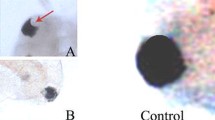
Abstract
The objective of this study was to evaluate the effects of AgNPs on Artemia salina and Allium cepa, evaluating the influence of the dilution solutions on the particle behavior. The AgNPs were synthesized by chemical reduction of AgNO3 (3 and 5 mmol L−1) with sodium borohydride and stabilized with PVA (polyvinyl alcohol) and CMC (sodium carboxymethyl cellulose). The toxicity of AgNPs was evaluated in Artemia salina (mortality) using Meyer’s solution as a diluent and in Allium cepa (chromosomal aberrations) using reconstituted hard water. AgNPs showed characteristic molecular absorption bands. Particles with CMC presented hydrodynamic radius between 4 and 102 nm and with PVA between 7 and 46 nm. The studied dispersions were toxic to A. salina species. Meyer’s solution, used as dilution water in the test, caused precipitation of Ag+ and also caused changes in CMC-stabilized AgNPs, changing the shape of the nanoparticles by depositing precipitates on their surface. These changes make the results of toxicity difficult to interpret. AgNPs stabilized with PVA remained unchanged. AgNPs affected cell division and caused the appearance of chromosomal aberrations on A. cepa. Higher numbers of chromosomal aberrations occurred in dispersions with smaller particle diameters (AgNPs3-PVA and AgNPs5-PVA, without dilution). In the studied conditions the dispersions were toxic to the tested organisms, the concentrations of precursors and the type of stabilizer used influenced the particle size and toxicity. In the test with A. cepa, the reconstituted hard water did not cause changes in the dispersions of AgNPs, whereas for A. salina the Meyer solution promoted aggregation of the particles and precipitation, in the dispersions stabilized with CMC, thus changing the samples.








Similar content being viewed by others
Data availability
The supporting data sets are restricted and not publicly available, and the manuscript details the methodologies used that can be reproduced.
References
An HJ, Sarkheil M, Parka HS, Yuc J, Johari SA (2019) Comparative toxicity of silver nanoparticles (AgNPs) and silver nanowires (AgNWs) on saltwater microcrustacean, Artemia salina. Comp Biochem Physiol C 218:62–69
Angel BM, Batley GE, Jarolimek CV, Rogers NJ (2013) The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 93:359–365
APHA - American Public Health Association (1998) American Water Works Association (AWWA): Water Pollution Control Federation (WPCF). Standard methods for the examination of water and wastewater, 20 ed. Water Environmental Federation, Washington DC, 1085
APHA - American Public Health Association (2005) American Water Works Association (AWWA): Water Pollution Control Federation (WPCF). Standard methods for the examination of water and wastewater, 21 ed. Water Environmental Federation, Washington DC, 1082
Ates M, Arslan Z, Demir V, Daniels J, Farah IO (2015) Accumulation and toxicity of CuO and ZnO nanoparticles through waterborne and dietary exposure of goldfish (Carassius auratus). Environ Toxicol 30:119–128
Bakare A, Mosuro A, Osibanjo O (2000) Effect of simulated leachate on chromosomes and mitosis in roots of Allium cepa (L.). J Environ Biol 21:263–271
Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY (2009) Toxicity assessment of multisized gold and silver nanoparticles in Zebrafish embryos. Small 16:1897–1910
Barrena R, Casals E, Colón J, Font X, Sánchez A, Puntes V (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75:850–857
Becaro AA, Jonsson CM, Puti FC, Siqueira MC, Mattoso LHC, Correa DS, Ferreira MD (2015) Toxicity of PVA-stabilized silver nanoparticles to algae and microcrustaceans. Environ Nanotechno Monit Manage 3:22–29
Benn TM, Werterhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42:4133–4139
Bradford A, Handy RD, Readman JW, Atfield A, Muhling M (2009) Impact of silver nanoparticle contamination on the genetic diversity of natural bacterial assemblages in estuarine sediments. Environ Sci Technol 43:4530–4536
Burić P, Jakšić Ž, Štajner L, Sikirić MD, Jurašin D, Cascio C, Calzolai L, Lyons DM (2015) Effect of silver nanoparticles on Mediterranean sea urchin embryonal development is species specific and depends on moment of first exposure. Mar Environ Res 111:50–59
Cabrera GL, Rodriguez DMG (1999) Genotoxicity of soil from farmland irrigated with wastewater using three plant bioassays. Mutat Res 426:211
Chae YJ, Pham CH, Lee J, Bae E, Yi J, Gu MB (2009) Evaluation of the toxic impact of silver nanoparticles on Japanese medaka (Oryzias latipes). Aquat Toxicol 94:320–327
Chambers BA, Afrooz ARMN, Bae S, Aich N, Katz L, Saleh NB, Kirisits MJ (2013) Effects of chloride and ionic strength on physical morphology, dissolution, and bacterial toxicity of silver nanoparticles. Environ Sci Technol 48:761–769
Chen KL, Elimelech M (2009) Relating colloidal stability of fullerene (C-60) nanoparticles to nanoparticle charge and electrokinetic properties. Environ Sci Technol 43:7270–7276
Chinnapongse SL, MacCuspie RI, Hackley VA (2011) Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci Total Environ 409:2443–2450
Choi O, Deng KK, Hu Z (2008) The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Research 42:3066–3074
Cumberland SA, Lead JR (2009) Particle size distributions of silver nanoparticles at environmentally relevant conditions. J Chromatogr A 1216:9099–9105
Delay M, Dolt T, Woellhaf A, Sembritzki R, Frimmel FH (2011) Interactions and stability of silver nanoparticles in the aqueous phase: influence of natural organic matter (NOM) and ionic strength. J Chromatogr A 1218:4206–4212
Dietz K, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16:582–589
El Badawy AM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM (2010) Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol 44:1260–1266
El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM (2011) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45:283–287
El-Temsah YS, Joner EJ (2012) Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol 27:42–49
Fabrega J, Fawcett SR, Renshaw JC, Lead JR (2009) Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ Sci Technol 43:7285–7290
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531
Falugi C, Aluigi MGA, Faimali M, Ferrando S, Gambardella C, Gatti AM, Ramoino P (2013) Dose dependent effects of silver nanoparticles on reproduction and development of different biological models. Int J Environ Qual 8:61–65
Feulgen R, Rossenbeck H (1924) Mikroskopisch-chemischer nachweis einer nucleinsäure vom typus der thymonucleinsaure und die darauf beruhende elektive farbung vom zelikernen in mikroskopischen praparaten. Zts Phys Chem 135:203–248
Fiskesjo G (1997) Allium test for screening chemicals; evaluation of cytological parameters. In: Wang W, Gorsuch JW, Hughes JS (Eds) Plants for Environmental Studies. CRC Lewis Publishers, Boca Raton, New York, NY, p 307–333
Gaiser BK, Fernandes TF, Jepson M, Lead JR, Tyler CR, Stone V (2009) Assessing exposure, uptake and toxicity of silver and cerium dioxide nanoparticles from contaminated environments. Environ Health 8:1–4
Gangadharan D, Harshvardan K, Gnanasekar G, Dixit D, Popat KM, Anand PS (2010) Polymeric microspheres containing silver nanoparticles as a bactericidal agent for water disinfection. Water Research 44:5481–5487
Grant WF (1982) Chromosome aberration assays in Allium: a report of the U.S. environmental protection agency gene-tox program. Mut Res 99:273–291
Greilhuber J, Ebert I (1994) Genome size variation in Pisum sativum. Genome 37:646–655
Hamilton MA, Russo RC, Thurston RV (1997) Trimmed Spearman Karber statistical method for estimating median lethal concentration in toxicity bioassays. Environ Sci Technol 11:714–719
Hänsch M, Emmerling C (2010) Effects of silver nanoparticles on the microbiota and enzyme activity in soil. J Plant Nutr Soil Sci 173:554–558
Hoshina M, Marin-Morales MA (2009) Micronucleus and chromosome aberrations induced in onion (Allium cepa) by a petroleum refinery effluent and by river water that receives this effluent. Ecotoxicol Environ Saf 72:2090–2095
Hund-rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ Sci Pollut Res Int 13:225–232
Huynh KA, Chen KL (2011) Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ Sci Technol 45:5564–5571
ISO/TS 20787 (2017) Nanotechnologies - Aquatic Toxicity Assessment of Manufactured Nanomaterials in Saltwater Lakes Using Artemia sp. Nauplii. ISO, Geneva
Jin R, Cao YW, Mirkin CA, Kelly KL, Schatz GC, Zheng JG (2001) PhotoInduced conversion of silver nanospheres to nanoprisms. Science 94:1901–1903
Johari SA, Rasmussen K, Gulumian M, Ghazi-Khansari M, Tetarazako N, Kashiwada S, Asghari S, Park JW, Yu IJ (2019) Introducing a new standardized nanomaterial environmental toxicity screening testing procedure, ISO/TS 20787: aquatic toxicity assessment of manufactured nanomaterials in saltwater lakes using Artemia sp. Nauplii. Toxicol Mech Methods 29:95–109
Kaegi I, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M, Siegrist H (2011) Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ Sci Technol 45:3902–3908
Kanaya N, Gill BS, Grover IS, Murin A, Osiecka R, Sandhu SS, Andersson HC (1994) Vicia faba chromosomal aberration assay. Mutat Res 310:231–247
Khaydarov RR, Khaydarov RA, Estrin Y, Evgrafova S, Scheper T, Endres C, Cho SY (2009) Silver nanoparticles environmental and human health impacts. In: Linkov I, Steevens J (eds.) Nanomaterials: Risks and Benefits. Springer Science + Business Media B.V. pp 287-297
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851
Kumar N, Shah V, Walker VK (2011) Perturbation of an arctic soil microbial community by metal nanoparticles. J Hazard Mater 190:816–822
Kumar P, Selvi SS, Prabha L, Selvaraj M, Rani LM, Palanisamy SP, Boominathan SD, Munisamy G (2012) Antibacterial activity and in-vitro cytotoxicity assay against brine shrimp using silver nanoparticles synthesized from Sargassum ilicifolium. Dig J Nanomater Biostruct 7:1447–1455
Kumari M, Mukherjee A, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246
Kwok KW, Auffan M, Badireddy AR, Nelson CM, Wiesner MR, Chilkoti A, Liu J, Marinakosc SM, Hinton DE (2012) Uptake of silver nanoparticles and toxicity to early life stages of Japanese medaka (Oryzias latipes): effect of coating materials. Aquat Toxicol 120:59–66
Lee M, Meisel D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86:3391–3395
Leff DV, Ohara PC, Heath JR, Gelbart WM (1995) Thermodinamic control of gold nanocrystal size: experiment and theory. J Phys Chem 99:7036–7041
Levard C, Hotze EM, Colman BP, Dale AL, Truong L, Yang XY, Bone AJ, Brown GE, Tanguay RL, Di Giulio RT, Bernhardt ES, Meyer JN, Wiesner MR, Lowry GV (2013a) Sulfidation of silver nanoparticles: natural antidote to their toxicity. Environ Sci Technol 47:13440–13448
Levard C, Hotze EM, Lowry GV, Brown Jr GE (2012) Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 46:6900–6914
Levard C, Mitra S, Yang T, Jew AD, Badireddy AR, Lowry GV, Brown GE (2013b) Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli. Environ Sci Technol 47:5738–5745
Li X, Lenhart JJ, Walker HW (2010) Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 26:16690–16698
Libralato G (2014) The case of Artemia spp. in nanoecotoxicology. Mar Environ Res 101:38–43
Lish RAD, Johari SA, Sarkheil M, Yu IJ (2019) On how environmental and experimental conditions affect the results of aquatic nanotoxicology on brine shrimp (Artemia salina): a case of silver nanoparticles toxicity. Environ Pollut 255:113358
Liu W, Zhou Q, Liu J, Fu J, Liu S, Jiang G (2011) Environmental and biological influences on the stability of silver nanoparticles. Chin Sci Bull 56:2009–2015
Liu Z, Zhou Y, Maszenan AM, Ng WJ, Liu Y (2013) pH-dependent transformation of Ag nanoparticles in anaerobic processes. Environ Sci Technol 47:12630–12631
Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PKH, Chiu JF, Che CM (2007) Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem 12:527–534
Ma R, Levard C, Judy JD, Unrine JM, Durenkamp M, Martin B, Jefferson B, Lowry GV (2013) Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processed biosolids. Environ Sci Technol 48:104–112
Magesky A, Ribeiro CAO, Beaulieu L, Pelletier E (2016) Physiological effects and cellular responses of metamorphic larvae and juveniles of sea urchin exposed to ionic and nanoparticulate silver. Aquat Toxicol 174:208–227
Magesky A, Ribeiro CAO, Beaulieu L, Pelletier E (2017) Silver nanoparticles and dissolved silver activate contrasting immune responses and stress-induced heat shock protein expression in sea urchin (Report). Environ Toxicol Chem 36:1872–1886
Manfra L, Savorelli F, Pisapia M, Magaletti E, Cicero AM (2012) Long-term lethal toxicity test with the crustacean Artemia franciscana. J Vis Exp 62:3790
Marambio-Jones C, Hoek EMV (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551
Maynard AD (2006) Nanotechnology: a research strategy for addressing risk. Woodrow Wilson International Center for Scholars, Washington, D.C
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, Mclaughlin JL (1982) Brine Shrimp: a convenient general bioassay for active plant constituents. J Med Plants Res 45:31–34
Miao AJ, Luo Z, Chen CS, Chin WC, Santschi PH, Quigg A (2010) Intracellular uptake: a possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS ONE 5:e15196. https://doi.org/10.1371/journal.pone.0015196
Mock JJ, Barbic CM, Smith DR, Schultz DA, Schultz S (2002) Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J Chem Phys 116:6755–6759
Mulfinger L, Solomon SD, Bahadory M, Jeyarajasingam AV, Rutkowsky SA, Boritz C (2007) Synthesis and study of silver nanoparticles. J Chem Educ 84:322. https://doi.org/10.1021/ed084p322
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Oliver ALS, Croteau MN, Stoiber TL, Tejamaya M, Römer I, Lead JR, Luoma SN (2014) Does water chemistry affect the dietary uptake and toxicity of silver nanoparticles by the freshwater snail Lymnaea stagnalis? Enviorn Pollut 189:87–91
Panyala NR, Pena-Mendez EM, Havel J (2008) Silver or silver nanoparticles: a hazardous threat to the environment and human health? J Appl Biomed 6:117–129
Quadros ME, Marr LC (2010) Environmental and human health risks of aerosolized silver nanoparticles. J Air Waste Manage Assoc 60:770–781
Rahaman MN (2003) Ceramic processing and sintering. 2rd ed. Marcel Dekker, New York, NY, p 181–247
Ratte HT (1999) Bioaccumulation and toxicity of silver compounds: a review. Environ Toxicol Chem 18:89–108
Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet JB (2008) Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J Antimicrob Chemother 61:869–876
Roh J-Y, Sim SJ, Yi J, Park K, Chung KH, Ryu D-Y, Choi J (2009) Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ Sci Techno 43:3933–3940
Selvaraj M, Kumar TS, Rao MV (2017) Anticancer activity of biogenic nanosilver and its toxicity assessment on Artemia salina - evaluation of mortality, accumulation and elimination: An experimental report. J Environ Chem Eng 5:1685–1695
Šiller L, Lemloh ML, Mendis BG, Horrocks BR, Brümmer F, Davorin Medaković D (2013) Silver nanoparticle toxicity in sea urchin Paracentrotus lividus. Environl Pollut 178:498–502
Skoog DA, West DM, Holler FJ (2003) Fundamentals of analytical chemistry. Saunders College Pub, New York, NY
Stampoulis D, Sinha SK, White JC (2009) Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol 43:9473–9479
Starov VM (2010) Nanoscience: colloidal and interfacial aspects. CRC Press, Boca Raton
Thuptimdang P, Limpiyakorn T, McEvoy J, Prüß BM, Khan E (2015) Effect of silver nanoparticles on Pseudomonas putida biofilms at different stages of maturity. J Hazard Mater 290:127–133
Tian J, Wong KKY, Ho CM, Lok CN, Yu WY, Che CM, Chiu JF, Tam PKH (2007) Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2:129–136
Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M (2010) An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ 408:999–1006
Verano-Braga T, Miethling-Graff R, Wojdyla K, Wezesinska-Rogowska A, Brewer JR, Erdmann H, Kjeldsen F (2014) Insights into the cellular response triggered by Ag nanoparticles using quantitative proteomics. ACS publications nano 8:2161–2175
Wang H, Burgess RM, Cantwell MG, Portis L, Perron MM, Wu FC, Ho KT (2014a) Stability and aggregation of silver and titanium dioxide nanoparticles in seawater: role of salinity and dissolved organic carbon. Environ Toxicol Chem 33:1023–1029
Wang H, Ho KT, Scheckel KG, Wu F, Cantwell MG, Katz DR, Horowitz DB, Boothman WS, Burgess RM (2014b) Toxicity, bioaccumulation, and biotransformation of silver nanoparticles in marine organisms. Environ Sci Technol 48:13711–13717
Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gösens I, Van de Meent D, Dekkers S, De Jong WH, Van Zijverden M, Sips AJAM, Geertsma RE (2009) Nano-silver — a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3:109–138
Wirth SM, Lowry GV, Tilton RD (2012) Natural organic matter alters biofilm tolerance to silver nanoparticles and dissolved Silver. Environ Sci Technol 46:12687–12696
Xiu Z-M, Ma J, Alvarez PJJ (2011) Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol 45:9003–9008
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45:2360–2367
Zhang L, Li X, He R, Wu L, Zhang L, Zeng J (2015) Chloride-induced shape transformation of silver nanoparticles in a water environment. Environ Pollut 204:145–151
Zhao CM, Wang WX (2010) Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna. Environ Sci Technol 44:7699–7704
Zhao CM, Wang WX (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30:88–892
Funding
The authors are grateful to National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES) of Brazil for financial support.
Author information
Authors and Affiliations
Contributions
SMP coordinated the research and supervised the master student JCBA and graduated students LKF and MTMD in laboratory activities, interpretation of results, writing of reports, and in the master’s dissertation; EAC co-supervised the master’s student and contributed to the characterization of silver nanoparticles. Co-author MTV collaborated in interpreting the results, writing the paper and revising it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
All authors participated in the preparation of the paper and agree with the publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palácio, S.M., de Almeida, J.C.B., de Campos, É.A. et al. Silver nanoparticles effect on Artemia salina and Allium cepa organisms: influence of test dilution solutions on toxicity and particles aggregation. Ecotoxicology 30, 836–850 (2021). https://doi.org/10.1007/s10646-021-02393-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02393-7