Abstract
Soft rot and blackleg can cause severe economic losses in potato production in South Africa and Zimbabwe depending on climatic conditions. The aim of the study was to identify the predominant bacteria causing potato soft rot and blackleg in these countries. Samples, comprising of stems and tubers from potato plants with blackleg and soft rot symptoms were collected from 2006–2009 from potato production areas where disease outbreaks occurred. The isolates from these plants and tubers yielded Gram negative, pectinolytic bacteria on crystal violet pectate and inoculated tubers. Identification was based on biochemical and phenotypic characteristics, rep-PCR, Amplified Fragment Length Polymorphisms and sequences of gyrB and recA genes. Isolates from Zimbabwe were identified as Pectobacterium carotovorum subsp. brasiliensis (Pcb) (21 isolates), Dickeya dadantii subsp. dadantii (Dd) (20 isolates), P. c. subsp. carotovorum (Pcc) (16 isolates) and P. atrosepticum (Pa) (4 isolates). Pcb, Pcc and Dd subsp. dadantii were isolated from samples collected from all the regions, while Pa was isolated from Nyanga the coolest region in Zimbabwe. In South Africa, however, Pcb was the most common causal agent of soft rot and blackleg. P. atrosepticum was the only pathogen isolated from samples collected in Nyanga, Zimbabwe, and was not isolated from any South African samples. AFLP analysis separated the Pcb strains into 12 clusters, reflecting subdivision in terms of geographic origin, and Pcc isolates were clearly differentiated from Pcb isolates. A large degree of DNA polymorphism was evident among these 12 clusters. The study identified all the pathogens associated with the blackleg/soft rot disease complex.




Similar content being viewed by others
References
Avrova, A. O., Hyman, L. J., Toth, R. L., & Toth, I. K. (2002). Application of amplified fragment length polymorphism fingerprinting for taxonomy and identification of the soft rot bacteria Erwinia carotovora and Erwinia chrysanthemi. Applied and Environmental Microbiology, 68, 1499–1508.
Brady, C. L., Cleenwerck, I., Venter, S. N., Vancanneyt, M., Swings, J., & Coutinho, T. A. (2008). Phylogeny and identification of Pantoea species associated with the environment, humans and plants based on multilocus sequence analysis (MLSA). Systematic and Applied Microbiology, 31, 447–460.
Cother, E. J., & Sivasithamparam, K. (1983). Erwinia: the “carotovora” group. In P. C. Fahy & G. J. Persley (Eds.), Plant bacterial diseases. A diagnostic guide (pp. 87–101). Sydney: Academic.
Czajkowski, R., Grabe, G. J., & Van der Wolf, J. M. (2009). Distribution of Dickeya spp. and Pectobacterium carotovorum subsp. carotovorum in naturally infected seed potatoes. European Journal of Plant Pathology, 125, 263–75.
Czajkowski, R., Pérombelon, M. C. M., van Veen, J. A., & van der Wolf, J. M. (2011). Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathology, 60, 999–1013.
Darrasse, A., Priou, S., Kotoujansky, A., & Bertheau, Y. (1994). PCR and restriction fragment length polymorphism of a pel gene as a tool to identify Erwinia carotovora in relation to potato diseases. Applied and Environmental Microbiology, 60, 1437–1447.
De Boer, S. H. (2003). Characterisation of pectolytic erwinias as highly sophisticated pathogens of plants. European Journal of Plant Pathology, 109, 893–899.
De Boer, S. H., & McNaughton, M. E. (1987). Monoclonal antibodies to the lipopolysaccharide of Erwinia carotovora subsp. atroseptica serogroup-1. Phytopathology, 77, 828–832.
De Boer, S. H., & Ward, L. J. (1995). PCR detection of Erwinia carotovora subsp. atroseptica associated with potato tissue. Phytopathology, 85, 854–858.
De Haan, E. G., Dekker-Nooren, T. C. E. M., Van den Bovenkamp, G. W., Speksnijder, A. G. C. L., van der Zouwen, P. S., & Van der Wolf, J. M. (2008). Pectobacterium carotovorum subsp. carotovorum can cause potato blackleg in temperate climates. European Journal of Plant Pathology, 122, 561–568.
Duarte, V., De Boer, S. H., Ward, L. J., & De Oliveira, A. M. R. (2004). Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil. Journal of Applied Microbiology, 96, 535–5.
Gallois, A., Samson, R., Ageron, E., & Grimont, P. A. D. (1992). Erwinia carotovora subsp. odorifera subsp. nov associated with odorous soft rot of chicory (Gichorium intybus L). International Journal of Systematic Bacteriology, 4, 582–588.
Gardan, L., Gouy, C., Christen, R., & Samson, R. (2003). Elevation of three subspecies of Pectobacterium carotovora to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. International Journal of Systematic and Evolutionary Microbiology, 53, 381–391.
Hauben, L., Moore, E. R., Vauterin, L., Steenackers, M., Mergaert, J., Verdonck, L., & Swings, J. (1998). Phylogenetic position phytopathogens within Enterobacteriaceae. Systematic and Applied Microbiology, 21, 384–397.
Hèlias, V., Le Roux, A. C., Bertheau, Y., Andrivon, D., Gauthier, J. P., & Jouan, B. (1998). Characterization of Erwinia carotovora subspecies and detection of Erwinia carotovora subspecies atroseptica in potato plants, soil and water extracts with PCR-based methods. European Journal of Plant Pathology, 104, 685–699.
Hyman, L. J., Sullivan, L., Toth, I. K., & Pérombelon, M. C. M. (2001). Modified crystal violet pectate medium (CVP) based on a new polypectate source (Slendid) for the detection and isolation of soft rot erwinias. Potato Research, 44, 265–270.
Kang, H. W., Kwon, S. W., & Go, S. J. (2003). PCR-based specific and sensitive detection of Pectobacterium carotovorum ssp. carotovorum by primers generated from a URP-PCR fingerprinting-derived polymorphic band. Plant Pathology, 52, 127–133.
Keim, P., Kalif, A., Schupp, J., Hill, K., Travis, S. E., Richmond, K., Adair, D. M., Hugh-Jones, M., Kuske, C. R., & Jackson, P. (1997). Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. Journal of Bacteriology, 179, 818–824.
Kwon, S., Go, S., Kang, H., Ryu, J., & Jo, J. (1997). Phylogenetic analysis of Erwinia species based on 16S rRNA gene sequences. International Journal of Systematic Bacteriology, 47, 1061–1067.
Laurila, J., Ahola, V., Lehtinen, A., Joutsjoki, T., Hannukkala, A., Rahkonen, A., & Pirhonen, M. (2008). Characterization of Dickeya strains isolated from potato and river water samples in Finland. European Journal of Plant Pathology, 122, 213–225.
Lelliot, R. A. and Dickey, R. S. (1984). Genus VII. Erwinia Winslow, Broadhurst, Buchanan, Krumwiede, Rogers and Smith 1920. (In N. R. Krieg and J. G. Holt Baltimore (Eds) Bergey’s Manual of Systematic Bacteriology, 1, 469–476
Manzira, C. (2010). Potato production handbook. Potato Seed Association Zimbabwe. Harare: Jongwe Printers.
Masuka, A. J., Cole, D. L., & Mguni, C. (Eds.). (1998). List of plant diseases in Zimbabwe. Harare: Jongwe Printers.
Ngadze, E., Coutinho, T. A., & van der Waals, J. E. (2010). First report of soft rot of potatoes caused by Dickeya dadantii in Zimbabwe. Plant Disease, 94, 1263.
Pérombelon, M. C. M. (2002). Potato diseases caused by soft rot erwinias: an overview of pathogenesis. Plant Pathology, 51, 1–12.
Pitman, A. R., Harrow, S. A., & Visnovsky, S. B. (2010). Genetic characterisation of Pectobacterium wasabiae causing soft rot disease of potato in New Zealand. European Journal of Plant Pathology, 126, 423–435.
Rademaker, J. L. W., Louws, F. J., & de Bruijn, F. J. (2004). Characterization of the diversity of ecological important microbes by rep-PCR genomic fingerprinting. In F. J. de Bruijn, I. M. Head, A. D. Akkermans, & J. D. van Elsas (Eds.), Molecular manual (pp. 1–33). Dordrecht: Kluwer.
Samson, R., Legendre, J. B., Christen, R., Fischer-Le Saux, M., Achouak, W. and Gardan, L. (2005). Transfer of Pectobacterium chrysanthemi (Burkholder et al., 1953) Brenner et al., 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. known as Dickeya chrysanthemi comb. nov and Dickeya paradisiaca combi. nov. and delineation of four novel species, Dickeya dadantii sp nov., Dickeya dianthicola sp. nov., Dickeya diefferenbachiae sp. nov. and Dickeya zeae sp. nov. International Journal of Systematic and Evolutionary Microbiology, 55, 1415–1427
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Toth, I. K., Avrova, A. O., & Hyman, L. J. (2001). Rapid identification and differentiation of the soft rot Erwinias by 16S-23S intergenic transcribed spacer and restriction fragment length polymorphism analysis. Applied and Environmental Microbiology, 67, 4070–4076.
Toth, I. K., Bell, K. S., Holeva, M. C., & Birch, P. R. J. (2003). Soft rot Erwinia: from genes to genomes. Molecular Plant Pathology, 4, 17–30.
Toth, I. K., van der Wolf, J. M., Saddler, G., Lojkowska, E., Hèlias, V., Pirhonen, M., Tsror, L., & Elphinstone, J. G. (2011). Dickeya species: an emerging problem for potato production in Europe. Plant Pathology, 60, 385–399.
Tsror, L., Erlich, O., Hazanovsky, M., Ben Daniel, B., Zig, U., & Lebiush, S. (2012). Detection of Dickeya spp. latent infection in potato seed tubers using PCR or ELISA and correlation with disease incidence in commercial field crops under hot-climate conditions. Plant Pathology, 61, 161–168.
Van der Merwe, J. J., Coutinho, T. A., Korsten, L., & van der Waals, J. E. (2010). Pectobacterium carotovorum subsp. brasiliensis causing blackleg on potatoes in South Africa. European Journal of Plant Pathology, 126, 175–185.
Versalovic, J., Koeuth, T., & Lupski, R. (1991). Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Research, 19, 6823–6831.
Wegener, C. B. (2002). Induction of defense responses against Erwinia soft rot by an endogenous pectate lyase in potatoes. Physiological and Molecular Plant Pathology, 60, 91–100.
Wright, P. J. (1998). A soft rot of Calla (Zanthedeschia spp.) caused by Erwinia carotovora subspecies carotovora. New Zealand Journal of Crop and Horticultural Science, 26, 331–334.
Young, J. M., & Park, D.-C. (2007). Relationship of plant pathogenic enterobacteria based on atpD, carA and recA and individual and as concatenated nucleotide and peptide sequences. Systematic and Applied Microbiology, 30, 343–354.
Young, J. M., Takikawa, Y., Gardan, L., & Stead, D. E. (1992). Changing concepts in the taxonomy of plant pathogenic bacteria. Annual Review of Phytopathology, 30, 67–105.
Acknowledgements
This work was funded by Potatoes South Africa and the National Research Foundation of South Africa. The authors would like to acknowledge the help of Mr Charles Wairuri in sequence alignment and drawing of phylogenetic trees.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
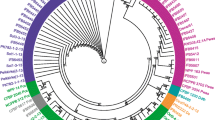
Phylogenetic tree based on recA gene sequence showing the phylogenetic relationship among Zimbabwean strains, D. dadantii subsp. dadantii and different Pectobacterium spp. The phylogram was produced by the neighbour-joining programme (Tamura et al. 2007). The numbers on the branches indicate bootstrap value support based on neighbour-joining analyses of 1000 bootstrap replication. Accession numbers of reference strains in GenBank are in parenthesis. T, type strain (PDF 32 kb)
Rights and permissions
About this article
Cite this article
Ngadze, E., Brady, C.L., Coutinho, T.A. et al. Pectinolytic bacteria associated with potato soft rot and blackleg in South Africa and Zimbabwe. Eur J Plant Pathol 134, 533–549 (2012). https://doi.org/10.1007/s10658-012-0036-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-0036-z