Abstract
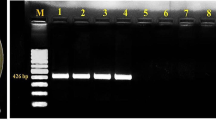
The genetic diversity of Xanthomonas campestris pv. campestris isolates from South Africa was evaluated using 28 isolates obtained from the Johannesburg Fresh Produce Market. Samples were collected from cabbage supplies from farms in Gauteng, Mpumalanga and North West Provinces. Strains were isolated from small sections of infected cabbage leaf samples and cultured on Yeast Dextrose Agar. Isolates identity was confirmed by ELISA and Pathogenicity test. Pathogenicity tests were performed by inoculating leaves of known susceptible cabbage seedlings. Infection symptoms induced could be categorized into three groups, ranging from typical to non-typical black rot symptoms. Four differential Brassica cultivars with known avirulence genes were used for race typing done by spray inoculation. Four races, namely 1, 3, 4 and 6, were identified. Of the 28 isolates, four were identified as race 1, two as race 3, 19 as race 4 and three as race 6. Repetitive DNA polymerase chain reaction-based fingerprinting using Eric- and Box-primers was used to assess the genetic diversity. Generated fingerprints of X. c pv. campestris were relatively similar. Cluster analysis could not strictly group isolates by their geographical origin, suggesting limited diversity of Xanthomonas campestris pv. campestris strains within cabbage producing regions in South Africa.




Similar content being viewed by others
References
Alvarez, A. (2000). Black rot of crucifers. In A. Slusarenko, R. S. S. Fraser, & L. C. van Loon (Eds.), Mechanisms of resistance to plant diseases (pp. 21–52). Dordrecht: Kluwer Academic Publishers.
Alvarez, A. M., Benedict, A. A., Mizumoto, C. Y., Hunter, J. E., & Gabriel, D. W. (1994). Serological, pathological, and genetic diversity among strains of Xanthomonas campestris infecting crucifers. Phytopathology, 84, 1449–1457.
Chidamba, L. (2011). Characterisation of Xanthomonas campestris pv. campestris isolates from South Africa using genomic DNA fingerprinting and pathogenicity tests. Masters Dissertation, North-West University, South Africa. 50p
de Bruijn, F. J. (1992). Use of repetitive (repetitive estrogenic palindromic and enterobacterial repetitive intergenic consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Applied and Environmental Microbiology, 58, 2180–2187.
Franken, A.J.M. (1992). Immunofluorescence microscopy and dilution plating for the detection of X. c pv. Campestris in crucifer seeds. Methods to determine seed health and seed infection. Ph.D. Thesis. Wageningen Agricultural University.
Ignatov, A., Kuginuki, Y., & Hida, K. (1998). Race-specific reaction of resistance to black rot in Brassica oleracea. European Journal of Plant Pathology, 104, 821–827.
Kamoun, S., Kamdar, H. V., Tola, E., & Kado, C. I. (1992). Incompatible interactions between crucifers and Xanthomonas campestris involve a vascular hypersensitive response: Role of the hrp X locus. Molecular Plant-Microbe Interactions, 5, 22–23.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Lelliot, R. A., & Stead, D. E. (1987). Methods for the diagnosis of bacterial diseases of plants. New York: Blackwell Scientific Publications.
Massomo, S. M. S., Hanne, N., Mansfield-Giese, K., Mabagala, R. B., Hockenhull, J., & Mortensen, C. N. (2003). Identification and characterisation of Xanthomonas campestris pv. campestris from Tanzania by pathogenicity tests, Biology, Eric- and Box-PCR and fatty acid methyl ester analysis. European Journal of Plant Pathology, 109, 775–789.
Pierce, L., Schroth, M. N., & McCain, A. H. (1990). Viscosity test for preliminary identification of strains of Xanthomonas campestris. Plant Disease, 74, 646–647.
Schaad, N. W., & Alvarez, A. (1993). Xanthomonas campestris pv. campestris cause of black rot of crucifers. In J. G. Swings & E. L. Civerolo (Eds.), Xanthomonas (pp. 51–55). London, UK: Chapman and Hall.
Schaad, N. W., Jones, J. B., & Lacy, G. H. (2001). Xanthomonas. Laboratory guide for identification of plant-pathogenic bacteria. St. Paul: American Phytopathological Society Press.
Scortichini, M., & Rossi, M. P. (2003). Genetic diversity of Xanthomonas arboricola pv. fragariae strains and comparison with some other X. arboricola pathovars using repetitive PCR genomic fingerprinting. Journal of Phytopathology, 151, 113–119.
Taylor, J. D., Conway, J., Roberts, S. J., Astley, D., & Vicente, J. G. (2002). Sources and origin of resistance to Xanthomonas campestris pv. campestris in Brassica genomes. Phytopathology, 92, 105–111.
Versalovic, J., Schneider, M., de Bruijn, F. J., & Lupski, J. R. (1994). Genomic fingerprinting of bacteria using repetitive sequence based polymerase chain reaction. Methods in Molecular and Cellular Biology, 5, 25–40.
Vicente, J. G. (2004). The black rot of crucifers. Portugal: Operations Center and National Horticultural Technology.
Vicente, J. G., Conway, J., Roberts, S. J., & Taylor, J. D. (2001). Identification and origin of Xanthomonas campestris pv. campestris races and related pathovars. Journal of Phytopathology, 91, 492–499.
Ward, J. H. (1963). Hierarchical grouping to optimise an objective function. Journal of the American Statistics Association, 58, 236–244.
Williams, P. H., Staub, T., & Sutton, J. C. (1972). Inheritance of resistance in cabbage to black rot. Phytopathology, 62, 247–252.
Williams, P. H. (1980). Black rot: A continuing threat to world crucifers. Plant Disease, 64, 736–745.
Acknowledgments
The authors would like to thank Nickerson-Zwaan Seed Company (Netherlands) for financial support, Agricultural Research Council of South Africa (Grain crops institute) for green house facilities, Deidre Fourie and Barend Greyling for assisting with the greenhouse studies, Walter de Millano for constructive discussions and suggestions as well as Maaike McIntyre for generating the map.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chidamba, L., Bezuidenhout, C.C. Characterisation of Xanthomonas campestris pv. campestris isolates from South Africa using genomic DNA fingerprinting and pathogenicity tests. Eur J Plant Pathol 133, 811–818 (2012). https://doi.org/10.1007/s10658-012-0002-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-0002-9