Abstract
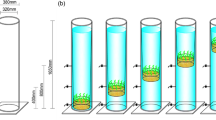
Sediment anoxia generally results from intense organic enrichment and is a limiting factor in the restoration of vegetation in eutrophic waters. To investigate the effect of sediment anoxia on a typical pollution-tolerant submerged macrophyte species, Hydrilla verticillata, and acclimation mechanisms in the plant, a gradient of sediment anoxia was simulated with additions of sucrose to the sediment, which can stimulate increased concentrations of total nitrogen, NH4 + and Fe in pore water. H. verticillata growth was significantly affected by highly anoxic conditions, as indicated by reduced total biomass in the 0.5 and 1 % sucrose treatments. However, slight anoxia (0.1 % sucrose addition) promoted growth, and the shoot biomass was 22.64 % higher than in the control. In addition to morphologic alterations, H. verticillata showed physiological acclimations to anoxia, including increased anaerobic respiration and changes in carbon and nitrogen metabolism in roots. The soluble protein and soluble carbohydrate contents in roots of the 1 % treatment were both significantly higher compared with those in the control. The increase in alcohol dehydrogenase activity and pyruvate content in the roots suggested that H. verticillata has a well-developed capacity for anaerobic fermentation. This study suggests that highly anoxic sediments inhibit the growth of H. verticillata and the species has a degree of tolerance to anoxic conditions. Further in situ investigations should be conducted on the interactions between sediment conditions and macrophytes to comprehensively evaluate the roles of sediment in the restoration of vegetation in eutrophic waters.







Similar content being viewed by others
References
Borum J, Sand-Jensen K, Binzer T, Pedersen O, Greve TM (2006) Oxygen movement in seagrasses. In: Larkum AWD et al (eds) Seagrasses: biology, ecology and conservation. Springer, Netherlands, pp 255–270
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein dye-binding. Anal Biochem 72:248–254
China Ministry of Environmental Protection (2002) Detection and analysis method of water and waste water. China Environmental Science Press, Beijing
Colmer TD, Flowers TJ (2008) Flooding tolerance in halophytes. New Phytol 179(4):964–974
Conley DJ, Humborg C, Rahm L, Savchuk OP, Wulff F (2002) Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ Sci Technol 36(24):5315–5320
Dai Y, Jia C, LiangW H, Wu Z (2012) Effects of the submerged macrophyte Ceratophyllum demersum L. on restoration of a eutrophic water body and its optimal coverage. Ecol Eng 40:113–116
Davey MW, Stals E, Panis B, Keulemans J, Swennen RL (2005) High-throughput determination of malondialdehyde in plant tissues. Anal Biochem 347(2):201–207
Gardner WS, McCarthy MJ, Carini SA, Souza AC, Lijun H, McNeal KS et al (2009) Collection of intact sediment cores with overlying water to study nitrogen- and oxygen-dynamics in regions with seasonal hypoxia. Cont Shelf Res 29(18):2207–2213
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:1–47
Giordani G, Bartoli M, Cattadori M, Viaroli P (1996) Sulphide release from anoxic sediments in relation to iron availability and organic matter recalcitrance and its effects on inorganic phosphorus recycling. Hydrobiologia 329(1–3):211–222
Goodman JL, Moore KA, Dennison WC (1995) Photosynthetic responses of eelgrass (Zostera marina L.) to light and sediment sulfide in a shallow barrier island lagoon. Aquat Bot 50(1):37–47
Hietanen S, Lukkari K (2007) Effects of short-term anoxia on benthic denitrification, nutrient fluxes and phosphorus forms in coastal Baltic sediment. Aquat Microb Ecol 49:293–302
Holmer M, Bondgaard EJ (2001) Photosynthetic and growth response of eelgrass to low oxygen and high sulfide concentrations during hypoxic events. Aquat Bot 70(1):29–38
Hossain MA, Uddin SN (2011) Mechanisms of waterlogging tolerance in wheat: morphological and metabolic adaptations under hypoxia or anoxia. Aust J Crop Sci 5(9):1094–1101
Ismail AM, Ella ES, Vergara GV, Mackill DJ (2009) Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Ann Bot 103(2):197–209
Jackson MB, Colmer TD (2005) Response and adaptation by plants to flooding stress. Ann Bot 96(4):501–505
Kristiansen KD, Kristensen E, Jensen EMH (2002) The influence of water column hypoxia on the behavior of manganese and iron in sandy coastal marine sediment. Estuar Coast Shelf Sci 55(4):645–654
Kumutha D, Sairam RK, Ezhilmathi K, Chinnusamy V, Meena RC (2008) Effect of waterlogging on carbohydrate metabolism in pigeon pea (Cajanus cajan L.): upregulation of sucrose synthase and alcohol dehydrogenase. Plant Sci 175(5):706–716
Lake BA, Coolidge KM, Norton SA, Amirbahman A (2007) Factors contributing to the internal loading of phosphorus from anoxic sediments in six Maine, USA, lakes. Sci Total Environ 373(2–3):534–541
Lou L, Gao N (2005) Determination of pyruvate by ultraviolet spectrophotometry. Anal Res 24(4):11–13
Maricle BR, Crosier JJ, Bussiere BC, Lee RW (2006) Respiratory enzyme activities correlate with anoxia tolerance in salt marsh grasses. J Exp Mar Biol Ecol 337(1):30–37
Matsui T, Tsuchiya T (2006) Root aerobic respiration and growth characteristics of three Typha species in response to hypoxia. Ecol Res 21(3):470–475
Møller CL, Sand-Jensen K (2011) High sensitivity of Lobelia dortmanna to sediment oxygen depletion following organic enrichment. New Phytol 190(2):320–331
Møller CL, Sand-Jensen K (2012) Rapid oxygen exchange across the leaves of Littorella uniflora provides tolerance to sediment anoxia. Freshw Biol 57(9):1875–1883
Mustroph A, Albrecht G (2003) Tolerance of crop plants to oxygen deficiency stress: fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol Plant 117(4):508–520
Pan Y, Xie Y, Chen X, Li F (2012) Effects of flooding and sedimentation on the growth and physiology of two emergent macrophytes from Dongting Lake wetlands. Aquat Bot 100:35–40
Pérez M, Invers O, Ruiz JM, Frederiksen MS, Holmer M (2007) Physiological responses of the seagrass Posidonia oceanica to elevated organic matter content in sediments: an experimental assessment. J Exp Mar Biol Ecol 344(2):149–160
Raun AL, Borum J, Sand-Jensen K (2010) Influence of sediment organic enrichment and water alkalinity on growth of aquatic isoetid and elodeid plants. Freshw Biol 55(9):1891–1904
Slomp CP, Van der Gaast SJ, Van Raaphorst W (1996) Phosphorus binding by poorly crystalline iron oxides in North Sea sediments. Mar Chem 52(1):55–73
Terrados J, Duarte CM, Kamp-Nielsen L, Agawin NSR, Gacia E, Lacap D et al (1999) Are seagrass growth and survival constrained by the reducing conditions of the sediments? Aquat Bot 65(1–4):175–197
Wang X (2006) The principle and technology of physiological and biochemical experiment of plant. Higher Education Press, Beijing
Wu J, Cheng S, Liang W, He F, Wu Z (2009) Effects of sediment anoxia and light on turion germination and early growth of Potamogeton crispus. Hydrobiologia 628(1):111–119
Wu J, Cheng S, Li Z, Guo W, Zhong F, Yin D (2013a) Case study on rehabilitation of a polluted urban water body in Yangtze River basin. Environ Sci Pollut R 20(10):7038–7045
Wu J, Cui N, Cheng S (2013b) Effects of sediment anoxia on growth and root respiratory metabolism of Iris pseudacorus: implications for vegetation restoration of eutrophic waters in China. Ecol Eng 53:194–199
Yang J, Wang C, Dai H (2008) Textbook of soil chemical analysis and environmental evaluation. China Land Press, Beijing
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508–514
Yin D, Chen S, Chen F, Guan Z, Fang W (2009) Morphological and physiological responses of two chrysanthemum cultivars differing in their tolerance to waterlogging. Envrion Exp Bot 67(1):87–93
Yu H, Ye C, Song X, Liu J (2010) Comparative analysis of growth and PhysIO-biochemical responses of hydrilla verticillata to different sediments in freshwater microcosms. Ecol Eng 36(10):1285–1289
Zhang X, Liu Z (2011) Interspecific competition effects on phosphorus accumulation by hydrilla verticillata and Vallisneria natans. J Environ Sci 23(8):1274–1278
Zimmerman RC, Alberte RS (1996) Effect of light/dark transition on carbon translocation in eelgrass Zostera marina seedlings. Mar Ecol Prog Ser 136:305–309
Zimmerman AR, Canuel EA (2000) A geochemical record of eutrophication and anoxia in Chesapeake bay sediments: anthropogenic influence on organic matter composition. Mar Chem 69(1–2):117–137
Acknowledgments
This study was supported by the National Natural Science Foundation (NSFC No. 51108334) and Project of National Major Program of Science and Technology (2011ZX07303-001). Other anonymous help and suggestions were also appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wu, J., Dai, Y., Rui, S. et al. Acclimation of Hydrilla verticillata to sediment anoxia in vegetation restoration in eutrophic waters. Ecotoxicology 24, 2181–2189 (2015). https://doi.org/10.1007/s10646-015-1549-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1549-y