Abstract
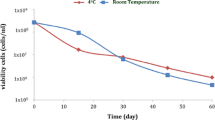
Entomopathogenic nematodes in the genus Steinernema are associated with Xenorhabdus spp. bacteria. When steinernematid colonise an insect host the nematode-bacterium association overcomes the insect immune system and kills the host within 48 h. Xenorhabdus spp. produce secondary metabolites that are antifungal to protect nematode-infected cadavers from fungal colonization. The concentrated, or cell-free metabolites of X. szentirmaii exhibit high toxicity against various fungal plant pathogens and show potential as natural bio-fungicides. In the current study, we determined 1) thermo-stability, 2) dose-response, and 3) shelf-life of antifungal metabolites of X. szentirmaii against Monilinia fructicola (cause of brown rot of peach and other stone fruit) and Glomerella cingulata (cause of antharacnose). Thermo-stability was determined by autoclaving bacterial culture broths (121 °C and 15 psi for 15 min) and measuring fungal growth on in potato dextrose agar (PDA) containing 10% of the supernatants. Autoclaving had no impact on the antifungal activity of the secondary metabolites. Over a test period of 9 months, the activity of both extract types did not decline when stored at 4 or 20 °C. A dose-response study (10, 20, 40, 60, 80 and 100% supernatant-containing metabolite) using both phytopathogens demonstrated that a greater dose of supernatant increased antifungal activity. The antifungal-metabolite containing supernatant of X. szentirmaii has potential as a bio-fungicide. These results demonstrate the metabolite(s) are thermo-stable, they have a long shelf-life and require no stabilizing formulation, even at room temperature.





Similar content being viewed by others
References
Akhurst, R. J. (1980). Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. Journal of General Microbiology, 121, 303–309.
Bock, C. H., Shapiro-Ilan, D. I., Wedge, D., & Cantrell, C. H. (2014). Identification of the antifungal compound, trans-cinnamic acid, produced by Photorhabdus luminescens, a potential biopesticide. Journal of Pest Science, 87, 155–162.
Bode, H. B. (2009). Entomopathogenic bacteria as a source of secondary metabolites. Current Opinion in Chemical Biololgy, 13, 1–7.
Boemare, N. E., & Akhurst, R. J. (2006). The genera Photorhabdus and Xenorhabdus. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, & E. Stackebrandt (Eds.), The prokaryotes (pp. 451–494). New York: Springer Science + Business Media Inc..
Boszormenyi, E., Ersek, T., Fodor, A., Fodor, A. M., Foldes, L. S., Hevesi, M., Hogan, J. S., Katona, Z., Klein, M. G., Kormany, A., Pekar, S., Szentirmai, A., Sztaricskai, F., & Taylor, R. A. J. (2009). Isolation and activity of Xenorhabdus antimicrobi- al compounds against the plant pathogens Erwinia amylovora and Phytophthora nicotianae. Journal of Applied Microbiology, 107, 764–759.
Brachmann, A. O., Forst, S., Furgani, G. M., Fodor, A., & Bode, H. B. (2006). Xenofuranones a and B: Phenylpyruvate dimers from Xenorhabdus szentirmaii. Journal of Natural Products, 69, 1830–1832.
Chen, G., Dunphy, G. B., & Webster, J. M. (1994). Antifungal activity of two Xenorhabdus species and Photorhabdus luminescens, bacteria associated with the nematodes Steinernema species and Heterorhabditis megidis. Biological Control, 4(2), 157–162.
Dowds, B. C. A., & Peters, A. (2002). Virulence mechanisms. In R. Gaugler (Ed.), Entomopathogenic nematology (pp. 79–98). CABI Publishing: New York.
Fang, X. L., Li, Z. Z., Wang, Y. H., & Zhang, X. (2011). In vitro and in vivo antimicrobial activity of Xenorhabdus bovienii YL002 against Phytophthora capsici and Botrytis cinerea. Journal of Applied Microbiology, 111(1), 145–154.
Fang, X., Zhang, M., Tang, Q., Wang, Y., & Zhang, X. (2014). Inhibitory effect of Xenorhabdus nematophila TB on plant pathogens Phytophthora capsici and Botrytis cinerea in vitro and in planta. Scientific Reports, 4, 1–7.
Glare, T. R., Jurat-Fuantes, J. L., & O’Callaghan, M. (2017). Basic and applied research: Entomopathogenic bacteria. In L. A. Lacey (Ed.), Microbial control of insect and mite pests (pp. 47–67). San Diego: Academic press.
Hazir, S., Kaya, H. K., Stock, S. P., & Keskin, N. (2003). Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biocontrol of soil pests. Turkish Journal of Biology, 27, 181–202.
Hazir, S., Shapiro-Ilan, D. I., Bock, C. H., Hazir, C., Leite, L. G., & Hotchkiss, M. W. (2016). Relative potency of culture supernatants of Xenorhabdus and Photorhabdus spp. on growth of some fungal phytopathogens. European Journal of Plant Pathology, 146, 369–381.
Hazir, S., Shapiro-Ilan, D. I., Bock, C. H., & Leite, L. G. (2017). trans-Cinnamic acid and Xenorhabdus szentirmaii metabolites synergize the potency of some commercial fungicides. Journal of Invertebrate Pathology (in press).
Hinchliffe, S. J., Hares, M. C., Dowling, A. J., & Ffrench-Constant, R. H. (2010). Insecticidal toxins from the Photorhabdus and Xenorhabdus bacteria. Open Toxicology Journal (special issue), 3, 83–100.
Houard, J., Aumelas, A., Noel, T., Pages, S., Givaudan, A., Fitton-Ouhabi, V., Villain-Guillot, P., & Gualtieri, M. (2013). Cabanillasin, a new antifungal metabolite, produced by en- tomopathogenic Xenorhabdus cabanillasii JM26. Journal of Antibiotics, 66, 617–620.
Hu, K., Li, J., Li, B., Webster, J. M., & Chen, G. (2006). A novel antimicrobial epoxide isolated from larval Galleria mellonella infected by the nematode symbiont, Photorhabdus luminescens (Enterobacteriaceae). Bioorganic & Medicinal Chemistry, 14, 4677–4681.
Kaya, H. K., & Stock, S. P. (1997). Techniques in nematology. In L. A. Lacey (Ed.), Manual of techniques in insect pathology (pp. 281–324). San Diego: Academic Press.
Lacey, L. A., Grzywacz, D., Shapiro-Ilan, D. I., Frutos, R., Brownbridge, M., & Goettel, M. S. (2015). Insect pathogens as biological control agents: Back to the future. Journal of Invertebrate Pathology, 132, 1–41.
Lewis, E. E., & Clarke, D. J. (2012). Nematode parasites and entomopathogens. In F. E. Vega & H. K. Kaya (Eds.), Insect pathology (2nd ed., pp. 395–424). Amsterdam: Elsevier.
Lewis, E. E., Hazir, S., Hodson, A., & Gulcu, B. (2015). Trophic relationships of entomopathogenic nematodes in agricultural habitats. In R. Campos-Herrera (Ed.), Nematode pathogenesis of insects and other pests (pp. 137–163). Switzerland: Springer International Pulishing.
Maxwell, P. W., Chen, G., Webster, J. M., & Dunphy, G. B. (1994). Stability and activities of antibiotics produced during infection of the insect Galleria mellonella by two isolates of Xenorhabdus nematophilus. Applied and Environmental Microbiology, 60, 715–721.
San-Blas, E., Carrillo, Z., & Parra, Y. (2012). Effect of Xenorhabdus and Photorhabdus bacteria and their exudates on Moniliophthora roreri. Archives of Phytopathology and Plant Protection, 45, 1950–1967.
SAS. (2002). SAS software: Version 9.1. Cary: SAS Institute.
Selvan, S., Gaugler, R., & Lewis, E. E. (1993). Biochemical energy reserves of entomopathogenic nematodes. Journal of Parasitology, 79, 167–172.
Shapiro-Ilan, D. I., Reilly, C. C., & Hotchkiss, M. W. (2009). Suppressive effects of metabolites from Photorhabdus and Xenorhabdus spp. on phytopathogens of peach and pecan. Archives of Phytopathology and Plant Protection, 42, 715–728.
Shapiro-Ilan, D. I., Bock, C. H., & Hotchkiss, M. W. (2014). Suppression of pecan and peach pathogens on different substrates using Xenorhabdus bovienii and Photorhabdus luminescens. Biological Control, 77, 1–6.
Shapiro-Ilan, D. I., Cottrell, T. E., Mizell III, R. F., Horton, D. L., & Abdo, Z. (2015). Field suppression of the peachtree borer, Synanthedon exitiosa, using Steinernema carpocapsae: Effects of irrigation, a sprayable gel and application method. Biological Control, 82, 7–12.
Shapiro-Ilan, D. I., Hazir, S., & Glazer, I. (2017). Basic and applied research: Entomopathogenic nematodes. In L. A. Lacey (Ed.), Microbial agents for control of insect pests: From discovery to commercial development and use (pp. 91–105). San Diego: Academic Press.
Solter, L. F., Hajek, A. E., & Lacey, L. A. (2017). Exploration for entomopathogens. In L. A. Lacey (Ed.), Microbial control of insect and mite pests (pp. 13–26). San Diego: Academic press.
Southwood, T. R. E. (1978). Ecological methods: With particular reference to the study of insect populations. London: Chapman and Hall.
Steel, R. G. D., & Torrie, J. H. (1980). Principles and procedures of statistics. New York, NY: McGraw-Hill Book Company.
Webster, J. M., Chen, G., Hu, K., & Li, J. (2002). Bacterial metabolites. In R. Gaugler (Ed.), Entomopathogenic nematology (pp. 99–114). London: CABI International.
Acknowledgements
We thank to Technical and Research Council of Turkey (TUBITAK) for supporting S. Hazir within the 2219 fellowship program. D. I. Shapiro-Ilan and C.H. Bock acknowledge the research was partially supported by the USDA National Programs for Crop Protection & Quarantine, and Crop Production (Project Numbers: 6042-22000-023-00D and 6042-21220-012-00D, respectively). We also thank Stacy Byrd, Amy Euston, Benjamin Graham, Domonique Roth-Alday, Unicka Stokes, Wanda Evans, Amy Eubanks and Seth Richards for their valuable technical assistance. This article reports the results of research only. Mention of a proprietary product does not constitute an endorsement or recommendation by the USDA for its use.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving animal and human rights
The research did not involve human participants and/or animals.
Rights and permissions
About this article
Cite this article
Hazir, S., Shapiro-Ilan, D.I., Bock, C.H. et al. Thermo-stability, dose effects and shelf-life of antifungal metabolite-containing supernatants produced by Xenorhabdus szentirmaii . Eur J Plant Pathol 150, 297–306 (2018). https://doi.org/10.1007/s10658-017-1277-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1277-7