation of Xcc via vascular tissues of silique and funicle into the developing seed, or alternatively via contact of seed with infected tissues of valves or septa. Van der Wolf et al. (2013) demonstrated that spray-inoculation of flower clusters of cabbage plants with Xcc resulted in a high incidence of infected seeds. In another study, Van der Wolf and Van der Zouwen (2010) showed that bluebottle flies (Calliphora vomitoria) artificially contaminated with Xcc were able to transmit the pathogen efficiently, resulting in a high percentage of infected seed lots. The relative importance of the different infection routes in commercial seed production fields is unknown and also which route could result in deep-seated seed infections that survive a hot water treatment.
This research was aimed to explore the seed infection process in Brassica plants after flower inoculation. A GFP-tagged Xcc strain was used for microscopic studies to establish the location of the bacteria in the infected seeds of plants of CrGC 3–1 (Brassica oleracea), a stock of rapid cycling Brassicas (RCB). RCB plants have been developed through careful selection and combining of genes of early-flowering Brassica types (Williams and Hill 1986). Under specific conditions, they are capable of cycling within a 5–9 weeks period, they remain small, and do not have a dormancy (Williams and Hill 1986), which makes them excellent model plants. From previous experiments it was known that RCB lineage CrGC 3-1 is susceptible to Xcc (unpublished results). Complementary data were collected by assessing population sizes of Xcc associated with flowers and siliques and by determining the percentage of seeds with external contamination and deep-seated infection.
Material and methods
Bacterial strains, buffer solutions and culture media
In the experiments Xcc wild type strain IPO 3078 and its GFP-tagged transformant strain IPO 3555 were used, derived from the work collection at Wageningen UR (https://www.wur.nl/en/show/Prime-Diagnostics-2.htm). The strain was isolated in 1986 in Wisconsin (USA) from Brassica oleracea as isolate PHW 824–1. Both strains were stored at −80 °C on beads (TS/70-YE, Protect bacterial preservers; www.tscswabs.co.uk). Xcc strain IPO 3078 was maintained at 17 °C on yeast dextrose chalk (YDC) agar (Duchefa Biochemie, NL) and inoculum was prepared from cultures grown on tryptone soya agar (TSA; Oxoid, UK) at 25 °C for 48 h. Suspensions of Xcc were prepared in ¼ strength Ringer’s solution (Oxoid) or water. Xcc strain IPO 3555 was maintained and cultured the same way, but on YDCK agar and TSAK (YDC agar and TSA supplemented with 50 μg mL−1 kanamycin monosulfate; Duchefa Biochemie).
Cells of Xcc strain IPO 3555 were extracted from petals and silique tissues in RT, which is ¼ strength Ringer’s solution supplement with 0.1% Tween-20 (Acros Organics, USA). The bacterium was extracted from ripe seeds in PBST (8 g L−1 NaCl, 13.5 g L−1 Na2HPO4.12H2O, 2 g L−1 NaH2PO4.2H2O and 0.2 ml L−1 Tween-20). Plant extracts were plated on FS and FSK (FS supplemented with 50 mg L−1 kanamycin monosulfate). FS is mFS medium (Duchefa Biochemie), supplemented with 15 mg L−1 methyl green (Sigma, USA), 3 mg L−1 D-methionine (Acros Organics), 1 mg L−1 pyridoxine-HCl (Duchefa Biochemie), 30 mg L−1 trimethoprim (Duchefa Biochemie) and 200 mg-L−1 cycloheximide (Duchefa Biochemie). After incubation (72 h, 25 °C) typical Xcc colonies were counted.
To improve microscopic detection of Xcc associated with RCB seeds, the selective growth of the pathogen in and on seeds was brought about by the incubation of seeds on blotters moistened with tryptone broth yeast (TBY; 10 g L−1 tryptone (Oxoid), 5 g L−1 yeast extract (Oxoid), 5 g L−1 NaCl, pH 7.5) supplemented with kanamycin monosulfate and cycloheximide.
Generation of GFP-tagged Xcc strain IPO 3555
Plasmid pHC60-GFP (Cheng and Walker 1998) was used for the generation of GFP-tagged Xcc strain IPO 3555. The plasmid was introduced to cells of strain IPO 3078 by electroporation, as described by Calvin and Hanawalt (1988). Briefly, 50 μL suspension of 1011 cfu mL−1 Xcc cells was mixed with 0.5 μL plasmid suspension (approximately 100 ng DNA μL−1) and electro-shocked at 2.5 kV for 1 to 2 s at 4 °C using a Bio-Rad Gene Pulser 200/2.0 (Bio-Rad, Hercules, USA). Electro-shocked cells were transferred to 500 μL nutrient broth (Difco, USA) and resuscitated by shaking on a TPM-2 microplate shaker (Sarstedt, DE) for 1 h at 500 rpm and 28 °C. One hundred μL suspension of transformed cells was plated on TSAK and incubated for 48 h at 28 °C. GFP-positive transformants were visualised using a Leica MZ FL III epifluorescence stereomicroscope (Leica, DE) equipped with a mercury high-pressure photo-optic lamp (Hg 50 W/AC) and GFP 2 filter cube. Strain IPO 3555 was isolated from a highly fluorescent colony.
Brassica plants and growth conditions
In all experiments, rapid cycling Brassica oleracea CrGC stock 3–1 (RCB) plants were used, which is an open pollinated variety. The seed was originally derived from the Wisconsin Fast Plants Program (https://fastplants.org/), but locally propagated for two years more by Syngenta Netherlands. RCB plants were kept in a greenhouse with a 16 h photoperiod maintained by supplemental lighting (high-pressure sodium lamps, 150 W m-2), a constant temperature of 24 °C and 70% relative humidity. RCB seeds produced by Syngenta Netherlands, were sown in Lentse seedling mix (Horticoop, NL). Seven days after sowing, seedlings were transplanted into 2 L-pots containing Lentse potting compost no 4 with pieces of bark (Horticoop, NL). In all developmental stages the plants were supported by Tonkin canes. Furthermore, lateral shoots were pruned.
To avoid seed losses, individual siliques were harvested twice a week shortly before they reached full ripeness. Siliques were collected per treatment in paper bags and air dried. Seeds were threshed and cleaned by hand. Between processing the harvests of different treatments, the equipment used, was disinfected with 70% ethanol. All seeds were stored in paper bags at room temperature in a dry place until further processing.
Pathogenicity test for strain comparison
To compare the pathogenicity of the GFP-tagged Xcc strain IPO 3555 with its untagged parental strain IPO 3078, twelve RCB plants of twenty days old (four true leaves stage) per strain were spray-inoculated. Bacterial suspensions were prepared in tap water from two-day-old cultures on TSA and adjusted to OD600 = 0.1 (ca. 108 cfu mL−1). Before inoculation, the wax layer of three leaves per plant was damaged by pinching the leaves with a rubber-gloved hand. Xcc suspensions were atomized on the leaves to runoff with a 0.5 L pump plant sprayer (Birchmeier, CH) held at 10–20 cm distance from the plants. Ten RCB plants were sprayed with tap water as a negative control. Disease assessments were performed at 5, 7, 9, 12, and 15 days after inoculation. For each assessment, three inoculated leaves from each plant were scored according to the disease scale proposed by Massomo et al. (2004). The score of 1 = no symptoms on the leaves; 2 = minute necrotic lesions at the hydathodes to discrete vein-restricted necrotic marginal panels with dark edges, panels 0.5 cm to several centimetres long and 0.5–2 cm wide, no chlorosis beyond the margin of the necrotic lesion; 3 = small marginal V-shaped lesions, 0.5–2 cm with narrow diffuse chlorotic margins, sometimes necrotic panels with diffuse chlorotic margins; 5 = small to medium V-shaped marginal lesions, necrotic centres, diffuse chlorotic margins, internal and marginal vein blackening, lesions rarely progressing half-way to midrib; 7 = expanding marginal lesions, frequently reaching midrib, prominent vein blackening; 9 = rapidly expanding marginal lesions frequently coalescing to give scorching and systemic invasion of the plant, frequently accompanied by severe stunting and death of the plant. The average disease scores for individual leaves was used to determine the area under the black rot progress curve (AUBRPC) according to Shaner and Finney (1977).
Flower inoculation of RCB plants with Xcc strain IPO 3555
Two experiments were carried out to evaluate the efficiency of seed infection by flower inoculation. The first experiment started in April 2013 and clusters of recently opened flowers of 64 RCB plants were spray-inoculated with Xcc strain IPO 3555. Xcc suspension was atomized with a Birchmeier 0.5 L pump plant sprayer held close to the flowers to be inoculated. However, unintentional deposition of inoculum on other plant tissues, such as nearby peduncles, pedicels and unopened flowers could not be avoided. The second experiment started in October 2013 whereby the flower clusters of 50 RCB plants were inoculated the same way with the same strain. In the first experiment, for inoculation, bacterial suspensions with an OD600 = 0.1 were prepared in tap water from two-day-old cultures on TSAK. The high dose resulted in high percentages of complete necrosis of young siliques and aborted seeds, therefore in the second experiment a ten-times dilution of a suspension with an OD600 = 0.1 was used. Inoculations started 5 days after the beginning of flowering and were repeated every 2–3 days until the end of the main flush of flowering.
During the 1st and 2nd experiment, clusters of recently-opened flowers were inoculated on 11 and 8 different days, respectively.
After the first inoculation, Minipol boxes (Koppert, NL) each containing a colony of about 40 bumblebees (Bombus terrestris L.), were placed inside the 16 m2 greenhouse compartment (3 boxes in the first experiment and 6 in the second one), for natural pollination of the RCB flowers. The bumblebees were removed from the greenhouse 3 days after the last inoculation and the peduncles were topped to remove non-inoculated flowers.
In each experiment, the flower clusters of 12 RCB plants were sprayed with tap water as negative controls. The mock-inoculated plants were kept in a 140 cm × 70 cm × 130 cm (length x depth x height) cage of transparent insect screen with a Minipol box to avoid cross-contaminations with Xcc-infected plants present in the glasshouse compartment.
Colonization of flowers by Xcc
In the second experiment, the population size of Xcc strain IPO 3555 on petals from Xcc- and mock-inoculated flowers was assessed approximately one hour after each inoculation and 2–3 days after each inoculation, just before the upcoming inoculation (a total of 16 assessments). During sampling, four fresh fully opened flowers from different inoculated plants were collected in Petri dishes using forceps. Three flowers from mock-inoculated plants were collected as negative controls. All the collected flowers were inspected using a MZ FL III epifluorescence stereo microscope (ESM) for the presence of GFP signal. Pictures were taken with an AxioCam MR05 camera (Carl Zeiss, DE) and AxioVision software programme Release 4.8 (Carl Zeiss). One petal was transferred from each inoculated flower to a 13 mL-tube with 5 mL RT and agitated in a tube rack mounted on a TPM-2 microplate shaker for 15 min at 900 strokes min−1. An aliquot of 100 μL of undiluted and another of 100-times diluted extracts were plated on FSK medium. After an incubation time of 72 h at 25 °C, typical Xcc colonies were counted.
Black rot incidence of siliques
In both experiments the occurrence of black rot on siliques was assessed at 62 days after potting, which was 11 days after the last/final inoculation. Four hundred siliques from 20 different plants inoculated with Xcc strain IPO 3555, and 240 siliques from 12 mock-inoculated plants were examined for the presence of symptoms (irregular shaped black spots) and the percentage of symptomatic siliques was calculated.
Infection loci on and population sizes of Xcc in green siliques
Green siliques with late stages of seed development and showing black lesions were sampled for microscopic and bacteriological analysis on the presence of Xcc. In the first experiment siliques were sampled at 16, 55, 62, 69, 76, 83 and 90 days after the first inoculation. In the second experiment sampling was done on day 42, 56, 63, 64, 70 and 77. Numbers collected depended on availability of suitable siliques and varied from 1 to 20 at a time. In total 48 siliques were collected in the first experiment and 42 siliques in the second experiment. At each sampling time 1–5 green siliques from the mock-inoculated plants were used as negative controls.
The surface of all siliques was inspected using ESM for the presence of a GFP-signal. GFP-positive siliques were cut longitudinally through the replum with a razor blade and the interior was also examined for the presence of GFP-signals. Seeds were removed from the opened siliques, counted and stored in 3.5 cm Petri dishes for up to a maximum of 5 days at 4 °C prior to microscopic and bacteriological analysis.
All the GFP-positive siliques from the first experiment and 10 GFP-positive siliques from the two first samplings of the second experiment were analysed bacteriologically. For dilution-plating the valves, septa and pedicels of each silique from the first experiment were processed separately. The different parts were subsequently weighed, transferred to a small plastic bag (60 × 80 × 0.09 mm) and crushed using a hammer. For each gram of tissue, 2 mL RT was added and mixed through the crushed tissues. The undiluted extract and 100 and 10.000-times dilutions in RT were plated on FS medium. In the second experiment the various silique parts mentioned above were not analysed separately. The parts of each individual silique were pooled before crushing and dilution-plated as described in the first experiment.
Infection loci and population sizes in immature seeds
Seeds from the green siliques of the first experiment were first inspected individually by ESM for the presence of a GFP-signal. Thereafter, each seed was transferred individually to a 1.5 mL Eppendorf tube containing 500 μL of RT and crushed with a disposable inoculation needle. The undiluted and 100-fold diluted extracts were examined for the presence of Xcc by plating on FS medium.
Seeds from the green siliques of the 2nd experiment were inspected for the presence of GFP-signal in two different ways. The seeds from the first 20 siliques collected were processed in the same way as seeds in the first experiment.
To increase the chance of detection, the seeds from 23 siliques collected later on were placed on blotting paper (11 × 11 cm and 1 mm thick; Schut papier, NL) wetted with 8 ml of 10x diluted TBY supplemented with 200 μg mL−1 cycloheximide and 50 mg L−1 kanamycin monosulphate. Seeds were arranged in lines of five seeds and incubated up to 2 days at 25 °C. At 24 h intervals the seeds were inspected by ESM for spots with a GFP-signal. Seeds showing a GFP signal were inspected in detail using confocal laser scanning microscopy (CLSM). Seeds lacking a GFP-signal after incubation were further processed for dilution-plating on FS medium as described above for seeds of the first batch of siliques.
GFP-positive seeds were inspected using CLSM (Leica DM5500Q; Leica, DE). They were cut by hand in three equally thick pieces and the central section was subsequently placed on a microscope slide, embedded in molten 0.2% multipurpose agarose (Agarose MP; Roche, CH) of 50 °C, covered with a cover glass and cooled to room temperature before using CLSM. For excitation of GFP a 488 nm blue laser was used and the emitted light was detected through a 505 nm filter. Pictures from tissues below the surface of the seed pieces were taken with the Leica Digital System (Leica) connected to the CLSM using 10, 63 and 100x objective lenses. In some cases pictures were composed by stacking multiple images. After CLSM inspection, seed parts were pooled with the two other corresponding parts and further processed similar to the seeds that did not have spots with GFP-signal during the ESM inspection.
Infection loci and population sizes in ripe seeds
The harvest of the first experiment was subdivided into 32 sub-samples of 25 seeds and 7 sub-samples of 25 seeds from plants inoculated with Xcc strain IPO 3555 and from mock-inoculated plants, respectively. In the second experiment, in total 100 sub-samples of 25 seeds from inoculated plants and 12 sub-samples of 25 seeds from mock-inoculated plants were processed. Each sub-sample was stored at 4 °C until analysis for external contamination of seeds and more deep-seated infections of seeds as outlined in Fig. 1.
Diagram of the procedure to analyse seeds harvested from mature siliques for external contaminations and deep-seated infections with Xanthomonas campestris pv. campestris. If the wash fluid of the seeds was positive in the dilution-plating assay, samples were considered externally contaminated. If seed extracts were positive in the dilution-plating assay after a hot water treatment or a fluorescent signal was observed in endosperm or embryo using confocal laser scanning microscopy, we assumed deep-seated infections
To analyse for external contaminations, 500 μL of PBST (pre-chilled at 2-4 °C) was added to each subsample. After agitation on a TPM-2 microplate shaker (1.5 mL Eppendorf tubes, 700 rpm, 10 min, room temperature) the washing buffer was removed, sampled for plating, and the seeds were air dried on filter paper in Petri dishes and stored at 4 °C until the results of the assessment of the external contaminations were known. The washing buffer was plated undiluted and 100-fold diluted on both FS and FSK media. The agar plates were incubated for 72 h at 25 °C before counting Xcc colonies.
Prior to assessing deep-seated infections, the seeds from each sub-sample with an external Xcc-contamination were incubated on blotters and inspected by ESM as described above for seeds of green siliques. Seeds showing a GFP-signal were inspected in detail by CLSM according to above description for GFP-positive seeds in green siliques. The assessment of the deep-seated Xcc infections of GFP-negative seeds included killing off external Xcc contamination using a hot water treatment (52 °C, 10 min) as described by Van der Wolf et al. (2013). After the decontamination, the PBST was removed and the seeds were subsequently air dried in a cross-flow on filter paper in 5.5 cm diameter Petri dishes, crushed in 60 × 80 × 0.09 mm plastic bags with a hammer. Directly after crushing the seeds, 500 μL PBST was mixed through the macerate and the samples were incubated for 10–15 min at room temperature followed by plating, as described above for assessing the external contamination. The incidences of externally contaminated seeds and seeds with deep-seated infections were estimated using the equation: I = {1-[(N-p)/N]1/n}×100 (De Boer 2002), where p is the number of sub-samples that tested positive for the presence of Xcc; N is the total number of sub-samples tested; and n is the number of seeds per sub-sample.
Data analysis
Numerical data were recorded and analysed in Microsoft Excel 2010, and when appropriate in the 18th edition of the statistical program Genstat (VSN International, Rothamsted UK). In the pathogenicity test, comparison of areas under the black rot progress curves of plants inoculated with the GFP-tagged Xcc strain IPO 3555 with those of plants inoculated with its parental strain IPO 3078 was made by General Analysis of Variance (ANOVA) and the Least Significant Difference (LSD, 5% level) of means was calculated. For monitoring the efficiency of flower inoculations, comparisons between densities of Xcc associated with petals from various inoculation moments, shortly after inoculation and 2–3 days post inoculation (dpi), were made by ANOVA and Duncan’s Multiple Range Test (DMRT).
Results
Pathogenicity test for strain comparison
In pathogenicity tests the GFP-tagged Xcc strain IPO 3555 was found to be less virulent than the parental strain IPO 3078 (Fig. 2). The average AUBRPC was 27 for strain IPO 3555, which was significantly (P = 0.05) lower than the average AUBRPC of 37 for strain IPO 3078 (data not shown). Although the virulence of the GFP-tagged strain IPO 3555 was reduced compared to the parental strain, the virulence was still considered sufficient for studying the infection process for seeds. All mock inoculated plants had a disease score = 1 (no symptoms) during the experiment, resulting in an AUBRPC = 10.
Progress of foliar symptoms of black rot after spray-inoculation of leaves of rapid cycling Brassica oleracea plants with either the GFP-tagged strain IPO 3555 of Xanthomonas campestris pv. campestris or its parental strain IPO 3078. Disease severities were assessed using the scale of Massomo et al. (2004) described in the section ‘Materials and Methods’
Colonization of flowers by Xcc
During the second experiment, approximately one hour after each inoculation and 2–3 days after each inoculation, just before the upcoming inoculation, opened flowers were inspected by ESM for the presence of a GFP-signal. One hour after inoculation, no green fluorescence was observed on any of the organs of these flowers (results not shown). No GFP signal developed on flowers of mock-inoculated plants (Fig. 3a3, b3), although the anther lobe of the stamen displayed a high yellow green autofluorescence (Fig. 3b3). In contrast, at 2–3 dpi, small bright green fluorescent loci were found on petals of open flowers indicating infection and multiplication (Fig. 3a4). Bright field illumination of flowers showed that the fluorescent loci were occasionally found in association with necrotic lesions, indicating symptomatic infection of petals by Xcc (Fig. 3a2). The necrotic lesions were not seen on petals of mock-inoculated flowers (Fig. 3a1). Also large weakly fluorescing spots were observed, not associated with necrosis, indicating superficially grown Xcc cells on petals (Fig. 3a4). Furthermore, bright green fluorescent spots were detected on the sepals (Fig. 3b4) of Xcc-inoculated flowers associated with necrotic lesions (Fig. 3b2), but not on mock-inoculated plants (Fig. 3 b1 and b3). A green fluorescence was also observed on the stigma of Xcc-inoculated flowers at 2–3 dpi, which was not found on the mock-inoculated flowers (Fig. 4).
Stereo photomicrographs of petals (a1–4) and sepals (b1–4) of mock-inoculated flowers (a1, a3, b1, b3) or of flowers inoculated with a GFP-tagged strain of Xanthomonas campestris pv. campestris (Xcc–inoculated; a2, a4, b2, b4) at 2–3 dpi. a1, a2, b1 and b2 are images made with low intensity bright field illumination. a3, a4, b3 and b4 are images made with UV light. On Xcc-inoculated petals (a2) and sepals (b2) occasionally necrotic spots were found (indicated with white triangles) which coincided with bright fluorescent spots (a4 and b4, also indicated with white triangles)
Stereo photomicrographs of styles of flowers from mock-inoculated plants (left column) or from plants inoculated with a GFP-tagged strain of Xanthomonas campestris pv. campestris (Xcc) (right column) at 3 dpi. The white triangle indicates a locus with a green fluorescent signal on the stigma, indicative for the presence of Xcc. a and b are images made with low intensity bright field illumination. c and d are images made with UV light
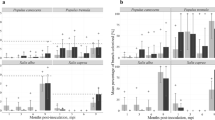
The population size of Xcc on petals of Xcc-inoculated flowers was assessed by dilution-plating one hour after each inoculation and 2–3 days after each inoculation, just before the upcoming inoculation. One hour after inoculation, Xcc was detected on all petals and the average Xcc population size on petals taken from different flowers during the experiment ranged from 7 104 to 1.2 107 cfu petal−1. At the day of inoculation the population sizes per petal did not differ significantly between the different inoculation days (P = 0.05), indicating a rather uniform administering of inoculum on the flowers in the course of the experiment (Table 1). Two to three days after the inoculations, Xcc was detected on 21 out of the total of 32 petals (66%) examined. Population sizes of the Xcc-positive petals of different flowers varied strongly ranging from 1 102 to 2.3 107 cfu petal−1.
Black rot incidence of siliques
The incidence of symptomatic siliques of flower-inoculated plants was 76.5% in the first experiment and 57.6% in the second experiment. No siliques with irregular shaped black spots or progressive rot were found on mock-inoculated plants. However, blackening of the pointed beaks of siliques was found both in Xcc- and mock-inoculated siliques (Fig. 5).
Infection loci on and population sizes in green siliques
Symptomatic green siliques from inoculated plants were inspected by ESM for the presence of a GFP signal. In the 90 siliques showing black spots that were analyzed during the first and second experiment, high GFP intensities were found on the receptacles, valves and/or septa (Fig. 6b, d, f, h). No GFP signal was found on siliques harvested from mock-inoculated plants (Fig. 6a, c, e, g).
Epifluorescence stereo photomicrographs of receptacle (a), valves (c and e) and septum (g) of green siliques from mock-inoculated plants, and receptacle (b), valves (d and f) and septum (h) of symptomatic siliques from plants inoculated with GFP-tagged Xanthomonas campestris pv. campestris (Xcc-inoculated) strain IPO 3555. White triangles indicate loci with a high fluorescent signal indicative for the presence of Xcc
After removal of seeds from symptomatic siliques in the first experiment, Xcc colonies were recovered on FS medium from 29 out of 48 siliques (60%), i.e. from 20 valves (42%), from 21 septa (44%) and from 21 pedicels (36%) (Table 2). Xcc was detected in all three parts in 10 siliques. Maximum densities of up to 3.109 cfu g−1 of tissue were recovered from valves and septum. In the second experiment, Xcc was recovered from all 10 siliques analyzed with densities of Xcc cells ranging from 6 104 to 2 107 cfu g−1 silique tissue. Xcc could not be detected in tissue extracts of mock-inoculated plants.
Infection loci and population sizes in immature seeds
The 29 siliques of the first experiment which were harvested 15–90 days after the first inoculation and found, using dilution-plating, to be infected by Xcc, yielded 215 seeds (Table 2). Although no GFP signal was detected during inspection by ESM, dilution-plating of the extracts of individual seeds on FS medium demonstrated the presence of Xcc in 7 seeds (3.3%) harvested from three siliques and sampled from three different plants. Population densities ranged from 1 to 3 104 cfu seed−1. No Xcc was detected in the 155 seeds harvested from 19 siliques which were found negative for Xcc.
During the second experiment, 163 seeds harvested 42–77 days after the first inoculation of the first series of 20 developing siliques were inspected by ESM, without prior incubation. None of these seeds showed a GFP signal.
To enhance detection of Xcc by ESM, the 174 seeds collected from the second series of 23 siliques were incubated on blotters prior to ESM. A GFP signal was detected in nine (5.2%) of these seeds, harvested from three severely infected siliques, of which seven seeds are shown in Fig. 7.
Epifluorescence stereo photomicrographs of a seed from a green silique of a mock-inoculated plant (a) and seeds (b – h) from symptomatic siliques of plants inoculated with GFP-tagged strain IPO 3555 of Xanthomonas campestris pv. campestris (Xcc). Seeds were incubated for 24 h at 25 °C on blotters with TBY. The green spots are loci with a high fluorescent signal indicative for the presence of Xcc
CLSM was applied for detailed observations. In seed harvested from symptomatic green siliques the presence of Xcc was observed in two seeds with CLSM after incubation on blotters (Fig. 8); the internal tissues of the other ESM positive seeds were decomposed. CLSM demonstrated the presence of Xcc cells in tissues of the embryo (Fig. 8b–d) and the external part of the seed coat (Fig. 8b). Mock-inoculated seeds did not expose a green fluorescent signal (Fig. 8a).
Confocal laser scanning photomicrographs of cross sections of seeds after incubation for 24 h at 25 °C on blotters with TBY. a: seed from green silique of a mock-inoculated plant; b – d: seeds from symptomatic green siliques of plants inoculated with GFP-tagged Xanthomonas campestris pv. campestris strain IPO 3555; E: ripe seed of a mock-inoculated plant; f-h: a ripe seed from an inoculated plant. Photomicrographs were taken at magnifications of 100x (a, b and f), 630x (c, e and g) and 1000x (d and h). The green or light blue spots are loci with a high fluorescent signal indicative for the presence of Xcc
Infection loci and population sizes in ripe seeds
In the first experiment, the seeds from four sub-samples with an external Xcc-contamination were incubated on blotters containing diluted TBY medium and inspected for the presence of GFP-signal using ESM. One seed out of the total of 100 seeds was found positive in the blotter test. CLSM revealed that Xcc was localized on the epidermis of the seed coat (Fig. 8f–h). No fluorescent signal was found in mock-inoculated seeds (Fig. 8e).
In both experiments, the sub-samples of 25 seeds were analyzed using dilution-plating on FS and FSK medium. The wash water of seeds without prior disinfection was analyzed to determine primarily external contamination, whereas deep-seated infection was detected subsequently after using a warm water treatment and crushing the seed with a hammer. The results are summarized in Table 3. Regarding plating results on FS medium, the percentages of sub-samples with an external Xcc contamination were 84.4 (first experiment) and 85 (second experiment). The percentages of sub-samples with deep-seated seed infections were 62.1 (first experiment) and 32 (second experiment). The percentages of positive sub-samples were considerably lower(7–32 fold) when extracts were dilution-plated on FSK medium, a medium selective for Xcc cells tagged with the plasmid pHC60-GFP, as opposed to FS medium without supplemented kanamycin. Based on the percentages of positive sub-samples on FS medium, the incidences of externally contaminated seeds were estimated at 7.2% (first experiment) and 7% (second experiment) and the estimated deep-seated seed infections at 3.8% (first experiment) and 2% (second experiment) (Table 3). None of the 25-seed subsamples from mock-inoculated plants were found to have externally contaminated seeds or seeds with deep-seated infections.
Discussion
In this study, we showed that spray-inoculation of flower clusters of rapid cycling Brassica plants with Xcc can result in infection of flower tissues, including sepals, petals and stamen, in high infection levels of silique tissues and in deep-seated seed infections By using confocal laser scanning microscopy, for the first time the pathogen could be visualized in the embryo and endosperm, demonstrating that deep-seated seed infections exist. These deep-seated seed infections form a risk in practice, as they cannot be eliminated by seed treatments to prevent seed from being a primary source of black rot in young Brassica crops.
Transmission of Xcc from the environment to the blossom can occur via aerosols, water splashes or by insects (Kuan et al. 1986; Poplawsky and Chun 1998). Inoculum can be present on leaves of infected Brassica plants nearby the seed production fields (Silva et al. 2017), alternative host plants, including weeds (Krauthausen et al. 2018), and in crop debris on soil (Köhl et al. 2011). In particular, pollinating insects may play a role in the occurrence of flower infections. Pollinating insects collecting the nectar produced by nectaries can release or pick up the bacterial pathogen and thus inadvertently spread the pathogen. In addition, they can carry high densities of the pathogen (van der Wolf and van der Zouwen 2010; Johnson et al. 1993), which can result in the deposition of high numbers of cells on floral tissues. Also in our studies, bumble bees may have increased infection prevalences by transmission of the pathogen. In previous studies, we showed that artificially contaminated bluebottle flies (Calliphora vomitoria) were able to transmit the pathogen efficiently to flowers of cauliflower plants, resulting in a high percentage of infected seed lots (van der Wolf and van der Zouwen 2010).
Translocation of phytopathogens from flower tissues into seeds may occur after penetration of the stigma, via stomata present on nectaries located between the sepals and stamens in the region of the filament (Denisow et al. 2016), on surfaces of sepals, on the style and on the abaxial surface of the anthers (Polowick and Sawhney 1986), and on siliques (Major 1975), or via wounds created during petal shedding. We cannot exclude any of these pathways as during multiple sprays, not only flowers were present on the peduncles Rapid cycling Brasicca plants don't have clusters of peduncles, but one single (sometimes branched) peduncle but also small green siliques. Even infections of leaves during spray-inoculation or due to contact with contaminated petals that drop on leaves during ageing of flowers cannot be excluded. However, in previous experiments, seed infections were frequently found after flower inoculation, but rarely if only leaves were inoculated (van der Wolf et al. 2013), indicating that likely the seed infections are predominantly due to flower infections.
In our studies, incidentally a specific GFP signal was observed on the stigma indicating multiplication of Xcc. The stigma can release nutrients enabling bacterial growth as has been shown for pomaceous flowers (Lawrence Pusey et al. 2008). Multiplication on the stigma of pepper plants by Xanthomonas euvesicatoria, the causative agent of bacterial spot, resulted in a high level of seed infection, possibly by translocation of the bacteria via the pollen tube (Dutta et al. 2013). Entry of the bacteria via stomata of wounds may result in systemic infections and a translocation of the pathogen through the vascular tissues of developing siliques into the seed. A strong signal in the suture of the silique is an indication for this systemic movement of the pathogen. Microscopic studies in the past indicated that inoculation of pedicels with Xcc can result in colonization of the xylem tissue of the funicles, suggesting a translocation via the vascular system into the seed (Cook et al. 1952a). In their studies, however, the transmission from funicles into the seed was never fully evidenced. Seed infections, however, may also be a result of contact with severely infected tissues of the valves or septum. In our studies, we found a specific GFP-signal both on the outer and inner tissues of the valves.
The significance of the different colonization pathways resulting in infections of Brassica seed with Xcc is unknown. For other phytobacteria, more detailed information has been generated on colonization routes (Dutta et al. 2012, 2016; Darsonval et al. 2008). For Acidovorax citrulli, a seed-transmitted pathogen in watermelon, both inoculation of pistils or the ovary pericarp of flowers resulted in seed infections (Dutta et al. 2012). Inoculation of pistils, however, resulted more often in deep-seated infections of endosperm and embryo. Deep-seated infections are considered of major epidemiological relevance, as it has been shown that A. citrulli survives for longer periods in the embryo or endosperm than in and on the seed coat (Dutta et al. 2016).
The pathogen translocated via the floral pathway may also escape the defense mechanism of the plant. In contrast to the inoculation of leaves, the percentage of melon seeds infected by A. citrulli after infection of flowers did not differ significantly between compatible and incompatible interactions, suggesting that the floral pathway of bacterial invasion may be less dependent on specific host-pathogen interactions (Dutta et al. 2014a). In addition, Darsonval et al. (2008) found that mutants of type III secretion systems (T3SS) of Xanthomonas citri pv. phaseoli var. fuscans, affected in their virulence, were still able to infect seeds by the floral pathway. However, unlike the parental strain, these mutants failed to infect seeds when inoculated in the plant vascular system. This shows that in the floral pathway, a functional T3SS is not necessary for transmission of the pathogen to the bean seeds.
The multiple spray-inoculations of flower clusters resulted in deep-seated RCB seed infections ranging from 2.0 to 3.8% (see Table 3). In comparison, in polytunnel experiments in which flowers were inoculated by contaminated blue bottle flies (Calliphora vomitoria) or using a brush, estimated seed infection incidences only were between 0.02 and 0.09% (Van der Wolf and Van der Zouwen 2010). Also in field experiments in the Netherlands, in which a single spray-inoculation of flowers of cauliflower plants was applied, the transmission of Xcc IPO 364 resulted in seed infections in lower incidences ranging from 0.034 to 0.21% than after multiple spray-inoculations (Van der Wolf et al. 2013). In practice, seed infection incidences of 0.01% are tolerated for direct seeded field crops, whereas for transplanted crops an even lower infection incidence can cause economic damage (Schaad et al. 1980; Roberts et al. 2007). Black rot due to the use of seed infected by Xcc can be influenced by various factors such as environmental conditions (Ignatov et al. 1999a; Kastelein et al. 2014), the bacterial strain (Guy et al. 2013; Chidamba and Bezuidenhout 2012; Kastelein et al. 2014), the genotype used (Ignatov et al. 1998, 1999b; van der Wolf et al. 2013), as well as pathogen densities. Seeds vacuum-infiltrated with Xcc suspensions as low as 1.4 cfu per seed were able to cause 1–4% black rot diseased plants (Roberts et al. 1999). Also in other studies, in which seeds were submerged in Xcc suspensions and sown in sand, 63% black rot diseased plants were found indicating a high level of transmission even when seeds have been externally contaminated (Cook et al. 1952b).
During the course of the experiments, a large part of the cells of IPO 3555 recovered from plant material had lost both their ability to express GFP and their resistance to kanamycin, indicating a loss of the plasmid. Further experiments indicated that after transferring suspensions of the transformed strain that were grown for one day under non-selective conditions four times into fresh broth, ca. 10% of the cells had lost the plasmid (data not shown). Other studies using bacteria tagged with a plasmid-based gfp construct also demonstrated that during colonization of substrates, part of the population lost their ability to express GFP (Gage et al. 1996; Stretton et al. 1998). To guarantee stable expression in long term in planta studies, the gene encoding GFP should be introduced into the bacterial chromosome, which is more stable than those plasmid-borne (Errampalli et al. 1999).
Transformation of the parental Xcc strain IPO 3078 with the plasmid pHC60-GFP into IPO 3555 resulted in a significant reduction of the virulence of the strain. Other studies have found that the fitness of bacteria can be negatively affected by expression of GFP (Füchslin et al. 2003). Despite the reduction in virulence, inoculation of flowers of RCB plants still resulted in a high incidence of diseased siliques and infected seeds. This may partly have been due to a conversion to the wild type strain in conjunction with the loss of the plasmid during colonization of siliques and seeds, which involved a period of 3–4 months.
It can be assumed that the risks for seed infections and the incidence of seed infection depends on the inoculum density after inoculation of flowers, as was found for Xanthomonas euvesicatoria on pepper (Dutta et al. 2014b). In our experiment relatively high densities of 108 or 107 cfu mL−1 were used. In practice, deposition of such high inoculum densities on flowers is unlikely. The minimum inoculum load on flowers able to cause seed infections needs still to be determined for Xcc in Brassicas. Nevertheless, for X. euvesicatoria it was found that inoculation with populations of bacteria as low as 10 cfu per blossom could result in seed infections.
Results indicated the potential risks of flower infections in a Brassica seed production crop. Therefore, besides other measures as outlined by Williams (2007), in a seed production crop treatment of flowers with biocides or biocontrol agents should be considered to control the disease. In other pathosystems whereby the role of flower infections were evident, these measures have been successfully applied, such as for Acidovorax avenae subsp. citrulli in water melon and Erwinia amylovora in pear (Fessehaie and Walcott 2005; Norelli et al. 2003).
References
Bailey, C. D., Koch, M. A., Mayer, M., Mummenhoff, K., O’Kane, S. L., Jr., Warwick, S. I., Windham, M. D., & Al-Shehbaz, I. A. (2006). Toward a global phylogeny of the Brassicaceae. Molecular Biology and Evolution, 23, 2142–2160.
Bradbury, J. F. (1986). Guide to plant pathogenic Bacteria. Wallingford: CAB International.
Calvin, N. M., & Hanawalt, P. C. (1988). High-efficiency transformation of bacterial cells by electroporation. Journal of Bacteriology, 170, 2796–2801.
Cheng, H. P., & Walker, G. C. (1998). Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. Journal of Bacteriology, 180, 5183–5191.
Chidamba, L., & Bezuidenhout, C. C. (2012). Characterisation of Xanthomonas campestris pv. Campestris isolates from South Africa using genomic DNA fingerprinting and pathogenicity tests. European Journal of Plant Pathology, 133, 811–818.
Cook, A. A., Larson, R. H., & Walker, J. C. (1952a). Relation of the black rot pathogen to cabbage seed. Phytopathology, 42, 316–320.
Cook, A. A., Walker, J. C., & Larson, R. H. (1952b). Studies on the disease cycle of black rot of crucifers. Phytopathology, 42, 162–167.
Dane, F., & Shaw, J. J. (1996). Survival and persistence of bioluminescent Xanthomonas campestris pv campestris on host and non-host plants in the field environment. Journal of Applied Bacteriology, 80, 73–80.
Darsonval, A., Darrasse, A., Meyer, D., Demarty, M., Durand, K., Bureau, C., Manceau, C., & Jacques, M. A. (2008). The type III secretion system of Xanthomonas fuscans subsp fuscans is involved in the phyllosphere colonization process and in transmission to seeds of susceptible beans. Applied and Environmental Microbiology, 74, 2669–2678.
De Boer, S. H. (2002). Relative incidence of Erwinia carotovora subsp. atroseptica in stolon end and peridermal tissue of potato tubers in Canada. Plant Disease, 86, 960–964.
Denisow, B., Masierowska, M., & Sebastian, A. (2016). Floral nectar production and carbohydrate composition and the structure of receptacular nectaries in the invasive plant Bunias orientalis L. (Brassicaceae). Protoplasma, 253, 1489–1501.
Dutta, B., Avci, U., Hahn, M. G., & Walcott, R. R. (2012). Location of Acidovorax citrulli in infested watermelon seeds is influenced by the pathway of bacterial invasion. Phytopathology, 102, 461–468.
Dutta, B., Gitaitis, R., Sanders, H., Booth, C., Smith, S., & Langston, D. B. (2013). Role of blossom colonization in pepper seed infestation by Xanthomonas euvesicatoria. Phytopathology, 104, 232–239.
Dutta, B., Gitaitis, R., Smith, S., & Langoston, D. (2014a). Interactions of seedborne bacterial pathogens with host and non-host plants in relation to seed infestation and seedling transmission. PLoS One, 9, 1–13.
Dutta, B., Gitaitis, R., Sanders, H., Booth, C., Smith, S., & Langston, D. B. (2014b). Role of blossom colonization in pepper seed infestation by Xanthomonas euvesicatoria. Phytopathology, 104, 232–239.
Dutta, B., Schneider, R. W., Robertson, C. L., & Walcott, R. R. (2016). Embryo localization enhances the survival of Acidovorax citrulli in watermelon seeds. Phytopathology, 106, 330–338.
Errampalli, D., Leung, K., Cassidy, M. B., et al. (1999). Applications of the green fluorescent protein as a molecular marker in environmental microorganisms. Journal of Microbiological Methods, 35, 187–199.
Fessehaie, A., & Walcott, R. R. (2005). Biological control to protect watermelon blossoms and seed from infection by Acidovorax avenae subsp citrulli. Phytopathology, 95, 413–419.
Füchslin, H. P., Rüegg, I., Van Der Meer, J. R., & Egli, T. (2003). Effect of integration of a GFP reporter gene on fitness of Ralstonia eutropha during growth with 2,4-dichlorophenoxyacetic acid. Environmental Microbiology, 5, 878–887.
Gage, D. J., Bobo, T., & Long, S. R. (1996). Use of green fluorescent protein to visualize the early events of symbiosis between Rhizobium meliloti and alfalfa (Medicago sativa). Journal of Bacteriology, 178, 7159–7166.
Gitaitis, R., & Walcott, R. (2007). The epidemiology and management of seedborne bacterial diseases. Annual Review of Phytopathology, 45, 371–397.
Guy, E., Genissel, A., Hajri, A., et al. (2013). Natural genetic variation of Xanthomonas campestris pv. campestris pathogenicity on Arabidopsis revealed by association and reverse genetics. mBio, 4, e00538–e00512.
Ignatov, A., Hida, K., & Kuginuki, Y. (1998). Black rot of crucifers and sources of resistance in Brassica crops. Jarq-Japan Agricultural. Research Quarterly, 32, 167–172.
Ignatov, A., Hida, K., & Kuginuki, Y. (1999a). Environmentally dependent change of disease symptoms caused by Xanthomonas campestris pv. campestris on brassicas. Acta Phytopathologica et Entomologica Hungarica, 34, 183–186.
Ignatov, A., Kuginuki, Y., & Hida, K. (1999b). Vascular stem resistance to black rot in Brassica oleracea. Canadian Journal of Botany-Revue Canadienne De Botanique, 77, 442–446.
Johnson, K. B., Stockwell, V. O., Burgett, D. M., Sugar, D., & Loper, J. E. (1993). Dispersal of Erwinia amylovora and Pseudomonas fluorescens by honey bees from hives to apple and pear blossoms. Phytopathology, 83, 478–484.
Kastelein, P., Krijger, M. C., van der Zouwen, P. S., van der Steen, J. J. M., Stevens, L. H., van der Wolf, J. M., Fernandes Vieira, J., & Amaral Villela, F. (2014). Transmission of Xanthomonas campestris pv. campestris in seed production crops of cauliflower. Acta Horticulturae, (1041), 197–204.
Köhl, J., Vlaswinkel, M., Groenenboom-de Haas, B. H., Kastelein, P., Van Hoof, R. A., Van der Wolf, J. M., & Krijger, M. (2011). Survival of pathogens of Brussels sprouts (Brassica oleracea Gemmifera group) in crop residues. Plant Pathology, 60, 661–670.
Krauthausen, H.-J., Hörner, G., Zimmermann, S., Voegele, R. T., & Brändle, F. (2018). Competence of Xanthomonas campestris from cruciferous weeds and wallflower (Erysimum cheiri) to induce black rot in cabbage. European Journal of Plant Pathology, 151, 275–289.
Kuan, T. L., Minsavage, G. V., & Schaad, N. W. (1986). Aerial dispersal of Xanthomonas campestris pv. campestris from naturally infected Brassica campestris. Plant Disease, 70, 409–413.
Lawrence Pusey, P., Rudell, D. R., Curry, E. A., & Mattheis, J. P. (2008). Characterization of stigma exudates in aqueous extracts from apple and pear flowers. Hortscience, 43, 1471–1478.
Major, D. J. (1975). Stomatal frequency and distribution in rape. Canadian Journal of Plant Science, 55, 1077–1078.
Massomo, S. M. S., Mortensen, C. N., Mabagala, R. B., Newman, M. A., & Hockenhull, J. (2004). Biological control of black rot (Xanthomonas campestris pv. campestris) of cabbage in Tanzania with Bacillus strains. Journal of Phytopathology, 152, 98–105.
Nega, E., Ulrich, R., Werner, S., & Jahn, M. (2003). Hot water treatment of vegetable seed - an alternative seed treatment method to control seed-borne pathogens in organic farming. Journal of Plant Diseases and Protection, 136, 220–234.
Norelli, J. L., Jones, A. L., & Aldwinckle, H. S. (2003). Fire blight management in the twenty-first century -using new technologies that enhance host resistance in apple. Plant Disease, 87, 756–765.
Polowick, P. L., & Sawhney, V. K. (1986). A scanning electron microscopic study on the initiation and development of floral organs of Brassica napus (cv. Westar). American Journal of Botany, 73, 254–263.
Poplawsky, A. R., & Chun, W. (1998). Xanthomonas campestris pv. campestris requires a functional pigB for epiphytic survival and host infection. Molecular Plant-Microbe Interactions, 11, 466–475.
Randhawa, P. S., & Schaad, N. W. (1984). Selective isolation of Xanthomonas campestris pv. campestris from crucifer seeds. Phytopathology, 74, 268–272.
Roberts, S. J., Hiltunen, L. H., Hunter, P. J., & Brough, J. (1999). Transmission from seed to seedling and secondary spread of Xanthomonas campestris pv. campestris in Brassica transplants: Effects of dose and watering regime. European Journal of Plant Pathology, 105, 879–889.
Roberts, S. J., Brough, J., & Hunter, P. J. (2007). Modelling the spread of Xanthomonas campestris pv. campestris in module-raised brassica transplants. Plant Pathology, 56, 391–401.
Schaad, N. W. (1983). Detection of Xanthomonas campestris pv. campestris in crucifer seeds. Seed Science and Technology, 11, 573–578.
Schaad, N. W., & Alvarez, A. (1993). Xanthomonas campestris pv. campestris: Cause of black rot of crucifers. In J. G. Swings & E. L. Civerolo (Eds.), Xanthomonas (pp. 51–55). London: Chapman & Hall.
Schaad, N. W., & Dianese, J. C. (1981). Cruciferous weeds as sources of inoculum of Xanthomonas campestris in black rot of cruciferes. Phytopathology, 71, 1215–1520.
Schaad, N. W., Sitterly, W. R., & Humaydan, H. (1980). Relationship of incidence of seedborne Xanthomonas campestris to black rot of crucifers. Plant Disease, 64, 91–92.
Shaner, G., & Finney, R. E. (1977). The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology, 67, 1051–1056.
Silva, J. C., Silva Júnior, T. A. F., Soman, J. M., Tomasini, T. D., Sartori, M. M. P., & Maringoni, A. C. (2017). Survival of Xanthomonas campestris pv. campestris in the phyllosphere and rhizosphere of weeds. Plant Pathology, 66, 1517–1526.
Stretton, S., Techkarnjanaruk, S., Mclennan, A. M., & Goodman, A. E. (1998). Use of green fluorescent protein to tag and investigate gene expression in marine bacteria. Applied and Environmental Microbiology, 64, 2554–2559.
Van der Wolf, J. M., & van der Zouwen, P. S. (2010). Colonization of cauliflower blossom (Brassica oleracea) by Xanthomonas campestris pv. campestris, via flies (Calliphora vomitoria) can result in seed infestation. Journal of Phytopathology, 158, 726–732.
Van der Wolf, J. M., van der Zouwen, P. S., & van der Heijden, L. (2013). Flower infection of Brassica oleracea with Xanthomonas campestris pv. campestris results in high levels of seed infection. European Journal of Plant Pathology, 136, 103–111.
Vicente, J. G., & Holub, E. B. (2013). Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Molecular Plant Pathology, 14, 2–18.
Walker, J.C. (1923). The hot water treatment of cabbage seed. Phytopathology, 13, 251–253.
Williams, P. H. (1980). Black rot: A continuing threat to world crucifers. Plant Disease, 64, 736–742.
Williams, P. H. (2007). Black rot. In R. S. Rimmer, V. I. Shattuk, & L. Buchwaldt (Eds.), Compendium of Brassica Diseases (pp. 60–62). St. Paul: APS Press.
Williams, P. H., & Hill, C. B. (1986). Rapid-cycling populations of Brassica. Science, 232, 1385–1139.
Acknowledgements
We are indebted to, and thank, the Dutch seed companies ENZA, Bejo, RijkZwaan and Syngenta for advice and financial support. The project was co-funded by the Dutch Ministry of Economic Affairs (BO-26.02-002-005) and the Brazilian government (Fapesp Process Number: 2012/22521-0). We thank Neima Ahmed and Beth Hyman for useful corrections and comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The corresponding author (Jan van der Wolf) has received research funding from Dutch seed growers. All other authors herewith declare that they have no conflict of interest.
This study does not contain studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van der Wolf, J., Kastelein, P., da Silva Júnior, T.A.F. et al. Colonization of siliques and seeds of rapid cycling Brassica oleracea plants by Xanthomonas campestris pv. campestris after spray-inoculation of flower clusters. Eur J Plant Pathol 154, 445–461 (2019). https://doi.org/10.1007/s10658-019-01668-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01668-4