Abstract
The use of primary care electronic health records for research is abundant. The benefits gained from utilising such records lies in their size, longitudinal data collection and data quality. However, the use of such data to undertake high quality epidemiological studies, can lead to significant challenges particularly in dealing with misclassification, variation in coding and the significant effort required to pre-process the data in a meaningful format for statistical analysis. In this paper, we describe a methodology to aid with the extraction and processing of such databases, delivered by a novel software programme; the “Data extraction for epidemiological research” (DExtER). The basis of DExtER relies on principles of extract, transform and load processes. The tool initially provides the ability for the healthcare dataset to be extracted, then transformed in a format whereby data is normalised, converted and reformatted. DExtER has a user interface designed to obtain data extracts specific to each research question and observational study design. There are facilities to input the requirements for; eligible study period, definition of exposed and unexposed groups, outcome measures and important baseline covariates. To date the tool has been utilised and validated in a multitude of settings. There have been over 35 peer-reviewed publications using the tool, and DExtER has been implemented as a validated public health surveillance tool for obtaining accurate statistics on epidemiology of key morbidities. Future direction of this work will be the application of the framework to linked as well as international datasets and the development of standardised methods for conducting electronic pre-processing and extraction from datasets for research purposes.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Background
Advancements in technology and healthcare systems has enabled large-scale collection of longitudinal electronic health records [1]. In the UK, there are many primary care databases (THIN, CPRD, QResearch and ResearchOne) of anonymised patient records [2, 3]. These datasets include information on demographics, practice registration related information, prescriptions, morbidity, lifestyle factors (height, weight, blood pressure, smoking and alcohol status) immunisation and laboratory test results [4]. The volume of data held in such datasets will continue to increase [5, 6].
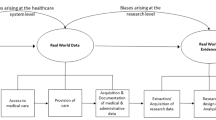
Generally, the data within primary care databases are derived from healthcare software system used to manage patient’s clinical data [7, 8]. These systems are designed for the end-user experience of helping healthcare professionals to access and manage clinical data rather than for research purposes. As such, these datasets present several challenges related to missing data, variation in definitions for diagnoses and incomplete and inadequate capturing of secondary care information [9,10,11]. However, the strength of these databases lies in their size, breadth, representativeness of the population, long-term follow-up and sufficient data quality. Primary care databases offer great research potential related to drug safety and effectiveness research, identification of disease risk factors, generation of algorithms for identification of high risk patients, evaluation of public health policies and surveillance of diseases [4, 12,13,14,15,16]. In recent years, there is an increase in trend of using such routinely available data in healthcare research [3, 17].
In research which utilises primary care databases, data pre-processing and extraction are important steps of transforming the available raw data into a format suitable for statistical analysis. The process of extraction of primary care data for research is expensive due to time, effort and expertise required [11]. Factors such as; database size, database structure, range of available data, level of detail, complexity of study designs and study variables (such as exposure, outcome, inclusion or exclusion criterion and potential confounders) makes data extraction a complex process. It requires experts with considerable clinical, scientific and technical expertise to interrogate primary care databases [18]. A sound communication and documentation process is also important between researchers and data extraction experts to reduce human induced errors, and to minimize any biases that may occur in extracted data set because of miscommunication or difference in understanding. Data suppliers may offer extraction services for a fee, or researchers may collaborate with in-house specialists available to carry out extraction. There are no standard methodologies one can follow to extract data from primary care databases. An initial extraction may be based on patient restrictions, inclusion and exclusion criteria and multiple other extractions are performed to obtain additional variables. Experts use various software to manually compile an analysable data set for each study. This non-standard and non-automatic way of data extraction is labour-intensive; adds constraints on accuracy and reproducibility of results; and has limited methods to verify the validity and integrity of the datasets generated. Some corporate as well as University bodies have made considerable progress in trying to deliver solutions to manipulating electronic health record data. The rEHR R package, and The European Health Data & Evidence Network’s (EHDEN) Observational Health Data Sciences and Informatics (OHDSI) platform ATLAS are examples of software available to support researchers in extracting data taken from primary care [19, 20]. However, these packages and tools requires substantial user manipulation, statistical background and programming expertise which clinicians may lack and/or lacks certain features such as data extraction based on particular study designs and the ability to match cohorts. Aetion is an example of an alternate corporate provider of readily analysable datasets using longitudinal healthcare data, however, the mechanism and schema of their approach has not yet been published, therefore the validity and reproducibility of their approach is currently unclear [21].
In spite of best efforts, data extraction from primary care databases poses a number of issues; technical, human dependent, non-automatic, time consuming, need for data cleaning, handling very large data sets and complex logic with no room for verification and validation of the generated data. With advances in technology, there is clear scope in improving current methods of designing studies and extracting data. In particular, considering the importance of data in medical research, it is crucial to create automated methods to extract verifiable and valid datasets to expedite research and to avoid human induced errors.
In this paper, we introduce DExtER, an extract transform load (ETL) based software framework that enables automated clinical epidemiological studies (ACES), in a reproducible and verifiable way. This system potentially allows the stakeholders to extract high quality, patient-based data from primary care databases and hence enables a large range of research possible with electronic primary care data that could be translated to other healthcare databases.
Methodology
Extract, transform and load (ETL)
ETL [22] processes are backbones of data warehousing. Application of ETL is not new in medical research [23,24,25,26]. ETL performs three distinct steps; extraction of data from source system, transformation of data and loading of data into target system.
‘Extraction’ is a simple process, in which depending on what stage the data is needed and what data is needed, tailored subset of data that is necessary for the subsequent transformation stage is extracted from specific parts of the data source. Extraction can be from multiple sources (multiple primary care databases or a combination of primary and secondary care databases) and from any form of technological infrastructure (or a combination of them) in which the data is being stored (e.g., RDBMS, NoSQL, spreadsheets and flat files).
During ‘Transformation’ a number of operations can take place e.g., conversion, filtering, reformatting, application of a number of special-purpose business rules and aggregation. It is important to identify and note some of the schema level and instance level challenges that may occur during transformation [27]. For example, as the system allows multiple data sources, conflicts in naming can occur where same name might identify different things in the data sources. Different primary care databases may use different clinical coding systems (Read codes vs ICPC: International Classification of Primary Care). Value level problems may exist such as different date formats or HbA1c being expressed in % as opposed to mmol/mol. Converting data structure and semantics of various databases into one common format, for example OMOP CMD [28], helps to solve some of these issues. The benefits of such unified data model has already been well researched [28,29,30].
Alternatively, several operations such as conversion, normalization and reformatting are accommodated in the transformation steps. Finally, in the ‘Load’ step, the resulting transformed data gets pushed into the target systems or file formats.
Flow of control and application to observational study design
Our system is based on observational (cohort, case–control, and cross-sectional) study designs. Although, the core principles of each observational studies are somewhat similar (observational in nature without introducing an intervention), they do have certain steps that do differ in terms of exposure definition, the need for controls and how they manage time. Hence the first step towards automating data extraction was to map out steps performed in these study designs. For example, a cohort study requires an exposed and unexposed group which are both followed up until a point of outcome or study end date.
Instead of creating a different ETL workflow for each study design, we identified common extraction steps and merged them while allowing necessary branches for steps unique to each design. This way we were able to create a single workflow model (Fig. 1) that is applicable for all the three study designs and understand the primary flow of control (ordered sequence of steps) that describes the route of data from the sources to the target, and intermediate transformations along the workflow. Defining this flow of control is essential for implementation and serves as the conceptual design of our novel model [31, 32] (i.e., the process of identifying the sources and the target systems, and determining the appropriate transformations).
Figure 1 also represents the ETL workflow and illustrates primary care database sources (PC DB1 and PC DB2), which could represent any electronic health record dataset. The raw data from each source propagates through different workflow stages of the system based on the study design and user input criteria. Depending on selected study design and input criteria, the extraction process may not involve all the transformation stages. For example, in a cross-sectional study, there is no control selection, outcome definition or matching required, and hence the extraction process would skip these stages. Before each transformation stage begins, an extract step will fetch tailored data from specific parts of the raw data sources based on the input criteria. After each stage of the transformation process, there are intermediate data stores that holds the transformed data until it propagates to the next stage. The intermediate data stores can be in-memory data structures (such as RAM) or can be stored in physical memory; the latter option are helpful in the case of a failure, where the whole process need not be started from the beginning. Ultimately, raw data is transformed into analysable datasets and loaded into the target system. An important process at the end of each transformation step is to document the reason why some patient records may be rejected and do not qualify for further processing. This documentation process is crucial and of immense importance, to ensure that data produced by the system is verifiable and valid, often a step which is not as easily achieved through other means. This process informs stakeholders of why patients were filtered out at various stages of data extraction and the reason for rejection. Another important feature of our model is its modular nature. Modularity of the system allows flexibility in terms of error handling, logic implementation, and provides ease to add new workflow stages (for example, a new workflow stage to clean the data set). A specific transformation stage of importance is added to encrypt the datasets using Advanced Encryption Standard (AES) 256-bit cipher as a data privacy step.
Stages of study design input using DExtER
A pre-requisite for using the system to extract data is to come up with a well-defined set of inputs, ranging from; study period, study population, study variables (exposure, outcome and covariates), requirement of controls with matching criteria and the baseline characteristics with the outcome(s) of interest. In our implementation of the system, we have built a web-based HTML UI for users to provide these inputs, and we save the input in database and supply it to the system for data extraction based on a FCFS (First Come, First Serve) queue and hence provide multiuser facilities. The primary care database we used was ‘The health improvement network’ (THIN) database [33]. We have been able to test and validate the system on the Clinical Practice Research Datalink (CPRD) GOLD [34] and CPRD Aurum [35]. Our system is applicable to any observational healthcare database which is similar in structure and semantics to CPRD, THIN or observational medical outcomes partnership (OMOP) common data model (CDM).
Stage 1: Defining study eligible period
The first stage of the system is the process of defining eligible period for the study (Table 1). This eligibility period is composed of the patient start and end date, and it is in between this time duration in which all the events of interest (exposure, outcome) take place. In this step, we define the age and sex requirements of the group (e.g. in a study about pregnant woman we usually require only females of the age group 13–50 years) and apply data quality filters (e.g. adding days to keys dates of adequate computer usage) to improve the integrity of the raw data [36, 37].
Stage 2: Defining the exposed group in cohort design or cases in case–control design
The next stage is to define the exposed/case group as the system assigns each patient an index date based on single or multiple exposure (Table 2). In this step we employ a recursive descent parser (a mathematical tool used to determine if a sequence of symbols such as sentence is syntactically correct) [38], and a regular grammar (set of rules used to define the syntax for a particular language) [39] to successfully identify any number of exposures. If the study has more than one exposure, then we propose two modes of parsing namely strict parsing and loose parsing. The former is used when the order of occurrence of each exposure is relevant to the study (for example, in one of our study the exposure was diabetic patients who were prescribed a particular medication [40]), if otherwise, the latter is selected. If the study requires unexposed group/controls, patients who do not belong to the exposed group are labelled as potential unexposed group/controls. In supplementary 1 we discuss the proposed regular grammar.
During implementation of the system we have trialled some advanced study designs such as whether the exposure considered is only incident patients compared to occasions where we have explored incidence and prevalent cases particularly where the exposure is rare [40,41,42,44]. Current work involves implementing pharmaco-epidemiolocal study designs such as new [45] and prevalent new-user designs [46].
During this stage it is also important to understand how to manage the outcome variables. If the study requires patients to be removed if an event of the outcome has occurred before the index date, then the workflow stage for determining outcome(s) is executed right after each time an index date is assigned to the patient and then patients are removed if the outcome has occurred before index date. If the study does not have any restriction on the patient based on the outcome the workflow stage for determining outcome(s) is executed before the last stage.
Stage 3 and 4: Defining the control group and matching
The control selection stage (stage 3) executes only if the controls are required for the study (Table 3). We note that unexposed/control group can still have exposure(s). Although the index date for controls depends on the clinical question and study design, in most studies the corresponding exposed patient’s index date is assigned to the control patient to mitigate immortality time bias [47].
The matching stage (stage 4) of the ETL workflow is employed where controls are required for the study and they need to be matched on specific parameters (Table 4). The matching criteria in each epidemiological study varies dependent on the study design. Parameters such as age, sex, and practice-based matching are common, some criteria such as matching disease duration (example diabetes duration), matching variable values (e.g. HbA1c or BMI) are also required at times. In our implementation of the system we have provided a range of matching criteria such as the number of unexposed/controls needed per exposed/case; should unexposed/control come from the same practice or from randomly selected practices; and what parameters they need to be matched on (e.g. age, gender and other variables described above).
We have developed the UI so that users can enter appropriate inputs for each of them. The matching stage of the workflow works in two steps. In the initial step, for each exposed/case in the study we identify and mark a list of unexposed/controls who pass the matching criteria. In the next step, we randomly (to avoid any biases) select the required number of controls for the exposed/case from the initial list and mark them as group by assigning them a unique number. Remaining unexposed/controls are unmarked and are available as potential controls for the other exposed/cases.
Stage 5: Determining outcome and defining patient exit date
This process introduces a new variable called the patient exit date (Table 5), which is by default the patient end date unless the study has an outcome in which case the patient exit date is set to the date on which the outcome occurred. This stage is also responsible to extract any outcome required for the study for all eligible patients.
Stage 6: Baseline variables and assembling an analysable dataset
The last stage of the workflow is extracting baseline variables that are required for the study (Table 6). This is a simple extract step where for each patient the required variables (e.g. BMI, glucose levels) are extracted from the raw data sources based on the input criteria (example if the latest value is required or if the earliest value is required). This stage also hosts a final Load step, which assembles all the study variables from corresponding intermediate data stores (previously extracted and transformed at different stages of the workflow) as the analysable dataset. We also propose encrypting the generated datasets to standards set by NHS to enforce data protection.
At the end of the process, we provide the reason for rejection of the discarded patients. This process is executed each time a patient is discarded. Here we employ a map data structure that maps reason for rejection to number of patients discarded for that reason. When the system encounters a patient, who is to be discarded, first it looks in the map to see whether it already contains the (key) reason for rejection which triggered the process. If present, then the value against this reason is incremented by one and patient is discarded. If the reason is not already present, then it is newly entered in the map, and the corresponding value is set to 1. This way we will be able to record all the patients who are rejected and the exact reason behind why they were rejected. Optionally, we can also log details of the patient to manually verify the rejection.
Implementation on site
We have implemented DExtER as a 3-tier web-based software system. We have built a website as the front end where stakeholders of the system can login and use the UI to submit their study design and data extraction requests which are stored in a database. The middleware of our system is the data extraction software written in Java. The middleware processes the data extraction requests on a first come, first serve basis and notifies users when the data extraction is complete. The backend of the system is Postgres RDBMS to store THIN database available to our institution.
Data protection and privacy
Data protection and privacy policies are important pre-requisites for any successful tool. Therefore, the web interface we developed for this tool are line with the University of Birmingham data regulation guidance and works within the principles set out by the Information Commissioners Office [48]. Prior to any data extraction, the study design must have gone through ethical approval. Following which, in accordance with the protocol sent for ethics, a minimum dataset is extracted. A data extraction log (audit) is created each time an extraction is attempted. Following extraction, the dataset is encrypted to AES 256 using a password supplied to the user.
Discussion
To date we have conducted a wide variety of epidemiological studies to both validate our tool and shed light on complex clinical questions. This research has been conducted as part of funded investigator led research, doctoral research and postgraduate taught course dissertations. The results of such studies have culminated in over 35 peer-reviewed publications in high impact factor general medical and specialist journals in the last 2 years, with more than 25 studies currently ongoing simultaneously. We highlight some of the studies, present comparable research elsewhere as a source of validity and discuss their clinical and public health importance below.
Utility and validity of DExtER
A summary of validation of the tool can be seen in supplementary 2. In our first study utilising the DExtER tool we explored the association between Type 1 Diabetes and subsequent risk of developing epilepsy using a cohort study design [49]. Previous literature were mainly case studies or case control in nature [49,50,52]. One study explored the association in a cohort design but the study was from a low-middle income country, where there are other confounding factors that may have resulted in the observed association. [53] In our study we identified exposed patients in THIN database with a diagnosis of Type 1 Diabetes and matched them to four unexposed patients by age, gender and general practice location. After following both sets of patients for, on average, just over 5 years, we were able to identify how many patients in each group developed epilepsy. The incidence rate (IR) of the development of epilepsy in the unexposed group was 44 per 100,000 person years. This rate is similar to published literature, as a recent systematic review described the IR to be 50/100,000 person years in developed countries globally [54] This was compared to an IR in our exposed group of 132/100,000 person years, resulting in an overall adjusted Hazard ratio (HR) of 3.01 (95% CI 1.93–4.68). This threefold increased risk was similar to cohort study from Taiwan (HR 2.84; 95% CI 1.95–4.69) [53].
We have conducted similar retrospective cohort study designs to report on outcomes including for rare diseases such as Achalasia [15] and IgA vasculitis [41]. For IgA vasculitis we identified patients with the child onset and adult onset IgA vasculitis and identified following adjustment that these patients had an increased risk of developing hypertension (Child onset: HR 1.52; 95% CI 1.22–1.89; Adult onset: HR 1.42; 95% CI 1.19–1.70) and chronic kidney disease (Child: HR 1.89; 95% CI 1.16–3.07; Adult: HR 1.54; 95% CI 1.23–1.93) [41]. With a similar study design, we identified that the diagnosis of the oesophageal condition achalasia was strongly associated with the development of oesophageal cancer and lower respiratory tract infections [15]. The findings in both of these studies are useful to clinicians as we can shed light on novel associations which are important to consider in clinical management and long-term surveillance.
These two manuscripts also highlight another application of the DExtER tool, which we have since utilised. We were also able to study yearly incidence and prevalence of these conditions using yearly cohort and cross-sectional study designs respectively. We noted that the incidence of IgA vasculitis was stable but the documented prevalence in the general population was increasing. In our Achalasia study, we were able to compare the documented IR from THIN data to that obtained from the Hospital Episodes Statistics. They were broadly similar (HES was 1.99 (95% CI 1.87–2.11) and 1.53 (95% CI 1.42–1.64) per 100 000 person-years in THIN), with observed difference potentially attributed to the differing population structure and incomplete recording of the condition in primary are settings. One of our publications on atrial fibrillation prevalence trends from 2000 to 2016 was extracted by a senior data scientist in the usual conventional manner by manually writing a programme using STATA software [55]. We compared the prevalence calculated by the data scientist to that obtained using DExtER, in the same year but using the latest version of the THIN database. Both were identical, for example both showed a prevalence of 3.3% in adults aged 35 years and older in 2016.
DExtER as a public health surveillance tool
The scope and use of automated cohort and cross-sectional designs has the potential of being an extremely important assert in Public Health settings and drug safety surveillance centres. Working with colleagues in Public Health England (PHE) we have compared the prevalence the tool generates to that reported in Quality Outcome Framework and again we found that they were similar to prevalence observed from other UK data sources. We have now implemented the tool in PHE for generating key incidence and prevalence figures that will aid with service planning and resource allocation, and for further independent evaluation. As part of the agreement with PHE, they will be independently validating the use of the tool. The tool can also be used for surveillance of beneficial and adverse effects of medications, early on after introduction of new therapeutic agents and over a long period of time for outcomes such as cancer and cardiovascular events. For example we were able to explore whether benefits from medications demonstrated in clinical trials (such as the Empagliflozin EMPA-REG trial [56]) can be replicated in real-world settings. Sodium-glucose transport protein 2 inhibitors were found to decrease mortality in patients with previous cardiovascular disease in this trial. We were able to explore this in THIN database and found that patients who were given SGLT2 Inhibitors were significantly less likely to die of any cause irrespective of baseline CVD status (adjusted IRR 0.50; 95% CI 0.33–0.75) [14]. This specific finding was replicated by another large real world evidence study (CVD-REAL HR 0.49; 95% CI 0.48–0.60 [57]).
Future directions, benefits, limitations and ethical considerations
We are now progressing the work towards automation for complex study designs through a work programme named Automated Clinical Epidemiology Studies (ACES), partly funded by Health Data Research (HDR) UK through a fellowship [58]. Additionally, through HDR UK, our team was involved in two successful digital innovation hubs (INSIGHT and PIONEER) [59]. As part of the INSIGHT hub funding, the tool is now also being adapted to include bespoke eye hospital data and provide further learning opportunities into the flexibility, benefits and limitations of the tool particularly for datasets not configured for use in observational research.
As part of the HDR fellowship work, we will apply the framework for pharmaco-epidemiology study designs, linked primary–secondary care databases and for databases with linked mothers and babies. How each of these can be incorporated into DExtER depends on what information should be extracted from them and can vary drastically depending on the research question. The important thing to note is that the modular nature of the tool allows the addition of new stages containing specific rules and complex scenarios to its current set to facilitate such datasets. For example, the tool has the capacity to conduct a study involving primary care and hospital episode statistics where a researcher maybe interested at looking readmissions to the hospital but may want to look in the primary care for the baseline variables. In such scenarios it is possible to add a new modular stage with bespoke UI to DExtER to facilitate this study design. We will also apply our tool to databases in other settings and countries, resulting in a global community who can collaborate and generate reproducible research across several databases. For example, we have evaluated its ability in the RNH (Registration Network for General Practitioners) database in Netherlands and found it to work seamlessly. The main benefits of the system are it provides researchers fast, efficient and reliable data extraction capacity. It eliminates the IT expertise required to extract datasets manually. The proposed ETL based architecture works as a standard to extract data for epidemiological studies and extracting data in this automated and standard way highly promotes reproducible research which is hard in epidemiology [60]. The documentation why patient records were discarded establishes data integrity, credibility and renders the dataset valid and verifiable. The tool has its limitation such as not being able to cater for all possible different subtle designs an epidemiologist may consider eliciting an association or methodologies to reduce biases in specific contexts. The tool can only be used with sound knowledge of epidemiological principles, otherwise may result in numerous spurious and potentially incorrect findings. The ability to conduct studies within hours could result in publication biases where researchers may choose to undertake studies with a prior knowledge of the likely outcome or chose to ignore pursuing studies with negative outcomes [61]. To avoid this, the team will aim to conduct workshops involving key stakeholders to build an ethical framework that mitigates these unintended consequences of ACES. To facilitate global research, currently the tool can be made available for research at academic institutions anywhere in the world, subject to a negotiated contractual licence with the University of Birmingham (contact the corresponding author for further details).
Conclusion
In the recent years, there is an increasing trend in the use of routinely available data in the field of epidemiology. Primary care databases supply researchers with large amounts of medical data and the potential to answer several different research questions using various study designs. However, nonstandard and manual data extraction from primary care databases is complex, labour intensive and time-consuming process. Currently existing solutions such as the rEHR package and EHDEN’s ATLAS [19, 20] attempt to overcome the need for manual extraction, but these options still require substantial programming skills (need for expertise and prone to human error) and are limited in its applicability to various study designs and matching options. Whereas in this paper, we have been able to present an ETL based framework (DExtER), a tool used to automate the process of data extraction for epidemiological research based on study designs which can utilise any longitudinal primary care electronic record source. Corporate solutions which can provide a similar service to DExtER, [21] are yet to publish the developed algorithms/programs, and hence they are limited in the validity of their techniques and reliability in the datasets generated for research. Whereas, DExtER provides a non-invasive solution to generate quality datasets in an analysable format through a process that can be verified and reproducible.
We anticipate this new architecture will expedite and reduce the costs of epidemiological and health services research by reducing the gap between medical researchers and electronic patient records. As a part of the future work, we want to develop concrete standards for each step in the data extraction process and work towards developing automated analytics with the vision to create an automated research pipeline for epidemiological studies.
References
Protti D. Comparison of information technology in general practice in 10 countries. Healthc Q. 2006;10:107–16.
Curcin V, Soljak M, Majeed A. Managing and exploiting routinely collected NHS data for research. J Innov Health Inform. 2013;20:225–31.
Vezyridis P, Timmons S. Evolution of primary care databases in UK: a scientometric analysis of research output. BMJ Open. 2016. https://doi.org/10.1136/bmjopen-2016-012785.
Cook JA, Collins GS. The rise of big clinical databases. Br J Surg. 2015. https://doi.org/10.1002/bjs.9723.
John O, Donoghue HJ. Data management within mHealth environments: patient sensors, mobile devices, and databases. J Data Inf Qual. 2012;4:1–20.
Murdoch TB, Detsky AS. The inevitable application of big data to health care. JAMA. 2013;309:1351–2.
Hippisley-Cox J, Stables D, Pringle M. QRESEARCH: a new general practice database for research. J Innov Health Inform. 2004;12:49–50.
Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44:827–36.
Cohen B, Vawdrey DK, Liu J, Caplan D, Furuya EY, Mis FW, Larson E. Challenges associated with using large data sets for quality assessment and research in clinical settings. Policy Polit Nurs Pract. 2015. https://doi.org/10.1177/1527154415603358.
Lin J-H, Haug PJ. Data preparation framework for preprocessing clinical data in data mining. In: AMIA annual symposium proceedings; 2006.
Wasserman RC. Electronic medical records (EMRs), epidemiology, and epistemology: reflections on EMRs and future pediatric clinical research. Acad Pediatr. 2011;11:280–7.
de Lusignan S, van Weel C. The use of routinely collected computer data for research in primary care: opportunities and challenges. Fam Pract. 2006;23:253–63.
Williams T, Van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf. 2012;3:89–99.
Toulis KA, Willis BH, Marshall T, et al. All-cause mortality in patients with diabetes under treatment with dapagliflozin: a population-based, open-cohort study in the health improvement network database. J Clin Endocrinol Metab. 2017;102:1719–25.
Harvey PR, Thomas T, Chandan JS, Mytton J, Coupland B, Bhala N, Evison F, Patel P, Nirantharakumar K, Trudgill NJ. Incidence, morbidity and mortality of patients with achalasia in England: findings from a study of nationwide hospital and primary care data. Gut. 2018;68:790–5.
Adderley NJ, Nirantharakumar K, Marshall T. Risk of stroke and transient ischaemic attack in patients with a diagnosis of resolved atrial fibrillation: retrospective cohort studies. BMJ. 2018;361:k1717.
Yao Q, Chen K, Yao L, Lyu P, Yang T, Luo F, Chen S, He L, Liu Z. Scientometric trends and knowledge maps of global health systems research. Health Res Policy Syst. 2014;12:26.
Hall GC, Sauer B, Bourke A, Brown JS, Reynolds MW, Lo CR. Guidelines for good database selection and use in pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2012;21:1–10.
Springate DA, Parisi R, Olier I, Reeves D, Kontopantelis E. rEHR: an R package for manipulating and analysing electronic health record data. PLoS ONE. 2017;12:e0171784.
The European Health Data & Evidence Network’s (EHDEN) (2015) The European Health Data & Evidence Network’s (EHDEN) OHDSI ATLAS.
Aetion. Aetion; 2020. https://www.aetion.com/. Accessed 8 Mar 2020.
Vassiliadis P, Simitsis A. Extraction, transformation, and loading. In: Encyclopedia of database systems. Berlin: Springer; 2009, pp 1095–1101.
Murphy S. Data warehousing for clinical research. In: Encyclopedia of database systems. Berlin: Springer; 2009, pp 679–84.
Pecoraro F, Luzi D, Ricci FL. Designing ETL tools to feed a data warehouse based on electronic healthcare record infrastructure. Studies Health Technol Inform. 2015;210:929–33.
Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011;44:266–76.
Lazarus R, Klompas M, Campion FX, McNabb SJN, Hou X, Daniel J, Haney G, DeMaria A, Lenert L, Platt R. Electronic support for public health: validated case finding and reporting for notifiable diseases using electronic medical data. J Am Med Inform Assoc. 2009. https://doi.org/10.1197/jamia.M2848.
Lenzerini M. Data integration: a theoretical perspective. In: Proceedings of the twenty-first ACM SIGACT-SIGMOD-SIGART symposium on principles of database systems. ACM; 2002, pp 233–246.
Reisinger SJ, Ryan PB, O’Hara DJ, Powel GE, Painter JL, Pattishall EN, Morris JA. Development and evaluation of a common data model enabling active drug safety surveillance using disparate healthcare databases. J Am Med Inform Assoc. 2010. https://doi.org/10.1136/jamia.2009.002477.
Zhou X, Murugesan S, Bhullar H, Liu Q, Cai B, Wentworth C, Bate A. An evaluation of the THIN database in the OMOP common data model for active drug safety surveillance. Drug Saf. 2013. https://doi.org/10.1007/s40264-012-0009-3.
Makadia R, Ryan PB (2014) Transforming the premier perspective hospital database into the observational medical outcomes partnership (OMOP) common data model. EGEMS (Washington, DC). https://doi.org/10.13063/2327-9214.1110.
Vassiliadis P, Simitsis A, Skiadopoulos S. Conceptual modeling for ETL processes. In: Proceedings of the 8th ACM international workshop on Data warehousing and OLAP. ACM; 2002, pp 14–21.
Trujillo J, Luján-Mora S (2003) A UML based approach for modeling ETL processes in data warehouses. In: International conference on conceptual modeling. Berlin: Springer, pp 307–20.
IQVIA. THIN-HES data linkage. 2016. https://www.iqvia.com/locations/uk-and-ireland/thin-hes-data. Accessed 28 Sep 2018.
Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, Smeeth L. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44:827–36.
Wolf A, Dedman D, Campbell J, Booth H, Lunn D, Chapman J, Myles P. Data resource profile: clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol. 2019;48:1740–1740g.
Horsfall L, Walters K, Petersen I. Identifying periods of acceptable computer usage in primary care research databases. Pharmacoepidemiol Drug Saf. 2013;22:64–9.
Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;18:76–83.
Okhotin A. Recursive descent parsing for Boolean grammars. Acta Inform. 2007;44:167–89.
Hopcroft JE, Motwani R, Ullman JD. Introduction to automata theory, languages, and computation, 2nd edition. ACM SIGACT News; 2001. https://doi.org/10.1145/568438.568455.
Toulis KA, Willis BH, Marshall T, Kumarendran B, Gokhale K, Ghosh S, Thomas GN, Cheng KK, Narendran P, Hanif W. All-cause mortality in patients with diabetes under treatment with dapagliflozin: a population-based, open-cohort study in THIN database. J Clin Endocrinol Metab. 2017;102(5):1719–25.
Tracy A, Subramanian A, Adderley NJ, Cockwell P, Ferro C, Ball S, Harper L, Nirantharakumar K. Cardiovascular, thromboembolic and renal outcomes in IgA vasculitis (Henoch–Schönlein purpura): a retrospective cohort study using routinely collected primary care data. Ann Rheum Dis. 2019;78:261–9.
Chandan JS, Thomas T, Lee S, Marshall T, Willis B, Nirantharakumar K, Gill P. The association between idiopathic thrombocytopenic purpura and cardiovascular disease: a retrospective cohort study. J Thromb Haemost. 2018. https://doi.org/10.1111/jth.13940.
Chandan JS, Thomas T, Bradbury-Jones C, Russell R, Bandyopadhyay S, Nirantharakumar K, Taylor J. Female survivors of intimate partner violence and risk of depression, anxiety and serious mental illness. Br J Psychiatry 1–6. 2019.
Chandan JS, Thomas T, Gokhale KM, Bandyopadhyay S, Taylor J, Nirantharakumar K. The burden of mental ill health associated with childhood maltreatment in the UK, using The Health Improvement Network database: a population-based retrospective cohort study. Lancet Psychiatry. 2019;6:926–34.
Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2:221–8.
Suissa S, Moodie EEM, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf. 2017;26:459–68.
Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087.
Information Commissioners Office. The Principles | ICO. In: ICO. 2018. https://ico.org.uk/for-organisations/guide-to-data-protection/guide-to-the-general-data-protection-regulation-gdpr/principles/. Accessed 8 Mar 2020.
Dafoulas GE, Toulis KA, Mccorry D, Kumarendran B, Thomas GN, Willis BH, Gokhale K, Gkoutos G, Narendran P, Nirantharakumar K. Type 1 diabetes mellitus and risk of incident epilepsy: a population-based, open-cohort study. Diabetologia. 2017;60:258–61.
McCorry D, Nicolson A, Smith D, Marson A, Feltbower RG, Chadwick DW. An association between type 1 diabetes and idiopathic generalized epilepsy. Ann Neurol. 2006;59:204–6.
O’Connell MA, Harvey AS, Mackay MT, Cameron FJ. Does epilepsy occur more frequently in children with Type 1 diabetes? J Paediatr Child Health. 2008;44:586–9.
Mancardi MM, Striano P, Giannattasio A, et al. Type 1 diabetes and epilepsy: more than a casual association? Epilepsia. 2010;51:320–1.
Chou I-C, Wang C-H, Lin W-D, Tsai F-J, Lin C-C, Kao C-H. Risk of epilepsy in type 1 diabetes mellitus: a population-based cohort study. Diabetologia. 2016;59:1196–203.
Neligan A, Sander JW. The incidence and prevalence of epilepsy. London: UCL Institute of Neurology; 2009.
Adderley NJ, Ryan R, Nirantharakumar K, Marshall T. Prevalence and treatment of atrial fibrillation in UK general practice from 2000 to 2016. Heart. 2019;105:27–33.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs. Circulation. 2017;136:249–59.
Health Data Research UK | HDR UK. https://www.hdruk.ac.uk/. Accessed 22 May 2019.
Health Data Research UK. The Hubs | HDR UK. 2019. https://www.hdruk.ac.uk/infrastructure/the-hubs/. Accessed 8 Mar 2020.
Peng RD, Dominici F, Zeger SL. Reproducible epidemiologic research. Am J Epidemiol. 2006;163:783–9.
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet (Lond Engl). 1991;337:867–72.
Acknowledgements
The authors would like to acknowledge and give their sincere thanks to the kind efforts and supervision provided by Dr Behzad Bordbar, previously a senior lecturer and Dr Ronan Ryan, previously a research fellow at University of Birmingham, during the initial phase of the development of DExtER.
Funding
KN is supported by a fellowship grant from Health data research UK.
Author information
Authors and Affiliations
Contributions
This piece of work forms part of the doctoral thesis for KG and builds on his MSc dissertation. KG led all parts of the development of DExtER including; conception, software development, methods development, production, implementation, testing, validation and writing of the final manuscript. KN conceived the idea to automate epidemiology study designs, contributed to all phases of the development of DExtER and supervised KG throughout the project with PT. JSC, KT and GG contributed to the implementation, testing and validation phase of the tool. All authors participated in revising the manuscript and have approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflict of interests to declare in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gokhale, K.M., Chandan, J.S., Toulis, K. et al. Data extraction for epidemiological research (DExtER): a novel tool for automated clinical epidemiology studies. Eur J Epidemiol 36, 165–178 (2021). https://doi.org/10.1007/s10654-020-00677-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-020-00677-6