Abstract
The primary intent of the research is to comprehensively assess the environmental benefits and cost dynamics associated with the adsorption process of CS–RHA (Copper Slag and Rice Husk Ash) to produce a novel geopolymer adsorbent material for application in wastewater treatment. The geopolymer forms a polyiron sialate network under alkali activation by dissolving fayalite, and aluminium silicate to ferro-ferri silicate hydrate gel. The mechanical strength, leaching characteristics, and microstructure of the geopolymer were determined using XRD and FTIR, and magnetic properties by VSM as well surface properties were derived from BET surface area and zeta potential. Recognizing the critical role of sodium iron silicate hydrate (NFS) in the sorption of methylene blue (MB) dyestuff, batch experiments were carried out using different adsorbents. The results indicated that the dye removal efficiency increased from 60% in control samples (FS) to 98% for the blend (FS1) under different pH values. The data was found to fit with the nonlinear form of Freundlich isotherm and follow pseudo-second-order kinetics. The active adsorption sites were deduced as –O–Fe–O–Si–O–Na and Si–OH groups. The addition of RHA increases the adsorption capacity of the geopolymer in a short time through chemical adsorption. The significant negative surface charge promotes MB adsorption via improved electrostatic attraction. The spent adsorbents were recovered through magnetic separation with a retrieval rate of 80–85% and active sites were rejuvenated by calcination. Consequently, waste copper slag emerges as a promising adsorbent with minimum potential ecological risk and high effective recycling capacity.
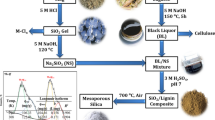
Graphical abstract














Similar content being viewed by others
References
Adediran, A., Yliniemi, J., Lemougna, P. N., Perumal, P., & Illikainen, M. (2023a). Recycling high volume Fe-rich fayalite slag in blended alkali-activated materials: Effect of ladle and blast furnace slags on the fresh and hardened state properties. Journal of Building Engineering, 63, 105436.
Adediran, A., Yliniemi, J., Moukannaa, S., Ramteke, D. D., Perumal, P., & Illikainen, M. (2023b). Enhancing the thermal stability of alkali-activated Fe-rich fayalite slag-based mortars by incorporating ladle and blast furnace slags: Physical, mechanical and structural changes. Cement and Concrete Research, 166, 107098.
Al-Mashaqbeh, A., El-Eswed, B., Banat, R., & Khalili, F. I. (2018). Immobilization of organic dyes in geopolymeric cementing material. Environmental Nanotechnology, Monitoring & Management, 10, 351–359.
Alter, H. (2005). The composition and environmental hazard of copper slags in the context of the basel convention. Resources, Conservation and Recycling, 43(4), 353–360.
Ambily, P. S., Umarani, C., Ravisankar, K., Prem, P. R., Bharatkumar, B. H., & Iyer, N. R. (2015). Studies on ultra high performance concrete incorporating copper slag as fine aggregate. Construction and Building Materials, 77, 233–240.
Ariffin, N., Abdullah, M.M., Zainol, R.R. (2017). Geopolymer as an adsorbent of heavy metal: A review. In AIP Conference Proceedings (vol 1885, no 1), (p. 020030).
Bah, A., Jin, J., Ramos, A. O., Bao, Y., Ma, M., & Li, F. (2022). Arsenic(V) immobilization in fly ash and mine tailing-based geopolymers: Performance and mechanism insight. Chemosphere, 306, 135636.
Bajpai, R., Shrivastava, A., & Singh, M. (2020). Properties of fly ash geopolymer modified with red mud and silica fume: A comparative study. SN Applied Sciences, 2(11), 1846.
Chindaprasirt, P., & Rattanasak, U. (2018). Fire-resistant geopolymer bricks synthesized from high-calcium fly ash with outdoor heat exposure. Clean Technologies and Environmental Policy, 20(5), 1097–1103.
Davidovits, J., & Davidovits, R. (2020). Ferro-sialate geopolymers (–Fe–O–Si–O–Al–O–). Geopolymer Institute Library. https://doi.org/10.13140/RG.2.2.25792.89608/2
El Alouani, M., Alehyen, S., & El Achouri, M. (2019). Preparation, characterization, and application of metakaolin-based geopolymer for removal of methylene blue from aqueous solution. Journal of Chemistry. https://doi.org/10.1155/2019/4212901
Fawer, M., Concannon, M., & Rieber, W. (1999). Life cycle inventories for the production of sodium silicates. The International Journal of Life Cycle Assessment, 4, 207–212.
Frischknecht, R., & Rebitzer, G. (2005). The ecoinvent database system: A comprehensive web-based LCA database. Journal of Cleaner Production, 13(13), 1337–1343.
Guo, B., Liu, B., Volinsky, A. A., Fincan, M., Du, J., & Zhang, S. (2017). Immobilization mechanism of Pb in fly ash-based geopolymer. Construction and Building Materials, 134, 123–130.
Hajira, T., Anas, M., Uroos, A., Rukhsana, B., & Masooda, Q. (2018). Structural modifications of surfactant-assisted alumina and their effectiveness for the removal of dyes. Iranian Journal of Chemistry and Chemical Engineering, 37(1), 47–60.
He, R., Zhang, S., Zhang, X., Zhang, Z., Zhao, Y., & Ding, H. (2021). Copper slag: The leaching behavior of heavy metals and its applicability as a supplementary cementitious material. Journal of Environmental Chemical Engineering, 9(2), 105132.
Henry, C. S., & Lynam, J. G. (2020). Embodied energy of rice husk ash for sustainable cement production. Case Studies in Chemical and Environmental Engineering, 2, 100004.
Jadhav, R., & Debnath, N. C. (2011). Computation of X-ray powder diffractograms of cement components and its application to phase analysis and hydration performance of OPC cement. Bulletin of Materials Science, 34(5), 1137–1150.
Jamaludin, L., Razak, R. A., Abdullah, M. M., Vizureanu, P., Bras, A., Imjai, T., Sandu, A. V., Abd Rahim, S. Z., & Yong, H. C. (2022). The suitability of photocatalyst precursor materials in geopolymer coating applications: A review. Coatings. https://doi.org/10.3390/coatings12091348
Jin, H., Zhang, Y., Wang, Q., Chang, Q., & Li, C. (2021). Rapid removal of methylene blue and nickel ions and adsorption/desorption mechanism based on geopolymer adsorbent. Colloid and Interface Science Communications, 45, 100551.
Jong, V., Hardjito, D, & Tang, F.E. (2010). Utilizing fly ash and bottom ash from Sejingkat coal fired power plant in Kuching in geopolymer mortar. In Proceedings of the World Engineering Congress, Conference on Natural Resources and Green Technology, Universiti Putra Malaysia.
Kamath, M., Prashant, S., & Kumar, M. (2021). Micro-characterisation of alkali activated paste with fly ash-GGBS-metakaolin binder system with ambient setting characteristics. Construction and Building Materials, 277, 122323.
Karuppaiyan, J., Mullaimalar, A., & Jeyalakshmi, R. (2023). Adsorption of dyestuff by nano copper oxide coated alkali metakaoline geopolymer in monolith and powder forms: Kinetics, isotherms and microstructural analysis. Environmental Research, 218, 115002.
Ke, Y., Liang, S., Hou, H., Hu, Y., Li, X., Chen, Y., Li, X., Cao, L., Yuan, S., & Xiao, K. (2022). A zero-waste strategy to synthesize geopolymer from iron-recovered Bayer red mud combined with fly ash: Roles of Fe, Al and Si. Construction and Building Materials, 322, 126176.
Kunaschk, M., Schmalz, V., Dietrich, N., Dittmar, T., & Worch, E. (2015). Novel regeneration method for phosphate loaded granular ferric (hydr)oxide—A contribution to phosphorus recycling. Water Research, 71, 219–226.
Lassinantti Gualtieri, M., Romagnoli, M., Pollastri, S., & Gualtieri, A. F. (2015). Inorganic polymers from laterite using activation with phosphoric acid and alkaline sodium silicate solution: Mechanical and microstructural properties. Cement and Concrete Research, 67, 259–270.
Lemougna, P. N., MacKenzie, K. J. D., Jameson, G. N. L., Rahier, H., & Chinje Melo, U. F. (2013). The role of iron in the formation of inorganic polymers (geopolymers) from volcanic ash: A 57 Fe Mössbauer spectroscopy study. Journal of Materials Science, 48, 5280–5286.
Luukkonen, T., & von Gunten, U. (2022). Oxidation of organic micropollutant surrogate functional groups with peracetic acid activated by aqueous Co(II), Cu(II), or Ag(I) and geopolymer-supported Co(II). Water Research, 223, 118984.
Marceau, M., Nisbet, M.A., Van Geem. M.G. (2006). Life cycle inventory of portland cement manufacture.
McLellan, B. C., Williams, R. P., Lay, J., van Riessen, A., & Corder, G. D. (2011). Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. Journal of Cleaner Production, 19(9), 1080–1090.
Medri, V., Papa, E., Landi, E., Maggetti, C., Pinelli, D., & Frascari, D. (2022). Ammonium removal and recovery from municipal wastewater by ion exchange using a metakaolin K-based geopolymer. Water Research, 225, 119203.
Mohd Basri, M. S., Mustapha, F., Mazlan, N., & Ishak, M. R. (2021). Rice husk ash-based geopolymer binder: Compressive strength, optimize composition, FTIR spectroscopy, microstructural, and potential as fire-retardant material. Polymers. https://doi.org/10.3390/polym13244373
Nikolov, A. (2020). Alkali and acid activated geopolymers based on iron-silicate fines—By-product from copper industry. International Scientific Journal Machines Technologies Materials, 14, 37–39.
Padmapriya, R., Sudarsan, J. S., Rohini, I., & Sunmathi, N. (2022). Geopolymer concrete with copper slag as fine aggregate a way towards developing green construction techniques. AIP Conference Proceedings, 2615(1), 020001.
Prem, P. R., Verma, M., & Ambily, P. S. (2018). Sustainable cleaner production of concrete with high volume copper slag. Journal of Cleaner Production, 193, 43–58.
Rajamane, N. P., Nataraja, M. C., Jeyalakshmi, R., & Nithiyanantham, S. (2015). Greener durable concretes through geopolymerisation of blast furnace slag. Materials Research Express, 2(5), 055502.
Rasaki, S. A., Bingxue, Z., Guarecuco, R., Thomas, T., & Minghui, Y. (2019). Geopolymer for use in heavy metals adsorption, and advanced oxidative processes: A critical review. Journal of Cleaner Production, 213, 42–58.
Revathi, T., & Jeyalakshmi, R. (2021). Fly ash–GGBS geopolymer in boron environment: A study on rheology and microstructure by ATR FT-IR and MAS NMR. Construction and Building Materials, 267, 120965.
Santa, R. A., Soares, C., & Riella, H. G. (2017). Geopolymers obtained from bottom ash as source of aluminosilicate cured at room temperature. Construction and Building Materials, 157, 459–466.
Siyal, A. A., Shamsuddin, M. R., Khan, M. I., Rabat, N. E., Zulfiqar, M., Man, Z., Siame, J., & Azizli, K. A. (2018). A review on geopolymers as emerging materials for the adsorption of heavy metals and dyes. Journal of Environmental Management, 224, 327–339.
Sousa, H. R., Silva, L. S., Sousa, P. A. A., Sousa, R. R. M., Fonseca, M. G., Osajima, J. A., & Silva-Filho, E. C. (2019). Evaluation of methylene blue removal by plasma activated palygorskites. Journal of Materials Research and Technology, 8(6), 5432–5442.
Tian, X., Xu, W., Song, S., Rao, F., & Xia, L. (2020). Effects of curing temperature on the compressive strength and microstructure of copper tailing-based geopolymers. Chemosphere, 253, 126754.
Turan, M. D., Sari, Z. A., & Demiraslan, A. (2019). Ultrasound-assisted leaching and kinetic study of blended copper slag. Metallurgical and Materials Transactions B, 50, 1949–1956.
Wang, J., Xiao, J., Zhang, Z., Han, K., Hu, X., & Jiang, F. (2021). Action mechanism of rice husk ash and the effect on main performances of cement-based materials: A review. Construction and Building Materials, 288, 123068.
Wang, M., Liu, Y., Feng, C., Zhang, D., Zhang, X., & Jiao, G. (2022). Pozzolanic activity enhancement of magnesium-rich nickel slag and geopolymer preparation. Journal of Material Cycles and Waste Management, 24(6), 2598–2607.
Zanoletti, A., Federici, S., Borgese, L., Bergese, P., Ferroni, M., Depero, L. E., & Bontempi, E. (2017). Embodied energy as key parameter for sustainable materials selection: The case of reusing coal fly ash for removing anionic surfactants. Journal of Cleaner Production, 141, 230–236.
Zein, R., Purnomo, J. S., Ramadhani, P., Alif, M. F., & Putri, C. N. (2023). Enhancing sorption capacity of methylene blue dye using solid waste of lemongrass biosorbent by modification method. Arabian Journal of Chemistry, 16(2), 104480.
Zhang, S., Zhu, N., Mao, F., Zhang, J., Huang, X., Li, F., Li, X., Wu, P., & Dang, Z. (2021). A novel strategy for harmlessness and reduction of copper smelting slags by alkali disaggregation of fayalite (Fe2SiO4) coupling with acid leaching. Journal of Hazardous Materials, 402, 123791.
Zhang, Y. J., Han, Z. C., He, P. Y., & Chen, H. (2020). Geopolymer-based catalysts for cost-effective environmental governance: A review based on source control and end-of-pipe treatment. Journal of Cleaner Production, 263, 121556.
Zheng, L., Wang, W., Qiao, W., Shi, Y., & Liu, X. (2015). Immobilization of Cu2+, Zn2+, Pb2+, and Cd2+ during geopolymerization. Frontiers of Environmental Science & Engineering, 9(4), 642–648.
Acknowledgements
This work was supported by the Department of Science and Technology, the Government of India (GOI), under the grant DST/TDT/WMT/2017 14/03/18, GOI. The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2024R436) King Saud University, Riyadh, Saudi Arabia. The authors greatly acknowledge the utilization of facilities provided by DST-FIST, Department of Chemistry and NRC, SRM Institute of Science and Technology, Kattankulathur, India. The authors thank the authorities of SRMIST for funding the research scholars.
Funding
This work was supported by the Department of Science and Technology, the Government of India (GOI), under the grant DST/TDT/WMT/2017 14/03/18, GOI received by corresponding author and author (6) express their sincere appreciation to the Researchers Supporting Project Number (RSP2024R436) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by, A. Mullaimalar, Rithikaa Thanigaiselvan, Janani Karuppaiyan, S. Kiruthika,R. Jeyalakshmi, Mohammed F Albeshr. The first draft of the manuscript was written by A. Mullaimalar and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The Researchers Supporting Project Number is corrected from ‘RSP2023R436’ to ‘RSP2024R436’.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mullaimalar, A., Thanigaiselvan, R., Karuppaiyan, J. et al. An efficient eco-friendly adsorbent material based on waste copper slag-biomass ash geopolymer: dye sorption capacity and sustainable properties. Environ Geochem Health 46, 110 (2024). https://doi.org/10.1007/s10653-024-01920-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10653-024-01920-9