Abstract
Several studies have related breeding success and survival of sea eagles to toxic or non-toxic stress separately. In the present investigation, we analysed single and combined impacts of both toxic and disturbance stress on populations of white-tailed eagle (Haliaeetus albicilla), using an analytical single-species model. Chemical and eco(toxico)logical data reported from laboratory and field studies were used to parameterise and validate the model. The model was applied to assess the impact of ∑PCB, DDE and disturbance stress on the white-tailed eagle population in The Netherlands. Disturbance stress was incorporated through a 1.6% reduction in survival and a 10–50% reduction in reproduction. ∑PCB contamination from 1950 up to 1987 was found to be too high to allow the return of white-tailed eagle as a breeding species in that period. ∑PCB and population trends simulated for 2006–2050 suggest that future population growth is still reduced. Disturbance stress resulted in a reduced population development. The combination of both toxic and disturbance stress varied from a slower population development to a catastrophical reduction in population size, where the main cause was attributed to the reduction in reproduction of 50%. Application of the model was restricted by the current lack of quantitative dose–response relationships between non-toxic stress and survival and reproduction. Nevertheless, the model provides a first step towards integrating and quantifying the impacts of multiple stressors on white-tailed eagle populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Populations of birds of prey have been affected by bioaccumulation of organochlorines like DDT and PCBs during the twentieth century (Grier 1982; Henny et al. 2009; Nygård and Gjershaug 2001; Helander et al. 2002). The relationships between DDT and PCBs exposure and reduced reproduction in sea eagles, i.e. white-tailed eagles (Haliaeetus albicilla) and bald eagles (Haliaeetus leucocephalus), have extensively been documented (Wiemeyer et al. 1993; Bowerman et al. 1995; Donaldson et al. 1999; Helander et al. 2008). Due to the ban on PCBs and DDT and due to conservation measures, most sea eagle populations are currently recovering (Hailer et al. 2006; Scharenberg and Struwe-Juhl 2006; Krüger et al. 2010). In some countries, however, reproduction rates of sea eagles are still low (Helander et al. 2002; Gill and Elliott 2003). In addition to toxic stress, non-toxic stress factors have been linked to low breeding success (Gende et al. 1997; Dykstra et al. 1998). Sea eagles are known to be sensitive to, for example, limited resource availability, climate change and poor habitat configuration, but also to frequent human disturbance, which can cause breeding failures (Buehler et al. 1991a; McGarigal et al. 1991; Grubb et al. 1992; Grim and Kallemeyn 1995; Steidl and Anthony 2000; Bowerman et al. 2005; Watts et al. 2008). To facilitate the return of sea eagles as an indigenous species in The Netherlands, impacts of toxic and non-toxic stress have to be integrated, quantified and assessed at population level endpoints (Sibly et al. 2005; Marris 2009). So far, most quantitative studies focussed on the impacts of single stressors. Besides, investigations of birds of prey, including sea eagle studies, usually assess impacts on reproduction, such as the number of young per pair, nestling brood size and fledging success, rather than population level endpoints.
The aims of the present study were to (1) develop a tool to quantify the impact of multiple stressors on the population development of white-tailed eagles; (2) determine if PCB and DDT were potentially limiting the establishment of white-tailed eagles in The Netherlands in the past; (3) assess the development potential of a white-tailed eagle population in The Netherlands. To that end, we extended an existing analytical single-species modelling framework to include the impacts of organochlorine contaminants as well as disturbance stress on white-tailed eagle populations. The model was parameterized using values collected from literature and validated on an independent field data set.
Model development and application
General approach
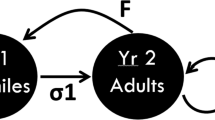
The toxic stress module of the model was based on a framework for assessing the impact of PCB contamination on reproduction, survival and population parameters of cormorants (Phalacrocorax carbo). The basic equations are briefly summarised, as details can be found elsewhere (Hendriks and Enserink 1996; Hendriks et al. 2005). The number of breeding individuals in a given year N(t) is calculated from the population in the preceding year N(t−Δt) by
where r(C,t) and r(0,t) represent the rates of increase at time t under contaminated conditions and under reference conditions respectively, N(∞) represents the carrying capacity, and the time step Δt was set at 1 year. The rate of increase under reference conditions r(0) was calculated by
with l(a) as the fraction surviving until at least age a and a max as the maximum age. The age-specific fecundity or reproduction rate m(a) represents the number of juveniles fledged per individual during interval da (Birch 1948; Hendriks and Enserink 1996). The rate of increase under contaminated conditions r(C) can be related to the exposure concentration C according to (Hendriks and Enserink 1996)
where the median lethal concentration (LC50) and median effect concentration (EC50) represent 50% reductions of l(a) and m(a), respectively. Parameter ß characterises the slope of the concentration–response curve; the terms 1/(1+(C/LC50)1/ß) and 1/(1+(C/EC50)1/ß) represent the fractions of the population which are unaffected by toxic stress through survival and reproduction, respectively. The ratio between the rate of increase r(C) at concentration C and the rate of increase under reference conditions r(0) is calculated according to (Hendriks and Enserink 1996; Hendriks et al. 2005)
with R(0) as the lifetime fecundity, i.e. the average number of offspring per individual per generation time, calculated as (Birch 1948; Hendriks and Enserink 1996)
Non-toxic environmental stressors, like disturbance, may affect the population size N(t) by further reducing the rate of increase r(C) (Hendriks et al. 2005). Under the assumption that effects of toxic stress and disturbance are purely additive, the population fractions unaffected by either stressor can be multiplied to determine the population fraction unaffected by both stressors combined (Traas et al. 2002). Disturbance stress can then be implemented in Eq. 4 to arrive at
where r(C,D)/r(0) represent the ratio of the rate of increase as a function of a chemical concentration C and disturbance D and the rate of increase in reference conditions r(0). The factors f L and f E represent the age independent population fractions which are unaffected by disturbance stress through survival and reproduction, respectively.
Ecological and toxicological parameters
The toxic stress module of the model was parameterised for the organic contaminants ∑PCB and DDE, which have been frequently linked to reduced reproduction in sea eagles (Wiemeyer et al. 1993; Bowerman et al. 1995; Donaldson et al. 1999; Helander et al. 2008). Where possible, white-tailed eagle data were used for model parameterization. If species-specific values were not available, values for the closely related bald eagle (Haliaeetus leucocephalus) were used instead. Assuming that bald eagle data can be applied instead of white-tailed eagle data seems reasonable because the life history patterns are sufficiently comparable. Besides, hazard values estimated for bald eagles have been used in previously published white-tailed eagle studies (Koistinen et al. 1997; Van Wezel et al. 1999). To cover variation and uncertainties in ecological data, the model was parameterised for both favourable and unfavourable conditions.
The rate of increase under reference conditions r(0) was obtained from age-specific reproduction rates of a bald eagle population living in uncontaminated areas and survival rates of white-tailed eagles (Table 1). Measured survival rates for juvenile white-tailed eagles of 0.86–0.95 were reported by Saurola et al. (2003). According to Nygård et al. (2000), survival rates for young birds in the first two years ranged from 0.90 to 0.95. Green et al. (1996) measured survival rates in a reintroduced population of 0.73 for young birds prior to settlement and 0.94 for established birds. In other studies, survival rates reported for white-tailed eagles range from 0.72 to 0.95 for juveniles and from 0.85 to 0.97 for adults (Evans et al. 2009; Krüger et al. 2010; Radovic and Mikuska 2009). Exceptionally low and high values could be attributed to location circumstances (e.g., Green et al. 1996) or were based on a small sample size (e.g. Krüger et al. 2010). Survival rates of 0.75–0.85 for juveniles and 0.90–0.95 for adults were found to be more common and were used for calculating the rate of increase under favourable and unfavourable conditions, respectively. For reproduction, we used data of a bald eagle population in Florida and Chesapeake Bay before widespread use of PCB and DDT (Broley 1947). The mean reproduction of 1.7 fledging juveniles per nest reported for 1936–1946 is among the highest published for sea eagles (Colborn 1991). Reproduction rates of 2 juveniles per nest over multiple years were common and some nests occasionally produced 3 fledging juveniles per year (Broley 1947). It was assumed that the somewhat lower average reproduction of 1.7 juveniles per nest included random nest failures due to inter-specific competition and bad weather (Broley 1947). Fledging success values of 1.7 (average) and 2 (maximum) juveniles per nest were converted following Bortolotti (1986) and Grim and Kallemeyn (1995) and used for parameterisation of the rate of increase under unfavourable and favourable reference conditions, respectively (Table 1). Following Watts et al. (2008) we assumed that the Chesapeake Bay bald eagle population had not reached its carrying capacity yet, implying that reproduction was not affected by intraspecific competition. The lifetime fecundity was based on the life history data given in Table 1.
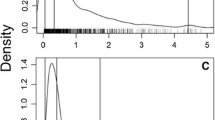
Lethal concentrations LC50 for ∑PCB and DDE were estimated by calculating the geometric mean of the highest concentration reported in bald eagles with a non-toxic cause of death and the lowest concentration found in sea eagles known to have died from ∑PCB and DDE (Table 2; Fig. 1). The median effect concentration EC50 and the slope constant ß (Table 2; Fig. 1) were derived by fitting a sigmoid dose–response curve on reproduction data from a field study of white-tailed eagle populations in Sweden (Helander et al. 2002). Reproduction rates apply to three different sub-populations, monitored in 1965–1997 (Fig. 1). During this period, 249 dead eggs from 205 clutches were analysed for ∑PCB and DDE residues. Measured lipid weight concentrations were converted to wet weight concentrations using the average lipid fraction of 0.05 noted for eggs of white-tailed eagles (Helander et al. 2002; 2008). Data from Lapland were not used because this subpopulation suffered from food shortages, human disturbance and bad weather (Helander et al. 2002).
The ratio r(C)/r(0) as a function of a ∑PCB and b DDE concentrations in white-tailed eagles for favourable and unfavourable conditions (Table 1) calculated according to Eq. 4. Concentration–response curves for average reproduction and survival were based on reproduction (dots) and survival (squares) data collected from literature
Model testing and data acquisition for validation
The equations and the parameter settings used in the toxic module of the model were tested by comparing exposure concentrations C(t) and population size N(t) with field data collected from the literature. Exposure concentrations C(t) were quantified by estimating residues in eggs from sediment concentrations, according to a method described by Hendriks and Enserink (1996). Sediment levels comprised PCB118 and DDE concentrations measured in Lake Erie’s western basin in 1971 and 1996 (Frank et al. 1977; Painter et al. 2001; Marvin et al. 2004). To obtain a continuous data set, linear interpolation was applied. Interpolated PCB118 concentrations in sediment organic matter were converted to concentrations of the standard congener PCB153 by multiplying them by 1.8 (Hendriks 1993). The PCB153 concentrations were used to derive levels in fish using a biota lipid-organic sediment accumulation factor of 1.8 and a lipid fraction of 0.05 for vertebrates (Hendriks 1995; Hendriks and Enserink 1996). Concentrations in fish, in turn, were used to determine concentrations in eagle eggs using a biomagnification factor from fish to egg of 28 as previously reported for bald eagles (Bowerman et al. 1995). The ∑PCB residue in eggs was calculated by multiplying the estimated PCB153 concentration by a factor of 5 (Helander et al. 2002). For DDE, we used a biota lipid-organic sediment accumulation factor of 11.6 reported for fish, a lipid fraction of 0.05 for vertebrates (Hendriks and Enserink 1996; Hendriks et al. 1998) and a biomagnification factor from fish to egg of 22, as reported for bald eagles (Bowerman et al. 1995).
Next, the chemical residues of ∑PCB and DDE predicted in eagle eggs from sediment concentrations were compared with ∑PCB and DDE residues measured in bald eagle eggs obtained from the Lake Erie area (Donaldson et al. 1999). The predicted egg residues were also used for estimating the population size N(t). Predicted population sizes were compared with observed population data of bald eagles from Lake Erie from 1977 to 1993 reported by Bowerman (1993), which were converted to breeding individuals according to Bortolotti (1986) and Grim and Kallemeyn (1995).
Population development for The Netherlands
The model was applied to estimate the past and future population developments of white-tailed eagles in The Netherlands. For the period up to 1988, environmental concentrations of PCB118 were derived from measurements in sediment core samples obtained from Lake Ketelmeer (Beurskens et al. 1993). PCB118 sediment concentrations were used to derive ∑PCB concentrations in white-tailed eagle eggs as described above. For 1989–2050, emissions as well as degradation of ∑PCB were assumed to be negligible. For DDE, no sediment core data were available for The Netherlands. Instead, we used concentration trends of ∑DDT in European eels (Anguilla anguilla) caught in Lobith from 1978 till 1997 (Hendriks and Pieters 1993). The ∑DDT concentrations were converted to DDE, assuming that DDD and DDT had been transformed to DDE. As the DDE peak is expected to have occurred before 1978, for which no measurements were available in The Netherlands, the DDE trends observed in Sweden (Helander et al. 2002) and Germany (Scharenberg and Struwe-Juhl 2006) were extrapolated to our study area. For 1998–2050, emissions as well as degradation of DDE were assumed to be negligible.
So far, mortality rates of sea eagles due to disturbance stress have not been quantified, although several authors have reported anthropogenic causes of white-tailed eagle and bald eagle mortality (Reichel et al. 1984; Elliott et al. 1996; Krone et al. 2006; Müller et al. 2007). We calculated a disturbance-induced mortality fraction of 0.016 based on the casualties and population size reported for white-tailed eagles in Germany, yielding a surviving fraction F L of 0.984 (Kollman et al. 2002; Krone et al. 2003). The non-toxic and unnatural causes of mortality underlying this fraction were collision with trains, tissue or bone destruction with unknown origin (trauma), electrocution and collision with wires (wind turbines and deliberate poisoning were not included). Unfortunately, no quantitative data could be found regarding the effects of disturbance stress on white tailed eagles’ reproduction success in The Netherlands. In addition, the degree of disturbance stress likely varies as it depends highly on variables such as distance, degree, frequency and type of disturbance (e.g. McGarigal et al. 1991; Grubb et al. 1992; Steidl and Anthony 2000). To cover some of the uncertainty and variability in the effects of disturbance stress, we used reductions in reproduction of 10 and 50% in the simulations, corresponding with unaffected fractions f E of 0.9 and 0.5, respectively.
As the white-tailed eagle has only recently begun to breed in The Netherlands, we did not simulate the population size N(t) from 1950 till 2005 starting from an arbitrary number. Instead, the ratio of r(C)/r(0) was used to assess the potential impacts of ∑PCB and DDE on white-tailed eagles. The model simulations for the period 2006–2050 start from one breeding pair in 2006 (Van Rijn et al. 2010). Within The Netherlands, the majority of wintering white-tailed eagles were recorded at large open wetlands, i.e. Oostervaardersplassen, Lauwersmeer and enclosed estuaries such as Biesbosch, Hollandsch Diep, Haringvliet, Grevelingen and Krammer-Volkerak (Van Rijn et al. 2010). These areas provide a total of 56300 ha of riverine forest, macrophyte marshland and shallow open water (Ministry of Agriculture, Nature and Food Quality 2005), which constitute suitable habitat for the species (Reijnen et al. 1995). Based on an average density of one white-tailed eagle breeding pair per 3750 ha of suitable habitat (Reijnen et al. 1995), following Bortolotti (1986) and Grim and Kallemeyn (1995), we calculated a minimum carrying capacity of N(∞) of 30 breeding individuals for The Netherlands.
Results
Model testing and validation
The difference between the estimated and measured ∑PCB concentration in eagle eggs was less than a factor of 2.5 (Fig. 2a). The measured population size followed the scenario with a favourable parameter setting based on ∑PCB concentrations in eggs (Fig. 2b). For DDE, the maximum difference between estimated and measured concentrations was a factor of 10.7 (Fig. 2c) and the measured population densities followed the scenario with an unfavourable parameter setting based on sediment concentrations (Fig. 2d).
Comparison of measured and simulated a ∑PCB and c DDE residues in eggs (Donaldson et al. 1999; Frank et al. 1977; Painter et al. 2001; Marvin et al. 2004) and observed population size N(t) (Bowerman 1993) compared with N(t) as a function of the simulated b ∑PCB and d DDE concentration trend for both favourable and unfavourable conditions (Table 1). Note that no carrying capacity N(∞) was applied in the model validation
Population development for The Netherlands
From 1950 till 1987, the simulated ∑PCB residues in white-tailed eagles exceeded the value of 6.45 × 104 μg·kg−1 wet weight, resulting in a negative ratio r(C)/r(0), corresponding to a population reduction (Fig. 3a). For DDE, ratios were close to 1, suggesting that the impact has been small even in unfavourable conditions (Fig. 3b). Therefore the impact of DDE was not assessed for the period 2006–2050.
The ratio of increase rates r(C)/r(0) for favourable (solid line) and unfavourable (dotted line) parameter setting as a function of a ∑PCB and b DDE concentrations in time. The grey area represents the concentrations simulated for white-tailed eagle eggs. Note that the impact of disturbance stress is not included
The white-tailed eagle population projected for 2006–2050 increased and levelled off to the carrying capacity for favourable reference conditions and showed a steady increase for unfavourable reference conditions (Fig. 4). Model simulations including ∑PCB exposure showed a reduced population growth, but eventually resulted in population sizes comparable to those in reference conditions (Fig. 4a). Population sizes predicted for ∑PCB exposure were in between the values predicted for 10 and 50% reductions in reproduction, respectively (Fig. 4b). Under the assumption of additive effects, the combination of toxic and disturbance stress resulted mostly in a reduced population growth. However, under unfavourable conditions, a 50% reduction in reproduction combined with toxic stress resulted in a negative rate of increase and extinction of the population (Fig. 4c).
Trends in white-tailed eagle population size N(t) projected for 2006–2050 as a function of a ∑PCB exposure, b 10 or 50% reduction in annual reproduction and 1.6% reduction in annual survival due to disturbance stress and c both stressors combined, for both favourable and unfavourable parameter setting (Table 1)
Discussion
Validation and assumptions
The model developed provides a tool to quantify the impact of toxic and other stressors, due to for example disturbance, on white-tailed eagle populations. For the prediction and evaluation of population trends related to toxic stress, most data were available in literature. Unfortunately, quantitative data that relate reproduction parameters to disturbance stress were not available for sea eagles. Hence, disturbance stress could not be included in the validation of the model. According to the validation of the toxic stress module of the model, the measured DDE concentrations were consistently higher than the simulated concentrations (Fig. 2c), but close to those estimated by Weseloh et al. (2002). Yet, the variability and uncertainty in ecological data (Table 1) have a larger effect on population development than uncertainty in the simulated egg concentrations (Fig. 2d).
Fixed fledging success values were used for calculating the reference rate of increase, which is a simplification. In reality, the fledging success often increases after first breeding and declines at the end of life (Best et al. 2010). Large variations were found in survival and reproduction rates. However, the survival and reproduction rates that were used resulted in reference rates of increase for favourable and unfavourable conditions (Table 1) that are close to the minimum and maximum rates of increase (0.07–0.19) reported for an exponentially growing bald eagle population (Buehler et al. 1991b).
The estimated EC50s and LC50s for ∑PCB and DDE are considered to be indicative because the values are based on reproduction data from field studies and they were not derived by standardised methods. The values may be overestimated due to cumulative effects of ∑PCB, DDE other chemicals, and other stressors. However, the threshold values for ∑PCB and DDE were close to those estimated for bald eagles (Wiemeyer et al. 1993; Elliott and Harris 2001; Best et al. 2010). In addition, the first signs of a reduction in reproduction were revealed at a ∑PCB concentration of 1 × 104 μg·kg−1 wet weight in eggs (Fig. 1a). This corresponds with ∑PCB concentrations in eggs of herring gull (Larus argentatus) and Caspian tern (Sterna caspia) that showed substantial PHA skin responses (Grasman 2002). An immuno-toxicological approach to threshold values is desirable, because immunologic and other sub-individual responses have been related with the establishment success and population development of birds (Grasman 2002; Møller and Cassey 2004).
Despite the assumptions and simplifications that were made in the validation simulation, like the exclusion of potential cumulative effects, a carrying capacity and possible density-dependence in survival and reproduction parameters, the predicted population densities showed no major deviations from field data.
Simulation of impact of ∑PCB, DDE and disturbance stress
In retrospection, the simulated ∑PCB and DDE concentrations in Dutch white-tailed eagle eggs (Fig. 3) are similar to levels previously reported for white-tailed eagle eggs from Sweden and Germany (Helander et al. 2002; Scharenberg and Struwe-Juhl 2006). From 2005 and further, the growing white-tailed eagle population is in line with the population dynamics of white-tailed eagles in Sweden (Helander et al. 2008), Germany (Scharenberg and Struwe-Juhl 2006; Krüger et al. 2010) and bald eagles in Canada and United States (Grier 1982; Bowerman et al. 1995; Donaldson et al. 1999; Buck et al. 2005).
For the simulation of the impact of disturbance stress, a mortality fraction of 0.016 per year was estimated based on mortality rates related to several anthropogenic, non-toxic causes reported for the German white-tailed eagle population (Kollman et al. 2002; Krone et al. 2003). A slightly lower mortality fraction of 0.01 was calculated for a white-tailed eagle population in Sweden, where electrocution and toxic stress were the major causes of death (Kollman et al. 2002; Krone et al. 2006). Yet, the mortality fraction of the German population was considered more representative, because of the resemblance of the infrastructure between Germany and The Netherlands. The calculated impact of a mortality fraction of 0.016 per year on the ratio of increase rates and on the population size N(t) was negligible. One may suggest that not all dead eagles were found the study of Krone et al. (2003) and that the mortality rate therefore is underestimated. However, tentative sensitivity analyses revealed that mortality fractions of 0.01, 0.04 and 0.08 yielded similar model outcomes N(2050) of 23, 23 and 24 in unfavourable reference conditions, respectively, indicating that the model is relatively insensitive to adult mortality.
Unfortunately, quantitative dose–response relationships relating reproduction parameters to human activities were not available for sea eagles, as such relationships are difficult to obtain for birds of prey (Steidl and Anthony 2000; McGarigal et al. 1991). To cover some of the uncertainty and variability in the effects of disturbance stress, we simulated reductions in reproduction of both 10 and 50%. This range is in line with reproduction reductions of 11% and 50–56% due to visitor and investigator disturbance previously reported for common eider (Somateria mollissima) and Leach’s storm-petrel (Oceanodroma leucorhoa), respectively (Bolduc and Guillemette 2003; Blackmer et al. 2004). Other reported values include 20% for black-legged kittiwake (Rissa tridactyla), 22% for golden plover (Pluvialis apricaria) and 44% for Egyptian vultures (Neophron percnopterus) (Beale and Monaghan 2005; Finney et al. 2005; Zuberogoitia et al. 2008). Relatively large impacts of disturbance stress were found for the 50% reduction in reproduction. Moreover, the model is sensitive to changes in the reproduction parameters (Hendriks and Enserink 1996), which further emphasises the need for quantitative dose–response relations between disturbing stress and reproduction parameters.
Conclusions
In the present study, we simulated the impacts of ∑PCB, DDE and disturbance stress on white-tailed eagle populations. The ∑PCB contamination from 1950 till 1987 was found to be too high to allow return of white-tailed eagle as a breeding species in The Netherlands. The impact of DDE concentrations was limited. Simulations of ∑PCB exposure for 2006–2050 indicated that the future population development is still hampered. Simulation of the impact of disturbance stress resulted in a reduced population development. The combination of both toxic and disturbance stress varied from a slower population development to a catastrophical reduction in population size, where the main cause was attributed to the reduction in reproduction of 50%. Despite the limited availability of dose–response relationships and the uncertainties in parameter setting and validation data, the model provides a first step in integrating and quantifying the impacts of multiple stressors on white-tailed eagle populations.
References
Beale CM, Monaghan P (2005) Modeling the effects of limiting the number of visitors on failure rates of seabird nests. Conserv Biol 19(6):2015–2019. doi:10.1111/j.1523-1739.2005.00256.x
Best DA, Elliott KH, Bowerman WW, Shieldcastle M, Postupalsky S, Kubiak TJ, Tillitt DE, Elliott JE (2010) Productivity, embryo and eggshell characteristics, and contaminants in bald eagles from the Great Lakes, USA, 1986 to 2000. Environ Toxicol Chem 29(7):1581–1592. doi:10.1002/etc.195
Beurskens JEM, Mol GAJ, Barreveld HL, Munster Bv, Winkels HJ (1993) Geochronology of priority pollutants in a sedimentation area of the Rhine River. Environ Toxicol Chem 12:1549–1566. doi:10.1002/etc.5620120905
Birch LC (1948) The intrinsic rate of natural increase of an insect population. J Anim Ecol 17(1):15–26. doi:10.2307/1605
Blackmer AL, Ackerman JT, Nevitt GA (2004) Effects of investigator disturbance on hatching success and nest-site fidelity in a long-lived seabird, Leach’s storm-petrel. Biol Conserv 116(1):141–148. doi:10.1016/S0006-3207(03)00185-x
Bolduc F, Guillemette M (2003) Human disturbance and nesting success of Common Eiders: interaction between visitors and gulls. Biol Conserv 110(1):77–83. doi:10.1016/S0006-3207(02)00178-7
Bortolotti GR (1986) Influence of sibling competition on nesting sex ratios of sexually dimorphic birds. Am Nat 127(4):495–507. doi:10.1086/284498
Bowerman WW (1993) Regulation of bald eagle (Halaieetus leucocephalus) productivity in the Great Lakes Basin: an ecological and toxicological approach. Dissertation, Michigan State University
Bowerman WW, Giesy JP, Best DA, Kramer VJ (1995) A review of factors affecting productivity of bald eagles in the Great Lakes region: implication for recovery. Environ Health Perspect 103(4):51–59. doi:10.2307/3432412
Bowerman WW, Grubb RG, Bath AJ, Giesy JP, Weseloh DVC (2005) A survey of potential bald eagle nesting habitat along the Great Lakes shoreline. Rocky Mountain Research Station, Fort Collins
Broley CL (1947) Migration and nesling of Florida Bald Eagles. Wilson Bull 59(1):1–68
Buck JA, Anthony RG, Schuler CA, Isaacs FB, Tillitt DE (2005) Changes in productivity and contaminants in bald eagles nesting along the lower Columbia River, USA. Environ Toxicol Chem 24(7):1779–1792. doi:10.1897/03-621.1
Buehler DA, Fraser JD, Seegar JKD, Therres GD, Byrd MA (1991a) Survival rates and population dynamics of bald eagles on Chesapeake Bay. J Wildl Manage 55(4):608–613. doi:10.2307/3809506
Buehler DA, Mersmann TJ, Fraser JD, Seegar JKD (1991b) Effects of human activity on bald eagles distribution on the Northern Chesapeake Bay. J Wildl Manage 55(2):282–290. doi:10.2307/3809151
Colborn T (1991) Epidemiology of Great Lakes bald eagles. J Toxicol Environ Health 33:395–453. doi:10.1080/15287399109531537
Donaldson GM, Shutt JL, Hunter P (1999) Organochlorine contamination in bald eagle eggs and nestlings from the Canadian Great lakes. Arch Environ Contam Toxicol 36:70–80. doi:10.1007/s002449900444
Dykstra CR, Meyer MW, Warnke DK, Karasov WH, Andersen DE, Bowerman WW, Giesy JP (1998) Low reproductive rates of Lake Superior bald eagles: low food delivery rates or environmental contaminants? J Great Lakes Res 24(1):32–44. doi:10.1016/S0380-1330(98)70797-x
Elliott JE, Harris ML (2001) An ecotoxicological assessment of chlorinated hydrocarbon effects on bald eagle populations. Rev Toxicol 4:1–60
Elliott JE, Wilson LK, Langelier KW, Norstrom RJ (1996) Bald eagle mortality and chlorinated hydrocarbon contaminations in livers from British Columbia, Canada, 1989–1994. Environ Pollut 94(1):9–18. doi:10.1016/S0269-7491(96)00106-6
Evans RJ, Wilson JD, Amar A, Douse A, MacLennan A, Ratcliffe N, Whitfield DP (2009) Growth and demography of a re-introduced population of white-tailed eagles Haliaeetus albicilla. Ibis 151(2):244–254. doi:10.1111/j.1474-919X.2009.00908.x
Finney SK, Pearce-Higgins JW, Yalden DW (2005) The effect of recreational disturbance on an upland breeding bird, the golden plover Pluvialis apricaria. Biol Conserv 121(1):53–63. doi:10.1016/j.biocon.2004.04.009
Frank R, Holdrinet M, Braun HE, Thomas RL, Kemp ALW, Jaquet JM (1977) Organochlorine insecticides and PCBs in sediments of Lake St. Clair (1970 and 1974) and Lake Erie (1971). Sci Total Environ 8(3):205–227. doi:10.1016/0048-9697(77)90022-5
Garcelon D, Thomas NJ (1997) DDE Poisoning in an adult Bald Eagle. J Wildl Dis 33(2):299–303
Gende SM, Wilson MF, Jacobsen M (1997) Reproductive success of bald eagles (Haliaeetus leucocephalus) and its association with habitat or landscape features and weather in southeast Alaska. Can J Zool-Rev Can Zool 75(10):1595–1604. doi:10.1139/z97-786
Gill CE, Elliott JE (2003) Influence of food supply and chlorinated hydrocarbon contaminants on breeding success of bald eagles. Ecotoxicology 12(1–4):95–111. doi:10.1023/A:1022549231826
Grasman KA (2002) Assessing immunological function in toxicological studies of avian wildlife. Integr Comp Biol 42(1):34–42. doi:10.1093/icb/42.1.34
Green RE, Pienkowski MW, Love JA (1996) Long-term viability of the re-introduced population of the white-tailed eagle Haliaeetus albicilla in Scotland. J Appl Ecol 33(2):357–368. doi:10.2307/2404757
Grier JW (1982) Ban of DDT and subsequent recovery of reproduction in bald eagles. Science 218(4578):1232–1235. doi:10.1126/science.7146905
Grim LH, Kallemeyn LW (1995) Reproduction and distribution of bald eagles in Voyageurs National Park, Minnesota, 1973–1993. National Biological Service, Washington, DC
Grubb TG, Bowerman WW, Giesy JP, Dawson GA (1992) Responses of breeding bald eagles, Haliaeetus leucocephalus, to human activities in Northcentral Michigan. Can Field-Nat 106(4):443–453
Hailer F, Helander B, Folkestad AO, Ganusevich SA, Garstad S, Hauff P, Koren C, Nygard T, Volke V, Vila C, Ellegren H (2006) Bottlenecked but long-lived: high genetic diversity retained in white-tailed eagles upon recovery from population decline. Biol Lett 2(2):316–319. doi:10.1098/rsbl.2006.0453
Helander B, Olsson A, Bignert A, Asplund L, Litzen K (2002) The role of DDE, PCB, coplanar PCB and eggshell parameters for reproduction in the white-tailed sea eagle (Haliaeetus albicilla) in Sweden. Ambio 31(5):386–403. doi:10.1579/0044-7447-31.5.386
Helander B, Bignert A, Asplund L (2008) Using raptors as environmental sentinels: monitoring the white-tailed sea eagle Haliaeetus albicilla in Sweden. Ambio 37(6):425–431. doi:10.1579/0044-7447(2008)37[425:URAESM]2.0.CO;2
Hendriks AJ (1993) Monitoring concentrations of microcontaminants in sediment and water in the Rhine Delta: a comparison to reference values. European Water Pollution Control 3(1):33–38
Hendriks AJ (1995) Modelling equilibrium concentrations of microcontaminants in organisms of the Rhine delta: Can average field residues in the aquatic food chain be predicted from laboratory accumulation? Aqua Toxicol 31:1–25. doi:10.1016/0166-445X(94)00052-R
Hendriks AJ, Enserink EL (1996) Modelling response of single-species populations to microcontaminants as a function of species size with examples for waterfleas (Daphnia magna) and cormorants (Phalacrocorax carbo). Ecol Model 88(1–3):247–262. doi:10.1016/0304-3800(95)00111-5
Hendriks AJ, Pieters H (1993) Monitoring concentrations of micro-contaminants in aquatic organisms in the Rhine delta: a comparison to reference values. Chemosphere 26:817–836. doi:10.1016/0045-6535(93)90358-C
Hendriks AJ, Pieters H, de Boer J (1998) Accumulation of metals, polycyclic (halogenated) aromatic hydrocarbons, and biocides in zebra mussel and eel from the Rhine and Meuse Rivers. Environ Toxicol Chem 17(10):1885–1898. doi:10.1002/etc.5620171001
Hendriks AJ, Maas-Diepeveen JLM, Heugens EHW, Van Straalen NM (2005) Meta-analysis of intrinsic rates of increase and carrying capacity of populations affected by toxic and other stressors. Environ Toxicol Chem 24(9):2267–2277. doi:10.1897/05-122.1
Henny CJ, Kaiser JL, Grove RA, Johnson BL, Letcher RJ (2009) Polybrominated diphenyl ether flame retardants in eggs may reduce reproductive success of ospreys in Oregon and Washington, USA. Ecotoxicology 18(7):802–813. doi:10.1007/s10646-009-0323-4
Koistinen J, Koivusaari J, Nuuja I, Vuorinen PJ, Paasivirta J, Giesy JP (1997) 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin equivalents in extracts of Baltic white-tailed sea eagles. Environ Toxicol Chem 16(7):1533–1544. doi:10.1002/etc.5620160730
Kollman R, Neumann T, Struwe-Juhl B (2002) Bestand und Schutz des Seeadlers (Haliaeetus albicilla) in Deutschland und seinen Nachbarländern. Corax 19(1):1–14
Krone O, Langgemach T, Sömmer P, Kenntner N (2003) Causes of mortality in white-tailed sea eagles from Germany. In: Helander B, Marquiss M, Bowerman W (eds) Sea Eagle 2000. Proceedings of the Swedish Society for Nature Conservation SNF, Stockholm, pp 211–218
Krone O, Stiernberg T, Kenntner N, Tataruch F, Koivusaari J, Nuuja I (2006) Mortalitv factors, helminth burden, and contaminant residues in white-tailed sea eagles (Haliaeetus albicilla) from Finland. Ambio 35(3):98–104. doi:10.1579/0044-7447(2006)35[98:MFHBAC]2.0.CO;2
Krüger O, Grünkorn T, Struwe-Juhl B (2010) The return of the white-tailed eagle (Haliaeetus albicilla) to northern Germany: modelling the past to predict the future. Biol Conserv 143(3):710–721. doi:10.1016/j.biocon.2009.12.010
Marris E (2009) Reflecting the past. Nature 462(7269):30–32. doi:10.1038/462030a
Marvin CH, Painter S, Charlton MN, Fox ME, Lina Thiessen PA (2004) Trends in spatial and temporal levels of persistent organic pollutants in Lake Erie sediments. Chemosphere 54(1):33–40. doi:10.1016/S0045-6535(03)00660-X
McGarigal K, Anthony RG, Isaacs FB (1991) Interactions of humans and bald eagles on the Columbia River estuary. Wildl Monogr 115:1–47
Ministry of Agriculture, Nature and Food Quality (2005) Handreiking Beheerplannen Natura 2000-gebieden. Ministerie van LNV, The Hague (in Dutch)
Møller AP, Cassey P (2004) On the relationship between T-cell mediated immunity in bird species and the establishment success of introduced populations. J Anim Ecol 73(6):1035–1042. doi:10.1111/j.0021-8790.2004.00879.x
Müller K, Altenkamp R, Brunnberg L (2007) Morbidity of free-ranging white-tailed sea eagles (Haliaeetus albicilla) in Germany. J Avian Med Surg 21(4):265–274. doi:10.1647/2007-001R.1
Nygård T, Gjershaug JO (2001) The effects of low levels of pollutants on the reproduction of golden eagles in western Norway. Ecotoxicology 10(5):285–290. doi:10.1023/A:1016759317738
Nygård T, Kenward RE, Einvik K (2000) Radio telemetry studies of dispersal and survival in juvenile white-tailed sea eagles (Haliaeetus albicilla) in Norway. In: Chancellor RD, Meyburg BU (eds) Raptors at risk. World working group of birds of prey, London, pp 487–497
Painter S, Marvin C, Rosa F, Reynoldson TB, Charlton MN, Fox M, Thiessen L, Estenik JF (2001) Sediment contamination in Lake Erie: A 25-year retrospective analysis. J Great Lakes Res 27(4):434–448. doi:10.1016/S0380-1330(01)70658-2
Radovic A, Mikuska T (2009) Testing the effect of persecution and permanent dispersion of sub-adult birds in long-term sustainability of White tailed eagles (Haliaeetus albicilla L.) population at different management options in Croatia. Acta Zool Acad Sci Hung 55(4):395–407
Reichel WL, Schmeling SK, Cromartie E, Kaiser TE, Krynitsky AJ, Lamont TG, Mulhern BM, Prouty RM, Stafford CJ, Swineford DM (1984) Pesticide, PCB, and lead residues and necropsy data for bald eagles from 32 states-1978–81. Environ Monit Assess 4(4):395–403. doi:10.1007/bf00394177
Reijnen R, Harms WB, Foppen RPB, De Visser R, Wolfert HP (1995) Rhine-Econet: ecological networks in river rehabilitation scenarios: a case study for the Lower Rhine. Publications and reports of the project `Ecological Rehabilitation of the Rivers Rhine and Meuse’. RIZA, Institute for Inland Water Management and Waste Water Treatment, Lelystad
Saurola P, Stjenberg T, Högmander J, Koivusaari J, Ekblom H, Helander B (2003) Survival of juvenile and sub-adult Finnish white-tailed sea eagles in 1991–1999: a preliminary analysis based on resightings of colour-ringed individuals. In: Helander B, Marquiss M, Bowerman WW (eds) Sea Eagle 2000. Swedish Society for Nature Conservation/SSF and Atta, Stockholm, pp 151–167
Scharenberg W, Struwe-Juhl B (2006) White-tailed eagles (Haliaeetus albicilla) in Schleswig-Holstein no longer endangered by organochlorines. Bull Environ Contam Toxicol 77(6):888–895. doi:10.1007/s00128-006-1227-0
Sibly RM, Akcakaya HR, Topping CJ, O’Connor RJ (2005) Population-level assessment of risks of pesticides to birds and mammals in the UK. Ecotoxicology 14(8):863–876. doi:10.1007/s10646-005-0033-5
Steidl RJ, Anthony RG (2000) Experimental effects of human activity on breeding Bald Eagles. Ecol Appl 10(1):258–268. doi:10.2307/2641000
Struwe-Juhl B (2002) Age-structure and productivity of a German white-tailed sea eagle (Haliaeetus albicilla) population. Corax 19(1):51–61
Traas T, Van de Meent D, Posthuma L, Hamers T, Kater BJ, De Zwart D, Aldenberg T (2002) The potentially affected fraction as a measure of ecological risk. In: Posthuma L, Suter GW, Traas TP (eds) Species sensitivity distributions in ecotoxicology. CRC Press, Boca Raton, pp 315–344
Van Rijn S, Zijlstra M, Bijlsma RG (2010) Wintering white-tailed eagles Haliaeetus albicilla in The Netherlands: aspects of habitat scale and quality. Ardea 98(3):373–382. doi:10.5253/078.098.0311
Van Wezel AP, Traas T, Polder M, Posthumus R, Van Vlaardingen P, Crornrnentuijn T, Van de Plassche EJ (1999) Maximum permissible concentrations for polychlorinated biphenyls. RIVM, Bilthoven
Watts BD, Therres GD, Byrd MA (2008) Recovery of the Chesapeake Bay bald eagle nesting population. J Wildl Manage 72(1):152–158. doi:10.2193/2005-616
Weseloh DV, Hughes KD, Ewins PJ, Best D, Kubiak T, Shieldcastle MC (2002) Herring gulls and great black-backed gulls as indicators of contaminants in bald eagles in Lake Ontario, Canada. Environ Toxicol Chem 21(5):1015–1025. doi:10.1002/etc.5620210518
Wiemeyer SN, Bunck CM, Stafford CJ (1993) Environmental contaminants in Bald eagle eggs 1980–84 and further interpretations of relationships to productivity and shell thickness. Arch Environ Contam Toxicol 24(2):213–227. doi:10.1007/BF01141351
Zuberogoitia I, Zabala J, Martinez JA, Martinez JE, Azkona A (2008) Effect of human activities on Egyptian vulture breeding success. Anim Conserv 11(4):313–320. doi:10.1111/j.1469-1795.2008.00184.x
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Korsman, J.C., Schipper, A.M., Lenders, H.J.R. et al. Modelling the impact of toxic and disturbance stress on white-tailed eagle (Haliaeetus albicilla) populations. Ecotoxicology 21, 27–36 (2012). https://doi.org/10.1007/s10646-011-0760-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0760-8