Abstract
Polycyclic aromatic hydrocarbons (PAHs) are among the most studied organic compounds in urban environments, due to their known threat to human health. This study extends the current knowledge regarding the ability of different vegetative parts of different tree species to accumulate PAHs. Moreover, exposure intensity to PAHs in areas frequented by population susceptible to adverse health effects of air pollution is evaluated. For this, leaves and barks of Sambucus nigra (S. nigra) and Acacia melanoxylon (A. melanoxylon) were collected at urban areas in the Andean city of Quito, at seven points near hospitals and schools. A methodology, previously developed, for the extraction, purification, and quantification of PAHs associated with the leaves and bark of S. nigra was employed and also validated for leaves and bark of A. melanoxylon. The total PAH level varied from 119.65 ng g−1 DW (dry weight) to 1969.98 ng g−1 DW (dry weight) with naphthalene (Naph), fluoranthene (Flt), pyrene (Pyr), chrysene (Chry), and benzo[a]pyrene (BaP) predominating in all samples. The results indicate that the leaves and bark of tree species studied have certain abilities to bio-accumulate PAH according to their molecular weight. The leaves of S. nigra and bark of A. melanoxylon showed the highest ability to accumulate PAHs, mainly those with high and medium molecular weight, respectively. The highest incidence of light molecular weight PAHs was found in the leaves of A. melanoxylon. Furthermore, coal combustion, biomass burning, and vehicle emissions were identified as the main PAHs sources. Concentrations of PAHs associated with tree species suggest an affectation in areas frequented by populations susceptible to air pollution. This fact shows the importance of regulatory scheme to significantly improve the air quality in the city integrating a knowledge-based decision-making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are important harmful pollutants to the environment and health (Hou et al., 2018; Perera et al., 2014); thus, there is a great interest in addressing the public health risk they cause, especially in urban areas frequented by populations susceptible to the adverse health effects of air pollution, with concentration diagnostics in the atmosphere and cost-effective action by means of the identification of pollutant sources.
PAHs are found in the gas phase or associated with particulate phase which contributes to their ubiquity in the environment. In general, low molecular weight PAHs (2- to 3-rings, LMW) are frequently found in the gas phase, medium molecular weight PAHs (4-rings, MMW) are generally present in both gas and particulate phase, and high molecular weight PAHs (5- and 6-rings, HMW) are mainly distributed in particulate phase (Dat & Chang, 2017). Common sources of PAHs in urban areas mainly include pyrogenic (or pyrolytic, e.g., vehicle emissions, typically associated with high molecular PAHs) and petrogenic (petroleum, e.g., crude oil, bitumen, asphalt, typically associated with low molecular PAHs) sources (Hwang et al., 2003; Ratola et al., 2011a).
In the last decades, the use of plants for biomonitoring of urban air pollution, including PAHs, has gained importance due to its easy access and low cost (De Nicola, et al., 2016; Prajapati & Tripathi, 2008; Sari et al., 2021). PAHs in the gas phase can be absorbed by stomatal uptake and/or they may diffuse through the wax layer and the cuticular membrane, while particle-bound PAHs are accumulated on the leaf surface due to the high interaction with the lipophilic waxy cuticle layer (De Nicola et al., 2016; Holoubek et al., 2000; Lehndorff & Schwark, 2004).
Several studies have shown the successful use of leaves of trees to measure the concentration of PAHs in an area (Alfani et al., 2001; Bakker et al., 2000; Fellet et al., 2016; van Drooge et al., 2014). Bark of trees has also shown good capacity to accumulate PAHs, due to its high lipid content, and porous and almost inert surface (Niu et al., 2019; Pereira et al., 2019; Ratola et al., 2009), although research with this type of matrix is scarcer. Accumulation of PAHs on vegetative parts is plant species dependent, mainly to the chemical, morphological and physiological characteristics of each tree species (Fellet et al., 2016; Pereira et al., 2019; Rodriguez et al., 2012). The vegetative parts of a same tree species have also shown to have different abilities to accumulate PAHs (Ratola et al., 2009, 2012; Yin et al., 2011). Identification of tree species, and different vegetative parts of a same tree species, with the best ability to accumulate air pollutants is still poorly known and constitutes an important research topic, mainly because data can allow to choose the most appropriate for a specific purpose and can also help to identify which factors control the PAH accumulation.
Sambucus nigra (S. nigra, tree or shrub of the family Adoxaceae) and Acacia melanoxylon (A. melanoxylon, tree of the family Fabaceae) are two widely distributed tree species in the Andean areas of Latin America. However, studies on their use as biomonitors are still very scarce. Although the literature shows evidence on the use of some vegetative parts of these two tree species as biomonitors of some air pollutants, such as heavy metals (Armijos et al., 2022; Kolodziej et al., 2012; Topolska et al., 2020), to our knowledge, there is no information to date on the evaluation of the potential use of their leaves and barks for PAHs. Thus, the present work aims to analyze the concentration of PAHs in leaf and bark samples of S. nigra and A. melanoxylon collected near Hospitals and Schools in the Andean city of Quito, Ecuador. In this way, this work addresses the existing gap by comparing the PAH incidence between different vegetative parts of a same tree species, and between tree species. Moreover, the levels of PAHs in areas frequented by populations susceptible to adverse health effects of air pollution are evaluated which could serve to assess the exposure intensity and to analyze the major emission sources and their contribution.
Materials and method
The methodology used was previously developed for leaf and bark samples of S. nigra (Viteri et al., 2023). In the present work, the performance of such methodology was also evaluated for leaf and bark samples of A. melanoxylon showing satisfactory results, as it will be seen later. A brief description of the methodology (sampling procedure, sample pretreatment and the quantitative determination of PAHs) is given below.
Sampling sites and sampling procedure
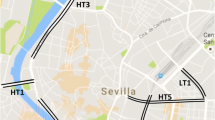
Tree leaf and bark samples were collected in urban areas of the city of Quito (2850 m.a.s.l.) at seven points (P1-P7) close to hospitals and schools that had both tree species at a maximum distance of 10 m from each other (Fig. 1): P1—Solca Hospital; P2—Baca Ortiz Pediatric Hospital; P3—Eugenio Espejo Hospital; P4—Carlos Andrade Marín Hospital and Francisco Febres Cordero LaSalle School; P5—Gran Colombia and Manuela Cañizares Schools; P6—La Inmaculada School; P7—24 de Mayo School. All the sampling points have a high flow of motor vehicles and some of them with food cooking services in the vicinity.
The sampling was carried out in May 2021. Briefly, a pruning shear was used to collect the leaves from all directions and the outer part of the trees at a height of about 2 m above the ground. For bark, a pre-cleaned stainless-steel knife was used to remove the external layer from all directions of the tree at the height of 1.5 m above the ground. Leaves with evidence of chlorosis or necrosis, as well as barks with moss and lichen on the surface, were avoided. A new pair of powder-free vinyl gloves for each sample was used to avoid cross-contamination.
Leaf and bark samples were placed in ziplock bags, labeled with sample information, wrapped in aluminum to protect from light and refrigerated in a cooler for transport to the laboratory. One fraction of the samples was used to measure the water content, while the other part was stored at -20◦C before sample treatment and analysis. The water content was measured by drying, in duplicate, 2 g of leaves and barks for each tree species and site, until constant weight in an oven at 70 ± 2◦C (Armijos et al., 2022).
Sample pretreatment (extraction and clean-up)
Prior to extraction, the samples were defrosted in a desiccator. Then, 2 g of leaves or barks was placed into a baker with 20 mL of a dichloromethane/hexane (1:1 v/v) mixture and immersed in a 420-W ultrasonic bath for 10 min. This procedure was repeated two more times, using fresh solvent mixture, for a total of 30 min of extraction and 60 mL of dichloromethane/hexane mixture. The three extracts were combined into the same round-bottom flask and evaporated up to approximately 1 mL in a Buchi rotary evaporator at 30 °C.
For clean-up of concentrated extract, each sample was transferred onto a Sep-Pak Alumina cartridge (6 cc, 1 g. Waters) previously conditioned with 10 mL of the dichloromethane/hexane mixture. The target analytes were eluted with 10 mL of the dichloromethane/hexane mixture and then with 5 mL of dichloromethane. After cleaning-up, the extracts were again evaporated by rotary evaporator to approximately 1 mL and concentrated using a vacuum concentrator (Genevac miVac) at 40 °C for 30 min. Finally, the samples were reconstituted in 1 mL of acetonitrile, filtered using PVDF syringe filters (32 mm, 0.22 μm) and transferred to 2 mL amber glass vial for subsequent high-performance liquid chromatography (HPLC) analysis.
Analysis of polycyclic aromatic hydrocarbons
In the present work, 14 PAHs were analyzed (see Table S1 for their structural formulas), namely naphthalene (Naph), acenaphthylene (Acy), phenanthrene (Phen), anthracene (Ant), fluoranthene (Flt), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chry), benzo[b]fluoranthene (BbF), benzo[k])fluoranthene (BkF), benzo[a]pyrene (BaP), dibenzo[a,h]anthracene (DahA), benzo[g,h,i]perylene (BghiP) and indeno[1,2,3-cd]pyrene (IcdP). The quantitative analysis of these PAHs was carried out by a HPLC (Agilent 1260 system) equipped with a ZORBAX Eclipse PAH column (4.6 × 50 nm, 3.5 µm) and an UV detector (Agilent 1260 DAD G4212B) set to wavelengths (λ) of 220 nm, 230 nm and 254 nm. Analytes were separated by gradient elution with acetonitrile (A) and water (B) as mobile phase, at a flow rate of 1.4 mL/min, the injection volume set to 20 μL and the column temperature at 20 °C. The elution program was defined as follows: 0–6 min isocratic 40:60 (v/v) A:B; 6–9.5 min linear gradient from 40 to 100% of A and 9.5–12 min isocratic 40:60 (v/v) A:B. Peak identification and integration were performed by external standard method with the ChemStation software (Agilent Technologies).
Quality assurance/quality control (QA/QC)
The details about the parameters of the analytical method (linearity, limit of detection (LOD), limit of quantification (LOQ), repeatability and recovery) can be found in Viteri et al. (2023). In brief, the linearity range was from 2.5 to 2500 μg L−1 with coefficient of determination (R2) greater than 0.9993. The LOD and LOQ values of individual PAHs were in the range of 0.20–13.7 μg L−1 and 0.6–41.5 μg L−1, respectively. Repeatability, studied as percent relative standard deviation (%RSD), ranged from 0.006 to 4.6% indicating good instrumental precision. Recovery results were in the 64.8–106.4% range for S. nigra and in the 48.2–91.3% range for A. melanoxylon (see Table 1).
To determine the possible background contamination during the treatment of the sample, including laboratory tools interference, procedural blanks (extraction and clean-up of reagents without vegetative material) were also analyzed. The concentration of PAHs in the samples was corrected based on the procedural blanks whenever they were above the respective LOD (Bakker et al., 2000; Birgül et al., 2011; Busso et al., 2018; Navarro-Ortega et al., 2012; Silva et al., 2015) and recoveries (Bakker et al., 2000; Tham et al., 2008; van Drooge et al., 2014). Moreover, to examine cross-contamination and interference in the HPLC and to check the accuracy of the determination of PAHs, blanks (only acetonitrile) and the standard solution at 100 μg L−1 were ran every ten samples. PAH concentrations in all the blank analyses were below the LOD.
Principal component analysis
Principal component analysis (PCA) has been widely used in biomonitoring studies of air pollution (Alexandrino et al., 2022; Alfani et al., 2001; Fellet et al.,; 2016) in which the pollutants in a same component may indicate a common source. In this work, PCA for each matrix type and tree species was performed to identify the emissions sources using the R software through the R commander interface and the Multivariate Exploratory Data Analysis and Data Mining-FactoMineR package.
Results and discussion
PAHs were identified in leaf and bark samples of S. nigra and A. melanoxylon collected at seven sites in urban areas of Quito near Hospitals and Schools. In this section, first, the total and individual concentration of the 14 PAHs in the leaf and bark samples of both tree species is discussed. Subsequently, a comparison of the PAHs levels between both vegetative parts and between tree species is presented. Finally, PAHs sources incidence and their contribution are identified.
Total and individual PAH concentration in the leaf and bark samples of both tree species
The total and individual PAH concentration in leaves and barks of S. nigra and A. melanoxylon at each site is plotted in Fig. 2. Total PAH refers to the sum of individual PAH \(({\sum }_{1}^{n:14}{PAHs}_{n} )\) found at each site studied. It is observed that the total PAH concentration ranges from 119.65 ng g−1 DW to 1969.98 ng g−1 DW which is within the same order of magnitude as the results found in the literature for different tree species (Alfani et al., 2001; Augusto et al., 2010; Holoubek et al., 2000; Navarro-Ortega et al., 2012; Niu et al., 2019; van Drooge et al., 2014; Wu et al., 2019; Wu et al., 2019; Yang et al., 2007).
It should be noted that DahA, BghiP and IcdP were not detected in the samples, as has also been reported in other works (Fernández et al., 2011; Ratola et al., 2011a; Fellet et al., 2016Orecchio et al., 2008). This could be because heavy PAHs, mainly particulate-bound compounds, are very likely to remain on the surface of leaves and barks rather than diffusing and accumulating in the inner compartment, becoming more prone to suffer the effect of external environmental factors such as heavy rain and wind (Amigo et al., 2011; Busso et al., 2018; Guidotti et al., 2003; Navarro-Ortega et al., 2012; Piccardo et al., 2005; Tomashuk et al., 2012; Wang et al., 2009).
To help to figure out which PAHs are the most concentrated in the samples, Fig. 3 shows the individual mean PAH profiles for leaves and barks of S. nigra and A. melanoxylon. Individual mean PAH refers to the mean of each individual PAH found at all sites studied in each matrix and tree species. Among all PAHs studied, Naph, Flt, Pyr, Chry and BaP show the highest concentrations. This result agrees with those observed in other works (Alfani et al., 2001; Busso et al., 2018; Ratola et al., 2009; Rodriguez et al., 2010), except for BaP. This PAH is the usual marker for carcinogenic levels of PAHs in environmental studies and its high levels could indicate a high human exposure risk (IARC, 2013). The high incidence of BaP could be attributed to gasoline exhaust that is known to contribute for more BaP emissions than diesel engines (Wu et al., 2012).
Differences between leaf and bark samples in PAH concentration
It is observed from Fig. 2a and 2b that the total PAH concentration in leaves of S. nigra is, in general, higher than that in bark. A t test (95% confidence interval) confirmed a statistical difference. This is in line with the result obtained by Ratola et al. (2009) in their work using needles and barks of Pinus pinaster Ait. and Pinus pinea L. On the other hand, in general, for A. melanoxylon, the total PAH concentration is higher in bark than in leaves (Fig. 2c and 2d), this being mainly due to the higher concentration of the 4-ring PAHs Flt, Pyr and Chry in bark (Fig. 3b), although a non-statistically significant difference is observed.
Due to the scarcity of comparative studies in the literature between the capacity of leaf and bark of a same tree species to accumulate PAH, a further comparison is not possible. However, studies reporting the potential of leaves and bark to accumulate metals also show contradictory results. For example, Dogan et al. (2010) indicated that the accumulation of metals in the bark of Pinus brutia was higher than that in needles. The same was observed by Sawidis et al. (2011), using needles and barks of Platanus orientalis and Pinus nigra. However, Solgi et al. (2020) indicated that levels of metals in leaves of Fraxinus excelsior and Pinus eldarica were higher than those in bark. Thus, the distribution of pollutants in different vegetative parts of a same tree seems to be species dependent. In this regard, further research is required to determine the ability of different vegetative parts to retain PAHs depending on the tree species.
The patterns of PAH incidence in the leaves and barks of both tree species are shown in Fig. 4, which indicates the distribution according to the molecular weight classification (contribution (%) of LMW, MMW, HMW to total PAH).
It is observed from Fig. 4 that, for the two tree species, 4-ring PAHs (MMW) are the dominant in both sample matrices, with a similar percentage in the leaves and bark of S. nigra (56.5 and 57.7%, respectively), and being almost double in the bark of A. melanoxylon than in the leaves (73.5 and 39.5%, respectively). The high incidence of 4-ring PAHs agrees with that observed in previous biomonitoring studies (Busso et al., 2018). 4-ring PAHs are present in both vapor and particulate matter and are easily undergoing wet and dry deposition enhancing the uptake levels (Amigo et al., 2011; Augusto et al., 2010; Busso et al., 2018). The incidence of LMW PAHs was not as high, mainly in leaves of S. nigra, maybe probably due to photodegradation or resuspension into the atmosphere due to their higher vapor pressures (higher volatility) (Amigo et al., 2011; Ratola et al., 2010). On the other hand, the percentage of HMW PAHs was relatively high, and it is mainly due to the high concentration of BaP found in the samples (Figs. 2 and 3).
The leaves and bark of S. nigra present the same trend of higher incidence of MMW PAHs, followed by HMW and finally by LMW PAHs, which is in line with that observed by Tian et al. (2019) in leaves of 8 common tree species in Shanghai, China. Moreover, leaves of S. nigra collect a higher proportion of HMW PAHs than bark (33.8% vs. 23.6%), and bark captures a higher proportion of LMW PAHs than leaves (18.7% vs. 9.7%). This is opposite to that observed in the work of Ratola et al. (2012) on the use of needles and barks of two pine species (Pinus pinaster Ait. and Pinus pinea L.) as biomonitors, which showed that LMW PAHs are predominant in needles, while HMW PAHs are predominant in bark. Moreover, Fig. 4 also shows that, although the leaves and bark of A. melanoxylon have the same trend of higher incidence of MMW PAHs, followed by LMW and finally by HMW PAHs, leaves of A. melanoxylon tend to accumulate more LMW and HMW PAHs than bark. The way leaves and bark of each tree species interact with the surrounding air could be an important factor controlling the PAH uptake. Some leaves and barks better retain particulate matter, while others interact mainly with the vapor phase, and this also has to do with the morphological, physiological and chemical properties of the vegetative parts of the tree species.
Differences between tree species in PAH concentration
It is clear that S. nigra and A. melanoxylon have different abilities to capture PAH. Specifically, a t test (95% confidence interval) indicates a statistically significant difference in the total concentration of PAHs between leaves of both tree species, with the mean concentration of individual PAHs being higher in the leaves of S. nigra than that in the leaves of A. melanoxylon, except for Naph (Fig. 3c). On the other hand, a non-statistically significant difference in the total concentration of PAHs between bark of both tree species is found. However, the mean concentrations of Pyr and Chry are found to be three times higher in the bark of A. melanoxylon than in S. nigra (Fig. 3d).
It is evident from Fig. 4 that leaves of S. nigra are more prone to capture MMW and HMW PAHs than those of A. melanoxylon, whereas leaves of A. melanoxylon are more prone to capture LMW PAHs. Regarding the bark samples, higher percentage of both LMW and HMW PAHs is observed in S. nigra than in A. melanoxylon, while higher percentage of MMW PAHs is observed in A. melanoxylon than in S. nigra. Differences between tree species toward PAHs were previously reported (Amigo et al., 2011; Baldantoni et al., 2014; Fellet et al., 2016; Navarro-Ortega, 2012; Piccardo et al., 2005; Ratola et al., 2010; Ratola et al., 2011b; Ratola et al., 2012; Rodriguez et al., 2012). Morphological, physiological and chemical characteristics, including stomatal density, specific area, surface roughness and lipid content, could be a reason for the differences in the distribution between tree species, as they have been identified to play an important role in the accumulation of air pollutants, including PAHs (Baldantoni et al., 2014; Franzaring & Eerden, 2000; Moeckel et al., 2008; Simonich & Hites, 1995; Wu et al., 2019; Yang et al., 2008). In fact, some studies indicate that, depending on the nature of the PAH, its accumulation will depend on one characteristics or another. For example, Tian et al. (2019) evaluated the effects of different leaf characteristics on their ability to accumulate PAHs and found that LMW PAHs were mainly affected by leaf morphological characteristics, whereas MMW and HMW PAHs were mainly affected by wax content. Moreover, Yang et al. (2008) found that specific surface area and stomata density affect the levels of HMW PAHs and lipid content the levels of LMW and MMW PAHs.
The experimental study of the characteristics of leaves and bark, including stomatal density, specific leaf area and lipid content, of S. nigra and A. melanoxylon is not considered in this work. However, values of some of these characteristics for these two tree species are reported in the literature. For example, the stomatal density on the abaxial surface of leaves of S. nigra was reported to be in the range of 71–139.32 mm2 (Amini et al., 2021; Atkinson & Atkinson, 1958), while that of A. melanoxylon was reported to be in the range of 306 – 473 mm2 (Scarr, 2011). Stomatal density has a high significant effect on the uptake rate of PAHs (Tao & Hornbuckle, 2001) and has been found to be positively (Fellet et al., 2016) and negatively (Tian et al., 2019), correlated with the content of PAHs. In the present work, the higher the stomatal density of leaves the lower the total PAH concentration, which is consistent with that observed by Tian et al. (2019). However, the leaves of A. melanoxylon are more prone to capture LMW PAHs than those of S. nigra (Fig. 4). This could be because light PAHs can penetrate more readily into leaf inner tissues through the stomata than heavy PAHs and then can be accumulated in higher proportions (Huang et al., 2018).
Future works should be focused on studying the morphological, physiological and chemical characteristics of the leaves and barks of S. nigra and A. melanoxylon in order to be able to identify a more accurate correlation between the concentration of PAHs and these characteristics and then to define those that most control the uptake and accumulation of PAHs in each vegetative part and tree species.
Analysis of sites and possible sources of PAHs
Difference between sites in total PAH concentration is observed from Fig. 2, which is expected given the main sources of atmospheric PAHs. Both leaves and bark of S. nigra indicate that sites P4, P5 and P7 have the highest total PAH levels, while both leaves and bark of A. melanoxylon indicate site P4 as being the one with the highest total PAH level. In this way, Fig. 5 shows the PAHs patterns for each site studied for leaves and barks of the two tree species. It is evident that, in general, at sites P5 and P7, 4-ring PAHs dominate, and it is due mainly to the high concentration of Flt that contributes largely to the total PAH concentration (Fig. 2). Fluoranthene is associated with pyrogenic activities, such as coal combustion and biomass burning (Pulster et al., 2019). In fact, in site 5 there are some soccer fields that make this place crowded by many people and for this reason it is normal to find street vendors preparing food on the street (using gas or coal) to sell. This could be contributing to PAH emissions, mainly pyrogenic PAHs, in that area. Also, at site 7, there is the presence of numerous restaurants than can also be contributing to the high presence of Flt in that area. Regarding site P4, in general, HMW PAHs dominate (Fig. 5). High contribution of HMW PAHs is often attributed to gasoline exhaust (Pereira et al, 2019). The dominance of HMW PAHs at site P4 is seemed to be due to the high concentration of BaP (Fig. 2), which, as mentioned above, is commonly associated with gasoline exhaust (Wu et al., 2012). At site P4 there is the Hospital Carlos Andrade Marin and Francisco Febres Cordero LaSalle School, which means a large circulation of vehicles in that area, in addition to the fact that the sampling point is next to a roundabout whose presence has been indicated to be relevant for PAH levels (Alexandrino et al., 2022).
To complement these observations, Table 2 and 3, and Figs. S1-S4 show the results for the PCA applied to the PAH concentrations found in the leaf and bark samples of S. nigra and A. melanoxylon from all the sites studied. The results of PAH concentrations were reduced to three (leaves of S. nigra and leaves and bark of A. melanoxylon) and four components (bark of S. nigra), whose eigenvalues were higher than 1. The components explained between 83.8 and 95.8% of data variability.
PCA patterns were not completely identical for all vegetative parts and species studied, which can partly be explained by the differences in morphological, physiological and chemical characteristics of the samples and by the sensitivity to processes causing the removal of deposited material (e.g., wash-off, wind abrasion). However, some similarities can be observed, mainly in leaf samples, in where Naph and BaP are in the same dimension suggesting the same emission source for these PAHs. Naph is linked to light vehicular traffic (Guidotti et al., 2003) and is also a component of diesel fuels (Preuss et al., 2003), while BaP is linked to gasoline exhaust (Wu et al., 2012). Moreover, in a previous work (Alexandrino et al., 2022), Naph and BaP were associated with traffic related emissions, specifically acceleration and braking activities, i.e., with the presence of traffic lights, roundabouts, intersections, curves, and speed bumps. Moreover, the PCA results show that, in general, Naph and BaP are related to the Site P4, which supports the above discussion that traffic emissions are pointed out as an important source of emissions in this urban area. On the other hand, in general, 3- and 4-ring PAHs, mainly Flt and Ant, are grouped in the same dimension which may originate from biomass burning, and diesel and natural gas combustion (Dias et al., 2016) and are more related to the Sites P5 and P7. These results indicate coal combustion, biomass burning, and vehicle exhaust as the main sources of PAHs emissions.
The implementation of tree planting as a strategy for ecological restoration in urban environments, particularly in areas with high levels of air pollutants such as PAHs, should take into account several key aspects. Firstly, monitoring of pollutants in urban areas is crucial. This involves identifying hotspots, distribution, types, and levels of pollutants. Our study demonstrates that the efficacy of aromatic remediation is dependent on the species of tree, hence characterization of pollutant presence is a crucial task. Secondly, it is essential to select tree species with the highest capacity for pollutant absorption. In our work, both tree species studied show good performance to accumulate PAHs. However, the selection of the best species and vegetative part depends on the type of PAHs (LMW, MMW, and HMW) that is to be identified, since the results show differences between tree species and vegetative parts to accumulate PAHs types. Thirdly, the planting of trees should be strategically planned in terms of density and height. Research indicates that dense and tall vegetation can significantly reduce pollutants, with some studies (Uribe et al., 2023) suggesting a reduction impact greater than 50%, particularly for vegetation belts approximately 10 m thick near pollution sources. Additionally, factors such as climate, geographical location, and landscaping should be considered in selecting the most suitable species. Avoid invasive species that can disrupt the ecosystem balance.
It is important to remember that tree planting is only one strategy among many for mitigating air pollutants like PAHs. Comprehensive mitigation efforts should include the control of stationary and mobile pollution sources, urban planning that incorporates integrated urban design, efficient monitoring and evaluation systems, and environmental education strategies, among others, to effectively reduce pollutants in the atmosphere.
Conclusions
This work extends the knowledge about the comparison of the incidence of PAHs between different vegetative parts of a same tree species (leaves and bark), and between tree species (S. nigra and A. melanoxylon), in areas with presence of vulnerable populations to air pollution. Both tree species studied show good performance as biomonitors for PAHs. However, the selection of the best species and vegetative part depends on the type of PAHs (heavy, medium, and light molecular weight PAHs) that is to be identified, since the results show differences between tree species and vegetative parts to accumulate PAHs types. The total PAH concentration in leaves of S. nigra was higher than that in bark due to a higher capacity of leaves to capture high molecular weight PAHs, while the total PAH concentration in bark of A. melanoxylon was higher than that in leaves. The 4-ring PAHs, mainly fluoranthene, pyrene, and chrysene, were dominant in all samples, with the highest incidence being in the bark of A. melanoxylon. The highest incidence of heavy, medium, and light molecular weight PAHs was found in leaves of S. nigra, bark of A. melanoxylon and leaves of A. melanoxylon, respectively. As chemical, morphological, and physiological characteristics of leaves and bark have been identified in the literature to play an important role in the accumulation of air pollutants, including PAHs, future investigations should be directed to investigate these characteristics of S. nigra and A. melanoxylon. Affectation by PAHs in areas frequented by populations susceptible to air pollution was observed, with coal combustion, biomass burning, and vehicle exhaust identified as the main sources of PAHs emissions.
Change history
25 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10653-024-01985-6
References
Alexandrino, K., Sánchez, N. E., Zalakeviciute, R., Acuña, W., & Viteri, F. (2022). Polycyclic Aromatic Hydrocarbons in Araucaria heterophylla Needles in Urban Areas: Evaluation of Sources and Road Characteristics. Plants, 11, 1948. https://doi.org/10.3390/plants11151948
Alfani, A., Maisto, G., Prati, M. V., & Baldantoni, D. (2001). Leaves of Quercus ilex L. as biomonitors of PAHs in the air of Naples (Italy). Atmospheric Environment, 35, 3553–3559. https://doi.org/10.1016/S1352-2310(01)00087-5
Amigo, J. M., Ratola, N., & Alves, A. (2011). Study of geographical trends of polycyclic aromatic hydrocarbons using pine needles. Atmospheric Environment, 45, 5988–5996. https://doi.org/10.1016/j.atmosenv.2011.07.058
Amini, E., Nasrollahi, F., Sattarian, A., Isazadeh-Araei, M., & Habibi, M. (2021). Morphological and anatomical study of the genus Sambucus L. (Adoxaceae) in Iran. Modern Phytomorphology, 15, 14–20.
Armijos, C., Tapia, W., & Alexandrino, K. (2022). Assessment of airborne metal pollution in urban parks and industrial areas using Callistemon citrinus and Acacia melanoxylon. Applied Geochemistry, 139, 105263. https://doi.org/10.1016/j.apgeochem.2022.105263
Atkinson, M.D., & Atkinson, E. Sambucus nigra L. List Br. Vasc. Pl. (1958) No. 487.2
Augusto, S., Máguas, C., Matos, J., Pereira, M. J., & Branquinho, C. (2010). Lichens as an integrating tool for monitoring PAH atmospheric deposition: A comparison with soil, air and pine needles. Environmental Pollution, 158, 483–489. https://doi.org/10.1016/j.envpol.2009.08.016
Bakker, M. I., Casado, B., Koerselman, J. W., Tolls, J., & Kolloffel, C. (2000). Polycyclic aromatic hydrocarbons in soil and plant samples from the vicinity of an oil refinery. Science of the Total Environment, 263, 91–100. https://doi.org/10.1016/S0048-9697(00)00669-0
Baldantoni, D., De Nicola, F., & Alfani, A. (2014). Air biomonitoring of heavy metals and polycyclic aromatic hydrocarbons near a cement plant. Atmospheric Pollution Research, 5, 262–269. https://doi.org/10.5094/APR.2014.032
Birgül, A., Tasdemir, Y., & Cindoruk, S. S. (2011). Atmospheric wet and dry deposition of polycyclic aromatic hydrocarbons (PAHs) determined using a modified sampler. Atmospheric Research, 101, 341–353. https://doi.org/10.1016/j.atmosres.2011.03.012
Busso, I. T., Tames, F., Silva, J. A., Ramos, S., Homem, V., Ratola, N., & Carreras, H. (2018). Biomonitoring levels and trends of PAHs and synthetic musks associated with land use in urban environments. Science of the Total Environment, 618, 93–100. https://doi.org/10.1016/j.scitotenv.2017.10.295
Capilla, X., Schwartz, C., Bedell, J. P., Sterckeman, T., Perrodin, Y., & Morel, J. L. (2006). Physicochemical and biological characterisation of different dredged sediment deposit sites in France. Environmental Pollution, 143, 106–116. https://doi.org/10.1016/j.envpol.2005.11.007
Dat, N. D., & Chang, M. B. (2017). Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Science of the Total Environment, 609, 682–693. https://doi.org/10.1016/j.scitotenv.2017.07.204
De Nicola, F., Concha, E., Aboal, J. R., Carballeira, A., Fernández, J., López, P., Prada, D., & Muniategui, S. (2016). PAH detection in Quercus robur leaves and Pinus pinaster needles: A fast method for biomonitoring purpose. Talanta, 153, 130–137. https://doi.org/10.1016/j.talanta.2016.01.067
Dias, A. P. L., Rinaldi, M. C. S., & Domingos, M. (2016). Foliar accumulation of polycyclic aromatic hydrocarbons in native tree species from the Atlantic Forest (SE-Brazil). Science of the Total Environment, 544, 175–184. https://doi.org/10.1016/j.scitotenv.2015.11.092
Dogan, Y., Ugulu, I., & Baslar, S. (2010). Turkish red pine as a biomonitor: A comparative study of the accumulation of trace elements in the needles and bark. Ekoloji, 19, 88–96. https://doi.org/10.5053/ekoloji.2010.7512
Fellet, G., Poscic, F., Licen, S., Marchiol, L., Musetti, R., Tolloi, A., Barbieri, P., & Zerbi, G. (2016). PAHs accumulation on leaves of six evergreen urban shrubs: A field experiment. Atmospheric Pollution Research, 7, 915–924. https://doi.org/10.1016/j.apr.2016.05.007
Fernández, R., Galarraga, F., Benzo, Z., Márquez, G., Fernández, A. J., Requiz, M. G., & Hernández, J. (2011). Lichens as biomonitors for the determination of polycyclic aromatic hydrocarbons (PAHs) in Caracas Valley. Venezuela. Journal of Environmental Analytical Chemistry, 91(2014), 230–240. https://doi.org/10.1080/03067310903198478
Franzaring, J., & Eerden, L. J. M. (2000). Accumulation of airborne persistent organic pollutants (POPs) in plants. Basic and Applied Ecology, 1, 25–30. https://doi.org/10.1078/1439-1791-00003
Guidotti, M., Stella, D., Owezarek, M., de Marco, A., & de Simona, C. (2003). Lichens as polycyclic aromatic hydrocarbons bioaccumulators used in atmospheric pollution studies. Journal of Chromatography A, 985, 185–190. https://doi.org/10.1016/S0021-9673(02)01452-8
Holoubek, I., Korˇinek, P., Šeda, Z., Schneiderová, E., Holoubková, I., Trˇiska, J., Cudlin, P., & Šaslawsky, J. (2000). The use of mosses and pine needles to detect persistent organic pollutants at local and regional scales. Environmental Pollution, 109, 283–292. https://doi.org/10.1016/s0269-7491(99)00260-2
Hou, J., Sun, H., Zhou, Y., Zhang, Y., Yin, W., Xu, T., Cheng, J., Chen, W., & Yuan, J. (2018). Environmental exposure to polycyclic aromatic hydrocarbons, kitchen ventilation, fractional exhaled nitric oxide, and risk of diabetes among Chinese females. Indoor Air, 28, 383–393. https://doi.org/10.1111/ina.12453
Huang, S., Dai, C., Zhou, Y., Peng, H., Yi, K., Qin, P., Luo, S., & Zhang, X. (2018). Comparisons of three plant species in accumulating polycyclic aromatic hydrocarbons (PAHs) from the atmosphere: A review. Environmental Science and Pollution Research, 25, 16548–16566. https://doi.org/10.1007/s11356-018-2167-z
Hwang, H.-H., Wade, T. L., & Sericano, J. L. (2003). Concentrations and source characterization of polycyclic aromatic hydrocarbons in pine needles from Korea, Mexico, and United States. Atmospheric Environment, 37, 2259–2267. https://doi.org/10.1016/S1352-2310(03)00090-6
IARC-International Agency for Research on Cancer . Outdoor Air Pollution/IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC-International Agency for Research on Cancer; Lyon, France: 2013. [(accessed on 02 February 2023)]. Available online: https://publications.iarc.fr/_publications/media/download/4317/b1f528f1fca20965a2b48a220f47447c1d94e6d1.pdf.
Kolodziej, B., Maksymiec, N., Drozdzal, K., & Antonkiewicz, J. (2012). Effect of traffic pollution on chemical composition of raw elderberry (Sambucus nigra L.). Journal of Elementology, 17, 67–78. https://doi.org/10.5601/jelem.2012.17.1.06
Lehndorff, L., & Schwark, L. (2004). Biomonitoring of air quality in the Cologne Conurbation using pines needles as a passive sampler—part II: Polycyclic aromatic hydrocarbons (PAH). Atmospheric Environment, 38, 3793–3808. https://doi.org/10.1016/j.atmosenv.2004.03.065
Moeckel, C., Thomas, G. O., Barber, J. L., & Jones, K. C. (2008). Uptake and storage of PCBs by plant cuticles. Environmental Science and Technology, 42, 100–105. https://doi.org/10.1021/es070764f
Moretto, L. M., Silvestri, S., Ugo, P., Zorzi, G., Abbondanzi, F., Baiocchi, C., & Lacondini, A. (2005). Polycyclic aromatic hydrocarbons degradation by composting in a soot-contaminated alkaline soil. Journal of Hazardous Materials, 126, 141–148.
Navarro-Ortega, A., Ratola, N., Hildebrandt, A., Alves, A., Lacorte, S., & Barceló, D. (2012). Environmental distribution of PAHs in pine needles, soils, and sediments. Environmental Science Pollution Research, 19, 677–688. https://doi.org/10.1016/j.jhazmat.2005.06.020
Niu, L., Xu, C., Zhou, Y., & Liu, W. (2019). Tree bark as a biomonitor for assessing the atmospheric pollution and associated human inhalation exposure risks of polycyclic aromatic hydrocarbons in rural China. Environmental Pollution, 246, 398–407. https://doi.org/10.1016/j.envpol.2018.12.019
Pereira, G. M., da Silva, S. E., Mota, E. Q., Parra, Y. J., Castro, P., & Vasconcellos, P. D. (2019). Polycyclic aromatic hydrocarbons in tree barks, gaseous and particulate phase samples collected near an industrial complex in Sao Paulo (Brazil). Chemosphere, 237, 124499. https://doi.org/10.1016/j.chemosphere.2019.124499
Perera, F. P., Chang, H. W., Tang, D., Roen, E. L., Herbstman, J., Margolis, A., Huang, T. J., Miller, R. L., Wang, S., & Rauh, V. (2014). Early-Life Exposure to Polycyclic Aromatic Hydrocarbons and ADHD Behavior Problems. PLoS ONE, 9, e111670. https://doi.org/10.1371/journal.pone.0111670
Piccardo, M. T., Pala, M., Bonaccurso, B., Stella, A., Redaelli, A., Paola, G., & Valerio, F. (2005). Pinus nigra and Pinus pinaster needles as passive samplers of polycyclic aromatic hydrocarbons. Environmental Pollution, 133, 293–301. https://doi.org/10.1016/j.envpol.2004.05.034
Prajapati, S. K., & Tripathi, B. D. (2008). Biomonitoring seasonal variation of urban air polycyclic aromatic hydrocarbons (PAHs) using Ficus benghalensis leaves. Environmental Pollution, 151, 543–548. https://doi.org/10.1016/j.envpol.2007.04.013
Preuss, R., Angerer, J., & Drexler, H. (2003). Naphthalene- an environmental and occupational toxicant. International Archives of Occupational and Environmental Health, 76, 556–576. https://doi.org/10.1007/s00420-003-0458-1
Pulster, E. L., Johnson, G., Hollander, D., McCluskey, J., & Harbison, R. (2019). Levels and sources of atmospheric polycyclic aromatic hydrocarbons surrounding an oil refinery in Curacao. Journal of Environmental Protection, 10, 431–453. https://doi.org/10.4236/jep.2019.103025
Ratola, N., Alves, A., & Psillakis, E. (2011a). Biomonitoring of Polycyclic Aromatic Hydrocarbons Contamination in the Island of Crete Using Pine Needles. Water, Air, and Soil Pollution, 5, 189–203.
Ratola, N., Amigo, J. M., & Alves, A. (2010). Levels and Sources of PAHs in Selected Sites from Portugal: Biomonitoring with Pinus pinea and Pinus pinaster Needles. Archives of Environmental Contamination and Toxicology, 58, 631–647. https://doi.org/10.1007/s00244-009-9462-0
Ratola, N., Amigo, J. M., Oliveira, M. S. N., Araújo, R., Silva, J. A., & Alves, A. (2011b). Differences between Pinus pinea and Pinus pinaster as bioindicators of polycyclic aromatic hydrocarbons. Environmental and Experimental Botany, 72, 339–347. https://doi.org/10.1016/j.envexpbot.2011.04.012
Ratola, N., Herbert, P., & Alves, A. (2012). Microwave-assisted headspace solid-phase microextraction to quantify polycyclic aromatic hydrocarbons in pine trees. Analytical and Bioanalytical Chemistry, 403, 1761–1769. https://doi.org/10.1007/s00216-012-5962-2
Ratola, N., Lacorte, S., Barcelo, D., & Alves, A. (2009). Microwave-assisted extraction and ultrasonic extraction to determine polycyclic aromatic hydrocarbons in needles and bark of Pinus inaster Ait. and Pinus pinea L. by GC–MS. Talanta, 77, 1120–1128. https://doi.org/10.1016/j.talanta.2008.08.010
Rodriguez, J. H., Pignata, M. L., Fangmeier, A., & Klumpp, A. (2010). Accumulation of polycyclic aromatic hydrocarbons and trace elements in the bioindicator plants Tillandsia capillaris and Lolium multiflorum exposed at PM10 monitoring stations in Stuttgart (Germany). Chemosphere, 80, 208–215. https://doi.org/10.1016/j.chemosphere.2010.04.042
Rodriguez, J. H., Wannaz, E. D., Salazar, M. J., Pignata, M. L., Fangmeier, A., & Franzaring, J. (2012). Accumulation of polycyclic aromatic hydrocarbons and heavy metals in the tree foliage of Eucalyptus rostrata, Pinus radiata and Populus hybridus in the vicinity of a large aluminium smelter in Argentina. Atmospheric Environment, 55, 35–42. https://doi.org/10.1016/j.atmosenv.2012.03.026
Rumana, H. S., Sharma, R. C., Beniwal, V., & Sharma, A. K. (2014). A retrospective approach to assess human health risks associated with growing air pollution in urbanized area of Thar Desert, Western Rajasthan, India. Journal of Environmental Health Science and Engineering, 12, 23. https://doi.org/10.1186/2052-336X-12-23
Sari, M. F., Esen, F., & Tasdemir, Y. (2021). Characterization, source apportionment, air/plant partitioning and cancer risk assessment of atmospheric PAHs measured with tree components and passive air sampler. Environmental Research, 194, 110508. https://doi.org/10.1016/j.envres.2020.110508
Sawidis, T., Breuste, J., Mitrovic, M., Pavlovic, P., & Tsigaridas, K. (2011). Trees as bioindicator of heavy metal pollution in three European cities. Environmental Pollution, 159, 3560–3570. https://doi.org/10.1016/j.envpol.2011.08.008
Scarr, M.J. The use of stomatal frequency from three Australian evergreen tree species as a proxy indicator of atmospheric carbon dioxide concentration. Thesis. Victoria University, Australia.
Silva, J. A., Ratola, N., Ramos, S., Homem, V., Santos, L., & Alves, A. (2015). An analytical multi-residue approach for the determination of semi-volatile organic pollutants in pine needles. Analytica Chimica Acta, 858, 24–31. https://doi.org/10.1016/j.aca.2014.12.042
Simonich, S. L., & Hites, R. A. (1995). Organic pollutant accumulation in vegetation. Environmental Science & Technology, 29, 2095–2103. https://doi.org/10.1021/es00012a004
Solgi, E., Keramaty, M., & Solgi, M. (2020). Biomonitoring of airborne Cu, Pb, and Zn in an urban area employing a broad leaved and a conifer tree species. Journal of Geochemical Exploration, 208, 106400. https://doi.org/10.1016/j.gexplo.2019.106400
Tao, Z., & Hornbuckle, K. C. (2001). Uptake of polycyclic aromatic hydrocarbons (PAHS) by broad leaves: Analysis of kinetic limitations. Water, Air, & Soil Pollution, 1, 275–283. https://doi.org/10.1023/A:1013136028586
Tham, Y. W. F., Takeda, K., & Sakugawa, H. (2008). Polycyclic aromatic hydrocarbons (PAHs) associated with atmospheric particles in Higashi Hiroshima, Japan: Influence of meteorological conditions and seasonal variations. Atmospheric Research, 88, 224–233. https://doi.org/10.1016/j.atmosres.2007.10.015
Tian, L., Yin, S., Ma, Y., Kang, H., Zhang, X., Tan, M., & H., & Liu, C. (2019). Impact factor assessment of the uptake and accumulation of polycyclic aromatic hydrocarbons by plant leaves: Morphological characteristics have the greatest impact. Science of the Total Environment, 652, 1149–1155. https://doi.org/10.1016/j.scitotenv.2018.10.357
Tomashuk, T. A., Truong, T. M., Mantha, M., & McGowin, A. E. (2012). Atmospheric polycyclic aromatic hydrocarbon profiles and sources in pine needles and particulate matter in Dayton, Ohio, USA. Atmospheric Environment, 51, 196–202. https://doi.org/10.1016/j.atmosenv.2012.01.028
Topolska, J., Kostecka-Gugała, A., Ostachowicz, B., & Latowski, D. (2020). Selected metal content and antioxidant capacity of Sambucus nigra flowers from the urban areas versus soil parameters and traffic intensity. Environmental Science and Pollution Research, 27, 668–677. https://doi.org/10.1007/s11356-019-06921-1
Uribe, D. M., Ortega, L. M., Grassi, M. T., Dolatto, R. G., & Sánchez, N. E. (2023). Lichns as bio-monitors of polycyclic aromatic hydrocarbons: Measuring the impact of features and traffic patterns. Heliyon, 9, e20087. https://doi.org/10.1016/j.heliyon.2023.e20087
Viteri, F., Sánchez, N. E., & Alexandrino, K. (2023). Determination of polycyclic aromatic hydrocarbons (PAHs) in leaf and bark samples of Sambucus nigra using high-Performance Liquid Chromatography (HPLC). Methods and Protocols., 6, 17. https://doi.org/10.3390/mps6010017
Yamamoto, S. S., Phalkey, R., & Malik, A. A. (2014). A systematic review of air pollution as a risk factor for cardiovascular disease in South Asia: Limited evidence from India and Pakistan. International Journal of Hygiene Environmental Health, 217, 133–144. https://doi.org/10.1016/j.ijheh.2013.08.003
van Drooge, B. L., Garriga, G., & Grimalt, J. O. (2014). Polycyclic aromatic hydrocarbons in pine needles (Pinus halepensis) along a spatial gradient between a traffic intensive urban area (Barcelona) and a nearby natural park. Atmospheric Pollution Research, 5, 398–403. https://doi.org/10.5094/APR.2014.046
Wang, Z., Chen, J., Yang, P., Tian, F., Qiao, X., Bian, H., & Ge, L. (2009). Distribution of PAHs in pine (Pinus thunbergii) needles and soils correlates with their gas-particle partitioning. Environmental Science and Technology, 43, 1336–1341. https://doi.org/10.1021/es802067e
Warwick, R. M. A., & biomass comparison method,. (2008). In S. V. Jorgensen & B. Fath (Eds.), Encyclopedia of Ecology (pp. 11–15). Elsevier.
Wu, C.Y., Cabrera-Rivera, O., Dettling, J., Asselmeier, D., McGeen, D., Ostrander, A., Lax, J., Mancilla, C., Velalis, T., Bates, J., Wong, P., & Doan, C. (2012). An Assessment of Benzo(a)pyrene Air Emissions in the Great Lakes Region.
Wu, X., Liu, H., Yuan, Z., Wang, S., Chen, A., & He, B. (2019). Concentration, exchange and source identification of polycyclic aromatic hydrocarbons in soil, air and tree bark from the Middle-Lower Yangtze. Atmospheric Pollution Research, 10, 1276–1283. https://doi.org/10.1016/j.apr.2019.02.011
Yang, P., Chen, J., Wang, Z., Qiao, X., Cai, X., Tian, F., & Ge, L. (2007). Contributions of deposited particles to pine needle polycyclic aromatic hydrocarbons. Journal of Environment Monitoring, 9, 1248–1253. https://doi.org/10.1039/B708508G
Yang, P., Wang, Z., Chen, J. W., Tian, F. L., & Ge, L. K. (2008). Influences of pine needles physiological properties on the PAH accumulation. Huanj Jing Ke Xue, 29, 2019–2023.
Yin, H., Tan, Q., Chen, Y., Lv, G., & Hou, X. (2011). Polycyclic aromatic hydrocarbons (PAHs) pollution recorded in annual rings of gingko (Gingko biloba L.): Determination of PAHs by GC/MS after accelerated solvent extraction. Microchemical Journal, 97, 138–143. https://doi.org/10.1016/j.microc.2010.08.008
Zapata-Carbonell, J., Bégeot, C., Carry, N., Choulet, F., Delhautal, P., Gillet, F., Girardclos, O., Mouly, A., & Chalot, M. (2019). Spontaneous ecological recovery of vegetation in a red gypsum landfill: Betula pendula dominates after 10 years of inactivity. Ecological Engineering, 132, 31–40. https://doi.org/10.1016/j.ecoleng.2019.03.013
Acknowledgements
This work was funded by Universidad de Las Américas (Grant number: AMB.KAF.21.02), Quito, Ecuador. The authors express their gratitude to the Environment Ministry (MAE) for providing permission to collect the vegetable species samples (authorization number MAAE-ARSFC-2021-1255).
Funding
This work was funded by Universidad de Las Américas (Grant number: AMB.KAF.21.02), Quito, Ecuador.
Author information
Authors and Affiliations
Contributions
FV and KA contributed to the study conception, design, and material preparation. Data collection and analyses were performed by KA. Interpretation of data was performed by FV, NES, and KA. The first draft of the manuscript was written by NES and KA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Article note
The original online version of this article was revised: The author ‘Fausto Viteri’ was incorrectly denoted as the corresponding author. The only corresponding author of the article should be Katiuska Alexandrino.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alexandrino, K., Sánchez, N.E. & Viteri, F. Levels and sources of polycyclic aromatic hydrocarbons (PAHs) near hospitals and schools using leaves and barks of Sambucus nigra and Acacia melanoxylon. Environ Geochem Health 46, 32 (2024). https://doi.org/10.1007/s10653-023-01825-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10653-023-01825-z