Abstract
Three submerged macrophytes, Hydrilla verticillata, Vallisneria natans, and Potamogeton crispus, were planted in winter and spring to investigate the environmental mechanism of phosphorus transformation during submerged macrophyte growth periods. The experimental buckets each contained 4,832.80 g of dry sediment (depth: 10 cm) and 100 L water. The phosphorus (P) fractions in the water and sediment were investigated along with the environmental factors, including pH, dissolved oxygen (DO), and redox potential (ORP). The concentrations of the different forms of P in the water and sediment were lower in the submerged macrophyte groups. Submerged macrophyte uptake was the primary reason for the decrease in P in the water and sediment. At the end of the experiment, the total amounts of phosphorus accumulated by H. verticillata, V. natans, and P. crispus were 156.05 g, 282.24 g, and 115.84 g, respectively, and the three submerged macrophytes enriched P by 4.18%, 7.56%, and 3.10%. The levels of the environmental factors pH, DO, and ORP were higher in the submerged macrophyte groups than the control groups. The environmental factors had different effects on the different forms of P during growth of the submerged macrophytes. H. verticillata primarily acted on the soluble reactive phosphorus (SRP) through environmental factors, while P. crispus acted on the dissolved total phosphorous, SRP, dissolved organic phosphorus, and particulate phosphorous through these factors. V. natans had a weak impact on P content in the water through the environmental factors. Hence, the transformation of P between the sediment and water was affected by environmental factors. Overall, the results of this study reveal the effects of environmental factors under submerged macrophyte growth on water purification as well as the migration and transformation of P in the sediment and on the surface and provide a theoretical basis for the ecological restoration of eutrophicated shallow lakes.
Article Highlights
-
Environmental factors had different effects on different forms of phosphorus transformation. The submerged macrophytes affected the migration and transformation of phosphorus by increasing pH, oxidation-redox potential and dissolved oxygen in the overlying water.
-
H. verticillata and P. crispus acted on the different forms of phosphorus through environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lake eutrophication is a serious problem that is largely caused by human modifications to the natural biogeochemical cycles of nitrogen and phosphorus (P). P is a primary cause of eutrophication because it amplifies positive feedback in eutrophication [1]. One of the most important factors influencing the P concentration of overlying water is the release of P from sediment, which may have a significant impact on water quality and result in continuing eutrophication[2]. P in overlying water may also be absorbed by sediment and accumulate on the bottom of lakes. Therefore, the factors that affect P transformation, bioavailability, and exchange between sediment and overlying water have been studied extensively. These factors have been identified as water temperature, water pH, oxidation-redox potential (ORP), dissolved oxygen (DO), and hydrological conditions [3].
Submerged macrophytes play an important role in P cycling, especially in shallow lakes, and have therefore attracted the interest of many researchers [4]. Various environmental factors, including pH, ORP, DO, and hydrological conditions, are influenced by the growth of submerged macrophytes and the mechanisms through which submerged macrophytes can act as P regulators in water have attracted considerable attention. It is generally accepted that submerged macrophytes reduce the concentration of P in the overlying water, primarily by the uptake of P from the water [5]. However, a recent mass balance study found that the total amount of P absorbed by submerged macrophytes was lower than the total decrease in P in the water, suggesting that the remaining P went into the sediment[6, 7]. P that has been absorbed by sediment may be influenced by environmental factors in submerged macrophyte-dominated aquatic systems [8]. Thus, an understanding of the relationship between environmental factors and P transformation between the sediment and water is necessary to study the influence of submerged macrophytes in aquatic systems.
However, not all P fractions can transfer between sediment and water. P remobilization from sediment is likely controlled by speciation, and it is important to know which portion of the stock can be mobilized [9]. Chemical extraction can be used to divide the P in sediment into different fractions [10], including labile, reductant, metal bound, occluded, and organic P. Moreover, in terms of the potential bioavailability, metal oxide-bound P, and calcium-bound P, the contents of different P fractions are useful for evaluating the potential P bioavailability in sediment. P in water can be divided into particulate phosphorous (PP) and dissolved total phosphorous (DTP). DTP can be sub-divided into dissolved organic phosphorus (DOP) and soluble reactive phosphorus (SRP). Although the phosphate absorption-release characteristics of sediment from shallow lakes have been extensively studied over the past 30 years, the majority of these studies were based on water–sediment systems [11]. Moreover, sediment was typically collected from lakes and delivered to the laboratory in these studies, after which environmental factors were controlled using chemical methods. Accordingly, many investigations have only investigated the influence of one environmental factor on P transformation, resulting in overemphasis of the effects of individual environmental factors [12]. However, P transformation is affected by a variety of factors, including pH, ORP, temperature, and hydrological conditions. In addition, while the results of these studies were based on water–sediment systems, many lake systems are water-macrophyte-sediment systems. Thus, these simulated laboratory results lack instructional and practical meaning.
The present study was performed under controlled, static conditions, with single species of macrophytes. Therefore, while the indoor experiment could imitate the condition of the field experiment, owing to the relatively tiny simulation system, many elements were difficult to regulate, resulting in fluctuations in many indicators of the water body. This should be connected to the lesser buffer capacity of the indoor simulation system.
In this study, a water-submerged macrophyte-sediment system was constructed and the P fractions and environmental factors were investigated during the submerged aquatic vegetation growth period to evaluate how P transformation precedes in a submerged macrophyte-dominated water environment. The specific aims of this study were as follows: (1) to evaluate which forms of P in water are affected by environmental factors under the growth of submerged macrophytes; (2) to study the relationship between environmental factors and P transformation; and (3) to evaluate the phosphorus enrichment ability of different submerged macrophytes.
2 Materials and methods
2.1 Seasonal experiments: sediment
Samples from the top 20 cm of the surface sediments were collected from the heavily polluted Linyi University Lake in Linyi, China (35°07′06.27″N, 118°16′54.39″E). The pollutants in the lake mainly come from the domestic sewage of the surrounding shops, and there were a few large submerged macrophytes in the water, such as P. crispus. This lake, which has a water surface area of 0.07 km2, has TP and TN concentrations of 0.74 and 38.92 mg L−1, respectively. The samples were collected using a core sampler that contained a Plexiglas cylinder tube (50 cm length × 10 cm diameter) in July 2020. Sediment samples were taken to the laboratory in sealed plastic bags on ice. Upon arrival at the laboratory, the samples were freeze-dried, passed through a 0.5 cm sieve to remove coarse debris, then mixed. The sand, slit, and clay of sediment were 9.71%, 79.49%, and 10.80%.
The homogenized sediment was added to high-density polyethylene 15 buckets (top diameter = 55 cm, bottom diameter = 45 cm, height = 75 cm). Each polyethylene bucket contained 4,832.80 g of dry sediment with a depth of 10 cm. A total of 100 L of deionized water was slowly added to the buckets, after which the sediments were allowed to equilibrate for one month, at which time samples were collected.
2.2 Submerged macrophyte materials
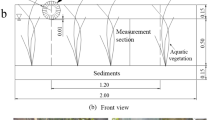
Potamogeton crispus germinates in autumn and grows during the winter and dies in the summer. Hydrilla verticillata and Vallisneria natans germinate in spring and grow during the summer and die in the autumn. So P. crispus was planted at the beginning of winter and H. verticillata and V. natans were planted in spring. The winter microcosm experiment was conducted from December 10, 2020 until April 29, 2021. Plant propagules of the submerged macrophyte, P. crispus, were collected from the Yi River in Linyi, China (35°05′N, 118°19′E) in May 2020. The propagules were stored in a laboratory incubator away from light until use. All of the plant propagules were centrifuged for approximately 15 s, then brushed carefully to remove adherent water and materials. Similarly, plant propagules were selected to determine the initial fresh weight (average fresh weight 251.63 g m−2). After weighing, plant propagules were assigned at random to plants in three buckets that contained sediment, with 60 propagules planted in each bucket (treatment condition). Additionally, three buckets were left unplanted as controls. All buckets received ambient greenhouse daylight and were kept at ambient temperature (Fig. 1). Water temperature did not differ by more than 2 °C among buckets. Losses in culture volume due to evapotranspiration were countered by the addition of deionized water to the original level every month.
The spring microcosm experiment was conducted from April 25, 2021 to September 12, 2021. The submerged macrophytes, H. verticillata and V. natans, were collected from the Yi River. The collected H. verticillata and V. natans had been cultured for more than one year. In this experiment, similar cultured submerged macrophyte apical shoots (30 cm long) were selected to determine the initial fresh weight. All of the submerged macrophytes were brushed carefully to remove adherent water and materials. After weighing, the plants were randomly assigned to buckets that contained sediments with 37 H. verticillata (average fresh weight 188.73 g m−2) and 17 V. natans (average fresh weight 188.73 g m−2) per bucket. H. verticillata and V. natans have different qualities. To ensure the same biomass in the experimental group, H. verticillata and V. natans were planted in different numbers. H. verticillata and V. natans were planted in separate buckets. They planted in the sediment. Additionally, three buckets were prepared for each treatment and control (Fig. 1b).
All other procedures were conducted in the same manner as for the winter experiment.
2.3 Sampling and analysis
Water samples were collected from 0.25 m below the surface on days 1, 20, 40, 60, 80, 100, 120, and 140 during the winter and spring experiments. One sample per bucket. Water samples (50 mL) were collected from the surfaces of the buckets and kept at 0–4 °C for analysis within 24 h, after which an appropriate amount of deionized water was added to compensate for the loss of water and evaporation.
Sediment samples were collected using a hand-driven polyethylene corer (patent number: ZL201420135437.1), sealed in plastic bags and immediately taken to the laboratory. One sample per bucket. Upon arrival in the laboratory, samples were put on ice, freeze-dried, and sieved (< 2 mm). Sediment samples (depth 10 cm, approximately 10 g) were collected from near the plant roots. All samples were kept at 0 °C–4 °C until analysis. The water total phosphorous (TP), particulate phosphorous (PP), dissolved total phosphorous (DTP), dissolved organic phosphorus (DOP), and soluble reactive phosphorus (SRP) in the water samples were determined using the molybdenum blue spectrophotometric method after filtration through a 0.45 μm cellulose acetate membrane. The TP subtracted from the DTP was defined as the sum of the PP fraction, while the DTP minus the SRP was defined as the sum of the DOP fraction. The detection limit for the SRP and DTP concentrations in the overlying water was 1 μg L−1. A continuous flow analyzer (FLOWSYS III, Systea, Italy) was used to determine the P concentrations. All materials used for these analyses were purchased from the Shanghai N&D Co. Ltd. (China). Triplicates of all the samples were analyzed, and the data were expressed as the means.
The water level was monitored to maintain the quantity after sampling and supplementation. The following equation was used to calculate the P concentration.
where Cn is the actual P concentration at the nth sampling time; V is the volume of the overlying water; and Vn is the volume of the deionized water added at the nth sampling time.
The sediment P fractionation of the TP, extraction of P with NaOH(NaOH-P), extraction of P with HCl(HCl-P), inorganic phosphorus (IP), and organic phosphorus (OP) was conducted based on the standards measurements and testing protocol, which consisted of three extraction procedures applied to 0.2 g aliquots of the sediment samples [13]. The TP in the microcosms minus the TP in water and sediment was defined as the sum of the TP in the macrophyte.
2.4 Environmental factors and submerged macrophyte biomass
The pH, (PHSJ-4A, Lei-ci, Shanghai, China), ORP (Lei-ci), and DO (YSI 5750, USA) in the overlying water were measured at 10:00 am prior to sampling using probes placed 0.25 m below the water surface.
The biomass could not be measured directly during the experiment; therefore, it was calculated using a model and data obtained in another experiment in which H. verticillata and P. crispus were grown under the same conditions as in this study(with similar light and temperature conditions). This is a limitation of this study. There is a certain deviation between the biomass calculated by the model and the actual biomass, but the error is within the acceptable range in the previous study [14]. We compared the modeled biomass at the end of the experiment with the measured biomass at the end of the experiment. The error between modeled biomass and the measured biomass of H. verticillata, V. natans and P. crispus were 6.23%, 5.21%, 6.14%.
The measured length included branches of the submerged macrophytes. The biomasses (W) of H. verticillata and P. crispus were then obtained using a single linear regression model (model 2):
where W is the biomass of the submerged macrophyte; X is the length of the submerged macrophyte; and a and b are constants. The biomasses of H. verticillata and P. crispus can be represented by:
When compared to H. verticillata and P. crispus, the canopy of V. natans differs in that leaves arise in clusters from their roots. Thus, the biomass (W/g), leaf length (X1/cm), and width (X2/cm) of V. natans were recorded. The biomass (W) of V. natans was obtained using a binary linear equation regression model (model 5):
where W is the biomass of the submerged macrophyte V. natans; X1 is the length of the submerged macrophyte leaves; X2 is the width of the submerged macrophyte leaves; and a, b, and c are constants. The biomass for V. natans can be represented by:
Following incubation, the plants were dried at 70 °C until they achieved a constant weight. The relative growth rate (RGR) was then calculated as the ratio of the difference between the dry weight at the end of incubation and that at the beginning of the experiment using Eq. 7.
where Wtend is the dry weight at the end of incubation and Wtbeginning is the dry weight at the beginning of the experiment.
2.5 Data analysis
In this study, the results are presented as an average of triplicates, and the significance of treatment effects was determined at a probability level of 0.05. Each sample was measured three times to get the average value after three samples were gathered from each experimental group. To guarantee the correctness of the data, the continuous flow analyzer sets three repeated measurements, and the results used are the average of the three measurement results. In addition, SPSS 16.0 was used to determine the Pearson correlation coefficients among different physical and chemical indicators. Principal component analysis of the environmental factors was also conducted. In addition, Z-score standardization of data and one-way analysis of variance (ANOVA) were performed.
3 Results
3.1 The RGR of submerged macrophytes
In the winter experiment, P. crispus grew relatively rapidly with an RGR of 4.67. During the spring experiment, V. natans had the highest RGR (10.75), while that of H. verticillata was lowest (0.68).
The biomasses of H. verticillata, V. natans, and P. crispus reached their maximum values on day 140 of the experiment (705.16 ± 15.21 g, 994.53 ± 20.45 g, and 354.34 ± 8.01 g, respectively, means ± SD). The rapid growth periods for H. verticillata and V. natans were days 20–60 and 20–80, respectively, while days 40–80 were the rapid growth period for P. crispus (Fig. 2). At the end of the experiment, the total P accumulated by H. verticillata, V. natans, and P. crispus was 156.05 mg, 282.24 mg, and 115.84 mg, respectively.
This study compared and analyzed the growth status of H. verticillata, V. natans, and P. crispus to compare and analyze the growth rates of plants growing in warm seasons and cold seasons. It was discovered that P. crispus can grow quickly and has a significant growth rate when the temperature rises in spring.
3.2 P concentration of water
3.2.1 Spring experiment
During the experimental period, P in the overlying water of the different experimental seasons exhibited different regulations. In the spring microcosm experiment, the TP, DTP, PP, and DOP in the control group reached maximum values of 0.24 ± 0.01, 0.09 ± 0.01, 0.18 ± 0.01, and 0.07 ± 0.00 mg L−1, respectively, during days 0–40, then gradually fell to 0.09 ± 0.01, 0.03 ± 0.00, 0.09 ± 0.01, and 0.02 ± 0.00 mg L−1 during days 40–140 (Fig. 3a, b, c, d and e).
The P concentration was low in the treatment groups. In the V. natans group, TP and PP exhibited an increase during the early growth stages (first 40 days), whereas DTP, SRP, and DOP exhibited a decrease in the same period. Thus, the increased amount of TP in the microcosm water was primarily affected by PP. A gradual decline in TP and PP from 0.18 ± 0.02 to 0.07 ± 0.01 mg L−1 and 0.16 ± 0.02to 0.03 ± 0.00 mg L−1, respectively, occurred during days 40–80. The DTP remained stable (0.02–0.03 mg L−1) during days 0–60, increased to 0.04 ± 0.00 mg L−1 on day 80 and then decreased to 0.01 ± 0.00 mg L−1 on day 140. The SRP exhibited a downward trend, decreasing to 0.0051 ± 0.0011 mg L−1 by the end of the experiment. The DOP maintained relatively stable levels during the experiment.
In the H. verticillata group, the change in TP concentration was different than in the V. natans group. The TP exhibited a downward trend, decreased to 0.01 ± 0.00 mg L−1 during days 0–40 and then increased during days 60–100. At the end of the experiment, the concentration of TP in the water was 0.11 ± 0.01 mg L−1. The DTP decreased to 0.02 ± 0.00 mg L−1 at the end of the experiment. The changes in the SRP were similar to those in the TP. Specifically, the SRP decreased to 0.0020 ± 0.0001 mg L−1 during days 0–40, then increased during days 60–100. At the end of the experiment, the concentration of SRP in the water was 0.0062 ± 0.0012 mg L−1. The PP increased during the experimental period, with a sharp increase occurring after day 60. The concentration of PP was 0.09 ± 0.01 mg L−1 at the end of the experiment. The change in the DOP concentration in the H. verticillata group was similar to that in the V. natans group and remained relatively stable during the experiment.
A one-way ANOVA (1–140 day)revealed that the concentrations of TP, SRP, PP, and DOP were significantly lower in the H. verticillata group than the control group (Table 1). However, the DTP did not differ significantly between the H. verticillata and the control group. The SRP and DOP were significantly lower in the V. natans group, while the TP, DTP, and PP exhibited no significant difference between the V. natans and the control group. The SRP and DOP in all of the treatment groups differed significantly from those of the control (SRP F = 14.60, P = 0.00; DOP F = 5.77, P = 0.01).
3.2.2 Winter experiment
In the winter microcosm experiment, the TP, DTP, SRP, and DOP in the control group reached maximum values of 0.21 ± 0.01, 0.18 ± 0.01, 0.10 ± 0.01 and 0.08 ± 0.01 mg·L−1, respectively, during days 0–100, then gradually fell to 0.14 ± 0.01, 0.10 ± 0.01, 0.09 ± 0.01, and 0.02 ± 0.00 mg L−1 during days 100–140. The PP exhibited a downward trend and decreased to 0.03 ± 0.00 mg·L−1 by the end of the experiment (Fig. 3f, g, h, i and j).
The opposite situation occurred in the P. crispus group, with the concentration of TP decreasing sharply from the beginning of the experiment to a minimum value of 0.02 ± 0.00 mg L−1 on day 60. The TP gradually increased to 0.08 ± 0.04 mg L−1 during days 60–140. The DTP and DOP remained stable in the first 60 days of the experiment, increased to peak levels (DTP 0.05 ± 0.01 mg L−1 and DOP 0.04 ± 0.01 mg L−1) on day 100, then gradually decreased. The PP decreased sharply from days 0–60 (PP 0.01 ± 0.00 mg L−1 on day 60), then remained stable from days 60–140.
A one-way ANOVA (Table 1) indicated the TP, DTP, SRP, and PP were significantly lower in the P. crispus group than the control group. The DOP did not differ significantly between the P. crispus and the control group. This has a direct connection to the experiment’s later decomposition and release of some phosphorus from P. crispus’s bottom leaves.
3.3 P concentration in sediment
The concentrations of the different P fractions are presented in Fig. 4. In the spring microcosm experiment, the TP, NaOH-P, HCl-P, IP, and OP were reduced in the treatment groups during the experimental period. Similar results were observed in the winter microcosm experiment.
In the spring microcosm experiment, the TP was significantly lower in both the H. verticillata and V. natans groups. At the end of the experiment, the TP varied greatly between the three treatment groups, ranging from 713.25 mg kg−1 (H. verticillata group) to 773.92 mg kg−1 (control group) in the following order: control group > V. natans group > H. verticillata group. The NaOH-P was significantly decreased in the V. natans group, but there was no significant difference between the H. verticillata and the control group. Compared to the control groups, the HCl-P was lower in the submerged macrophyte treatment groups, but there was no significant difference among treatment groups. The IP and OP were similar to the HCl-P and were lower in the submerged macrophyte treatment groups, but not significantly different among treatment groups(Fig. 4a, b, c, d and e).
In the winter microcosm experiment, nearly all of the P fractions showed no significant differences between the P. crispus and the control groups throughout the experimental period. The IP was significantly reduced in the P. crispus group. Overall, the P fractions in sediment from different treatment groups occurred in the following order: IP > OP, HCl-P > NaOH-P (Fig. 4f, g, h, i and j). The winter and spring experiments indicated that the submerged macrophytes greatly reduced the NaOH-P and IP in the sediment.
3.4 Water environmental factors
In the spring and winter microcosm experiments, the DO and ORP were significant higher (P < 0.05) in the treatment groups than the control during the experimental period (Fig. 5a, b and c). There was no significant difference in pH between the treatment and control groups in the spring microcosm experiment. However, the pH was significantly increased (P < 0.05) in the treatment groups during the winter microcosm experiment (Fig. 5d, e and f). The DO, pH, and ORP showed upward trends in the treatment groups; however, the DO, pH, and ORP of the control groups slowly rose or remained steady.
3.5 Phosphorus distribution
During the growth of submerged macrophytes, P in the sediment and overlying water accumulates in the plant. Therefore, the distribution ratios of P in the water and sediment in these experiments changed from the original sediment–water two-phase to the submerged macrophyte-sediment–water three phase. At the end of the experiment, the percentages of P in the three groups were as follows: H. verticillata, water 0.26%, submerged macrophytes 4.18%, and sediment 95.56%; V. natans, water 0.16%, submerged macrophytes 7.56%, and sediment 92.28%; and P. crispus, water 0.21%, submerged macrophytes 3.10%, and sediment 96.69% (Fig. 6).
3.6 Relationship between environmental factors and various forms of phosphorus based on principal component analysis
To study the relationship between environmental factors and different forms of P in water, the environmental factors of the treatment group were analyzed using principal component analysis. One environmental factor (E) was extracted from each plant treatment group, and the eigenvectors were determined (Table 2). E represents the combined effect of the environmental factors, pH, DO, and ORP.
EH.Verticillata = Principal component of the environmental factors in the H. verticillata group; EV. natans = Principal component of the environmental factors in the V. natans group; EP. crispus = Principal component of the environmental factors in the P. crispus group.
Therefore, the relationship between the principal components of the environmental factors and each index can be expressed as follows:
where ZpH, ZDO, and ZORP are the pH, DO, and ORP after Z-score standardization, respectively.
Correlation analysis showed that the principal component, E, of the environmental factors was significantly negatively correlated with the different sediment P, sediment P percentage, and water SRP in the H. verticillata and P. crispus groups (Table 3). The correlation coefficients between E and the different forms of P in the water and sediment were low in the V. natans group. Correlation analysis showed that the environmental factors had an important impact on the content of P in the water and sediments, as well as on the distribution of P between the sediments and water. Therefore, the environmental factors influenced the transformation of P between the water and sediments in the H. verticillata and P. crispus groups. The environmental factors in the V. natans group had no significant effect on P content and P transformation, possibly because of the higher biomass of V. natans or the crown morphology of the plants.
Correlation analysis showed that the environmental factors had different effects on different forms of P during the growth of submerged macrophytes. H. verticillata primarily acted on the SRP through environmental factors, while P. crispus acted on the DTP, SRP, DOP, and PP through these factors. V. natans had a weak impact on the water P content due to environmental factors.
4 Discussion
4.1 Effects of submerged macrophytes on phosphorus concentration in water and sediment
In the spring experiment, H. verticillata and V. natans entered a vigorous growth period after day 20 of the experiment, while P. crispus entered a vigorous growth period after day 40 in the winter experiment. This experimental system was a relatively closed environment that did not receive exogenous P, which is an important reason explaining why submerged macrophytes reduced the concentration of various forms of P in the overlying water. Uptake by submerged macrophytes was the primary reason for the decrease in P in the water and sediment in the vegetated macrocosms. Although plant uptake played a significant role in the removal of P, it did not account for all of the P loss from the system. At the end of the submerged macrophyte growth period, the biomass changed slightly, but the P concentration in the overlaying water remained low, indicating the possibility of biochemical and physicochemical processes functioning in the system.
Among the effects of submerged macrophytes on the sediment IP, the effect on NaOH-P was greater than that on HCl-P. This result was primarily related to the binding properties of sediment P. NaOH-P is P combined with iron, aluminum oxides, or hydroxides, which are prone to reduction in sediments. This portion of P is generally considered to be easily released and utilizable by organisms [15]. Nearly all of the root growth of the submerged macrophytes occurred in the sediments. During root growth, oxygen transport in roots increases the dissolved oxygen around the roots. This causes changes in the environmental factors around the roots (e.g., pH and ORP), which causes oxidization of the sediments around the roots. Hence, a change in environmental factors will inevitably affect the release of NaOH-P in sediments [16]. Studies have shown that a higher ORP and lower pH can promote the release of NaOH-P [17]. In this study, the sediment pH of the submerged macrophyte group showed a downward trend, while the ORP showed an upward trend. Hence, NaOH-P can be released in large quantities from sediments and used by submerged macrophytes.
HCl-P primarily refers to the existing form of sedimentary P related to P containing minerals, such as authigenic apatite, sedimentary calcium carbonate, and the biological skeleton, which primarily originate from the deposition of biological particles and fluorapatites formed by early diagenesis [18]. Although this is a relatively stable form of P in sediments, HCl-P can also be released from sediment when the pH is low. Under the experimental conditions, the pH in the overlying water was alkaline, while the pH in the sediment was maintained at a neutral level. Hence, there was no obvious fluctuation in HCl-P in the sediment. [7].
OP includes a refractory organophosphorus fraction composed of terrestrial emissions and a degradable organophosphorus fraction composed of dead aquatic residues. The OP in lake sediments has partial activity, but its structure is relatively stable. As a result, plant utilization is relatively difficult and the release rate of OP is small [19]. This may be one of the primary reasons for the small change in OP during the experiment. Most sediment OP appears in the form of phosphate esters (including inositol hexaphosphate), phospholipids, nucleic acids, phosphoproteins, phosphosaccharides, and some unknown compounds. Inositol hexaphosphate is an important form of organic P in sediments that is effectively mineralized into inorganic P during aerobic degradation, and its mineralization degree and rate are closely related to redox conditions [20].
Previous investigations of factors that affect the release of P from the sediment have revealed that they include temperature, pH, ORP, and DO [21].
Submerged macrophytes can absorb P in the overlying water and sediments through the stems, leaves, and roots to synthesize proteins, nucleic acids, and enzymes [22]. Therefore, plants will accumulate a large amount of P in their biomass during growth [23]. Following the experiment's conclusion, the amount of phosphorus in water and sediment decreased; therefore, using the total phosphorus balance, the amount of phosphorus accumulated by plants can be calculated. At the end of the experiment, the total amounts of P accumulated by H. verticillata, V. natans, and P. crispus were 156.05 g, 282.24 g, and 115.84 g, respectively. Overall, these findings indicate submerged macrophytes accumulate P from sediments and the overlying water, reducing the total amount of P involved in the exchange between sediments and water. This subsequently affects P transformation between sediments and water.
Performing an indoor experiment enabled analysis of the effects of the growth of submerged macrophytes on the transformation of P between the sediment and water. Although the study showed that the transformation of P was closely related to environmental factors, the interaction of the environmental factors had complex effects on the migration and transformation of P in the water. Hence, the micro mechanisms of these relationships require further investigation. Because of the complex environmental conditions and numerous influencing factors, it is impossible to observe the P transformation process at the micro level. Therefore, more detailed experiments are required to observe the process of P migration and transformation.
4.2 Effects of environmental factors on phosphorus transformation
The release of endogenous P is affected by water environmental conditions, such as temperature, DO, ORP, pH, and disturbance [24]. Large aquatic botanical species, especially submerged macrophytes, have important impacts on water environmental conditions [25]. Under the experimental conditions, the DO, pH, and ORP of the overlying water in the submerged macrophyte groups were higher than those in the control group in both the winter and spring experiments. A higher DO, pH, and ORP in water can inhibit the release of P in sediments, while each index has different effects on P migration [26].
The pH of the overlying water was alkaline during the submerged macrophyte growth period. This experiment showed that, when submerged macrophytes are present, an increase in pH caused by their growth led to a decrease in sediment P, which should be closely related to an increase in DO and ORP in the overlying water during the growth of submerged macrophytes. These results showed that an increase in DO and the ORP can effectively inhibit the release of P in sediments [27]. Therefore, increasing pH did not lead to increased P concentrations in the overlying water owing to the combined action of different environmental factors during the growth of submerged macrophytes [28].
The growth of submerged macrophytes also changes the environment around plant roots, thereby affecting the rate of diffusion of interstitial water to overlying water. Submerged macrophytes with developed roots can reduce the difference in the concentration of the P content between the interstitial water and the overlying water through absorption of P in the interstitial water by roots, which affects the rate of diffusion of interstitial water to overlying water [29]. This is one of the primary ways that submerged macrophytes with developed roots influence P content in overlying water. Although submerged macrophytes with degenerated roots can absorb P in interstitial water to some extent, the amount of absorption is relatively small; therefore, it has little effect on the rate of diffusion from the interstitial water to the overlying water. Accordingly, submerged macrophytes with degenerated roots primarily affect the levels of various forms of P in overlying water through absorption by stems and leaves.
Changes in environmental factors also have important effects on the migration and transformation of different forms of P in sediments [30]. The NaOH-P represents P bound to metal oxides (primarily Al and Fe) that is exchangeable with OH− and other inorganic P compounds that are soluble in base [31]. In the present study, the NaOH-P decreased significantly in the treatment groups during the experimental period, likely in response to an increase in the pH induced by the submerged macrophytes. The majority of the sediment IP in the studied samples was NaOH-P, and the concentration of NaOH-P in the treatment groups was significantly reduced compared to the control group. Taken together, these findings indicated that the uptake of P by the submerged macrophytes was important to this process. HCl-P represents the P fraction that is sensitive to low pH and assumed to primarily consist of apatite P (natural and detritus), including P bound to carbonates and traces of hydrolysable organic P. This P fraction is deemed a relatively stable fraction of IP in sediment [32]. Therefore, the concentration of HCl-P did not differ significantly between the treatment and control groups. NaOH-P and HCl-P comprised the primary P fraction of IP; thus, IP was caused by NaOH-P and HCl-P. OP is considered largely refractory, and only the labile portion can be released from surface sediment by bacterial re-mineralization [33]. Because this P fraction is relatively stable in sediment, the concentration of OP did not differ significantly between the treatment and control groups.
In the spring and winter experiments, the DO and ORP were higher in the treatment groups and were significantly increased by the submerged macrophytes. During the growing season, rooted macrophytes can transfer oxygen to roots through shoots and rhizomes [34]. Therefore, sediment near the roots can be oxidized by O2 and reduce P release. Oxygen near roots results in a higher ORP, which increases the oxidation of Fe(III) from Fe(II) to Fe(OOH). Under oxic and aerobic conditions, phosphate can bind with Fe3+ to form Fe2(PO4)3. At the same time, dissolved phosphate in the overlying water can be adsorbed by Fe(OH)3 in the sediments. As a result, P was not easily released from the sediment in this aerobic system.
The DO in the water body increases as a result of the oxygen produced by the photosynthesis of submerged macrophytes and the oxygen transport of submerged macrophyte roots during the growth period. These changes in the redox conditions and pH value of the sediment have an impact on the absorption and release of phosphorus in the sediment. To make their rhizosphere microenvironment more oxidizing, the roots of submerged macrophytes can secrete oxygen and other oxidizing agents like oxidase. This can encourage metal ions to transition from a reducing state to an oxidizing state, such as oxidizing Mn2+ and Fe2+ to form an iron and manganese oxide film, which can significantly increase the adsorption of phosphorus by the soil and decrease the release of phosphorus [35]. Diverse submerged macrophytes have varying capacities for releasing oxygen. Our studies have demonstrated that compared to H. verticellata and P. crispus, V. natans has roots that may release more oxygen, and the sediment in its root zone exhibits higher oxidation. As a result, V. natans is a better option for shallow lake ecological restoration.
During the growing season of submerged macrophytes, the pH of the water was alkaline. According to Jin et al. [36], phosphorus will be released from the sediment as the pH rises, however in our experiment, the concentration of different types of phosphorus in the water under the submerged macrophytes group did not substantially increase. This research assumes that the experimental setup is responsible for this. According to Jin's study strategy, other parameters like DO, ORP, etc. are controlled inside, and only the effect of pH on sediment phosphorus at the water and soil interface is taken into account. This technique ignores the complexity of different environmental elements that affect submerged macrophytes development.
This experiment demonstrates that, in the presence of submerged macrophytes, the rise in pH brought on by their development does not result in the release of phosphorus into the sediment, although this should happen given the rise in DO and ORP in the water above the submerged macrophytes. The study demonstrates that an increase in DO and ORP can successfully prevent phosphorus from releasing from sediment and can encourage sediment to adsorb phosphorus from overlying water. As a result, under the overall influence of various environmental factors during the development of submerged macrophytes, an increase in pH did not increase the concentration of phosphorus in the water above.
Overall, the existence of submerged macrophytes reduced the internal loading of P. The DO and ORP were higher in the treatment groups than in the control groups, indicating that they influenced the P concentration in the overlying water. Submerged macrophytes can maintain a clear water state through various buffering mechanisms. In our experiments, submerged macrophytes maintained a low P level relative to the control group because of the changing environmental factors.
5 Conclusions
Submerged macrophytes reduced the different forms of P in the overlying water and sediment during the growth period, and the metabolism and photosynthesis of the submerged macrophytes significantly altered the surrounding environmental factors. These environmental factors had different effects on different forms of P transformation.
The growing submerged macrophytes absorbed a large amount of P from the water and enriched plant P, which reduced the P content in the water and sediments. At the end of the experiment, the total P accumulated by H. verticillata, V. natans, and P. crispus was 156.05 mg, 282.24 mg, and 115.84 mg, respectively.
The submerged macrophytes affected the migration and transformation of P between the overlying water and sediment by increasing the DO, ORP, and pH in the overlying water. H. verticillata primarily acted on the SRP through environmental factors, while P. crispus acted on the DTP, SRP, DOP, and PP through these factors. V. natans had a weak impact on water P content through environmental factors.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Chen L, Zhao JJ, Zhang ZP, Shen ZW, Dong WM, Ma R, Chen J, Niu LL, Chen SQ, Wu D, Liu JB, Zhou AF (2022) Lake eutrophication in northeast China induced by the recession of the East Asian summer monsoon. Quat Sci Rev 281:107448
Zhang X, Zhao J, Ding L, Li Y, Liu H, Zhao Y, Fu G (2022) Eutrophication evolution trajectory influenced by human activities and climate in the shallow Lake Gehu China. Ecol Indic 138:108821
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Controlling eutrophication by reducing both nitrogen and phosphorus. Science 323:1014–1015
Maceda-Veiga A, MacNally R, Rodríguez S, Szabo S, Peeters ETHM, Ruff T, Salvadó H (2022) Effects of two submerged macrophyte species on microbes and metazoans in rooftop water-storage ponds with different labile carbon loadings. Water Res 211:117999
Finkler Ferreira T, Crossetti LO, Motta Marques DML, Cardoso L, Fragoso CR, van Nes EH (2018) The structuring role of submerged macrophytes in a large subtropical shallow lake: clear effects on water chemistry and phytoplankton structure community along a vegetated-pelagic gradient. Limnologica 69:142–154
Wang L, Liu Q, Hu C, Liang R, Qiu J, Wang Y (2018) Phosphorus release during decomposition of the submerged macrophyte Potamogeton crispus. Limnology 19(3):355–366
Verhofstad MJJM, Alirangues Núñez MM, Reichman EP, van Donk E, Lamers LPM, Bakker ES (2017) Mass development of monospecific submerged macrophyte vegetation after the restoration of shallow lakes: roles of light, sediment nutrient levels, and propagule density. Aquat Bot 141:29–38
Zhang W, Kim DK, Rao YR, Watson S, Mugalingam S, Labencki T, Dittrich M, Morley A, Arhonditsis GB (2013) Can simple phosphorus mass balance models guide management decisions? a case study in the Bay of Quinte, Ontario, Canada. Ecol Modell 257:66–79
Schad M, Halama M, Jakus N, Robbins LJ, Warchola TJ, Tejada J, Kirchhof R, Lalonde SV, Swanner ED, Planavsky NJ, Thorwarth H, Mansor M, Konhauser KO, Kappler A (2021) Phosphate remobilization from banded iron formations during metamorphic mineral transformations. Chem Geol 584:120489
Nürnberg GK, Fischer R, Paterson AM (2018) Reduced phosphorus retention by anoxic bottom sediments after the remediation of an industrial acidified lake area: Indications from P, Al, and Fe sediment fractions. Sci Total Environ 626:412–422
Vázquez-Ortega A, Perdrial N, Reinoso-Maset E, Root RA, O’Day PA, Chorover J (2021) Phosphate controls uranium release from acidic waste-weathered Hanford sediments. J Hazard Mater 416:126240
Chen G, Wang J, Yu F, Wang X, Xiao H, Yan B, Cui X (2022) A review on the production of P-enriched hydro/bio-char from solid waste: transformation of P and applications of hydro/bio-char. Chemosphere 301:134646
Ruban V, López-Sánchez JF, Pardo P, Rauret G, Muntau H, Quevauviller P (2001) Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—a synthesis of recent works. Fresenius J Anal Chem 370:224–228
Wang L, Song H, Wu X, An J, Wu Y, Wang Y, Li B, Liu Q, Dong B (2022) Relationship between the coprecipitation of phosphorus-on-calcite by submerged macrophytes and the phosphorus cycle in water. J Environ Manag 314:115110
Zhang L, Zhang Y, Liu L (2019) Effect of submerged macrophytes Vallisneria spiralis L. on restoring the sediment contaminated by enrofloxacin in aquaculture ponds. Ecol Eng 140:105596
Anda A, Simon B, Soos G, da Silva JAT, Kucserka T (2016) Effect of submerged, freshwater aquatic macrophytes and littoral sediments on pan evaporation in the Lake Balaton region, Hungary. J Hydrol 542:615–626
Chen Y, Li L, Zhang Y, Pan Y, Ni M, Yang WJ, Huang Y (2022) Phosphorus absorption and release in biofilm sequencing batch reactor: the combined action of cells and extracellular polymeric substances and the characteristics of polymer metabolism. J Water Process Eng 49:102979
Olsen M, Schaanning MT, Braaten HFV, Eek E, Moy FE, Lydersen E (2018) The influence of permanently submerged macrophytes on sediment mercury distribution, mobility and methylation potential in a brackish Norwegian fjord. Sci Total Environ 610–611:1364–1374
Zhu YR, Zhang RY, Wu FC, Qu XX, Xie FZ, Fu ZY (2013) Phosphorus fractions and bioavailability in relation to particle size characteristics in sediments from Lake Hongfeng Southwest China. Environ Earth Sci 68(4):1041–1052
Percival LME, Bond DPG, Rakociński M, Marynowski L, Hood AVS, Adatte T, Spangenberg JE, Föllmi KB (2020) Phosphorus-cycle disturbances during the Late Devonian anoxic events. Global Planet Change 184:103070
Xiang S, Zhou W (2011) Phosphorus forms and distribution in the sediments of Poyang Lake China. Int J Sedim Res 26(2):230–238
Gao J, Xiong Z, Zhang J, Zhang W, Mba FO (2009) Phosphorus removal from water of eutrophic Lake Donghu by five submerged macrophytes. Desalination 242(1–3):193–204
Dong B, Qin B, Gao G, Cai X (2014) Submerged macrophyte communities and the controlling factors in large, shallow Lake Taihu (China): sediment distribution and water depth. J Great Lakes Res 40(3):646–655
Gaoa L (2012) Phosphorus release from the sediments in Rongcheng swan lake under different pH conditions. Proc Environ Sci 13:2077–2084
Herrera A, D’Imporzano G, Acién Fernandez FG, Adani F (2021) Sustainable production of microalgae in raceways: Nutrients and water management as key factors influencing environmental impacts. J Clean Prod 287:125005
Song K, Winters C, Xenopoulos MA, Marsalek J, Frost PC (2017) Phosphorus cycling in urban aquatic ecosystems: connecting biological processes and water chemistry to sediment P fractions in urban stormwater management ponds. Biogeochemistry 132(1):203–212
Barik SK, Purushothaman CS, Mohanty AN (2015) Phosphatase activity with reference to bacteria and phosphorus in tropical freshwater aquaculture pond systems. Aquaculture Res 32(10):819–832
Wang H, Wen ZH, Zhang ZH, Zhang XL, Fu H, Cao Y, Ni LY, Cao T, Li KY (2020) Environmental vs. spatial drivers of submerged macrophyte community assembly in different seasons and water depths in a mesotrophic bay of Erhai Lake China. Ecol Indic 117:106696
Amorim CA, Moura AN (2020) Effects of the manipulation of submerged macrophytes, large zooplankton, and nutrients on a cyanobacterial bloom: a mesocosm study in a tropical shallow reservoir. Environ Pollut 265:114997
Kor K, Ershadifar H, Ghazilou A, Koochaknejad E (2021) Seasonal variations, potential bioavailability, and ecological risk of phosphorus species in the coastal sediments of the Makran. Mar Pollut Bull 173:113125
Fan W, Ao L, Zhang S (2022) Sedimentary phosphorus fractions in typical lacustrine wetland of Changshou Lake flowing into the three-gorges reservoir. Bull Environ Contam Toxicol 109(5):706–712
Hassrick JL, Korman J, Kimmerer WJ, Gross ES, Grimaldo LF, Lee C, Schultz AA (2023) Freshwater flow affects subsidies of a copepod (Pseudodiaptomus forbesi) to low-salinity food webs in the upper San Francisco estuary. Estuaries Coasts 46(2):450–462
Sylvan J, Dortch Q, Nelson D, Brown A, Morrison W, Ammerman J (2006) Phosphorus limits phytoplankton growth on the Louisiana shelf during the period of hypoxia formation. Environ Sci Technol 40(24):7548–7553
Younis AM, Soliman NF, Elkady EM, Mohamedein LI (2022) Distribution and ecological risk evaluation of bioavailable phosphorus in sediments of El Temsah Lake. Suez Canal Oceanologia 64(2):287–298
Horppila J, Nurminen L (2003) Effects of submerged macrophytes on sediment resuspension and internal phosphorus loading in Lake Hiidenvesi (southern Finland). Water Res 37(18):4468–4474
Jin XC, Wang SR, Pang Y, Chang WuF (2006) Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake. China Environmental Pollution 139(2):288–295
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province, China (ZR2021MD045, ZR2021MD003, and ZR2020MD102) and the National Natural Science Foundation of China (41977067, 32071630, 41303061, and 41601283)
Funding
This work was supported by the Natural Science Foundation of Shandong Province, China (ZR2021MD045, ZR2021MD003 and ZR2020MD102), the National Natural Science Foundation of China (41977067, 32071630, 41303061 and 41601283).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by LZW, XYW, BD and HLS. The first draft of the manuscript was written by LZW, and the data analysis was performed by J A, YZW, YW. BL and QJL,WNY commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Wu, X., Song, H. et al. Effect of environmental factors on phosphorus transformation during the growth of submerged macrophytes. SN Appl. Sci. 5, 111 (2023). https://doi.org/10.1007/s42452-023-05334-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05334-7