Abstract
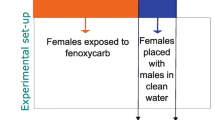
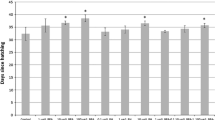
Bisphenol A is a known endocrine disruptor in vertebrates that mimics the action of estrogens by interacting with hormone receptors. It also affects reproduction and development in many invertebrate animals, though mechanisms of action are unclear. Terrestrial insects, despite their abundance and profound ecological significance, have been largely overlooked as a group that might be affected by vertebrate endocrine disrupting chemicals. We evaluated potential effects of bisphenol A on the ring-legged earwig, Euborellia annulipes, as a model for terrestrial arthropods. Dosages of 0, 0.12, 1.2 and 12 μg bisphenol A were injected over a 6 day period into newly eclosed males and newly mated (7-day) females. The lowest dosage (0.12 μg) was most effective in eliciting significant effects including reducing weight gain while increasing testis size and seminal vesicle size; higher dosages were less effective or ineffective. In females, treatment with 0.12 μg bisphenol A enhanced clutch size but higher dosages were required to affect the duration of embryogenesis in offspring of treated mothers. Hatching success and the onset of the second reproductive cycle were not affected by treatments. No gross abnormalities were observed as a result of treatment in the reproductive structures of either males or females. Similarly, injection of varying concentrations of estradiol into males enhanced testis length, though it had no effect on seminal vesicle size. Lastly, we administered bisphenol A in drinking water for up to 2 weeks. Surprisingly, as little as 1 μg/L inhibited testis growth; 100 μg/L inhibited ovarian growth.







Similar content being viewed by others
References
Alonso-Magdalena P, Morimoto S, Ripoll C, Fuetes E, Nadal A (2006) The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect 114:106–112
Canesi L, Lorusso LC, Ciacci C, Betti M, Zampini M, Gallo G (2004) Environmental estrogens can affect the function of mussel hemocytes through rapid modulation of kinase pathways. Gen Comp Endocrinol 138:58–69
Canesi L, Borghi C, Ciacci C, Fabbri R, Vergani L, Gallo G (2007) Bisphenol-A alters gene expression and functional parameters in molluscan hepatopancreas. Mol Cell Endocrinol 276:36–44
Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ (2007) An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 24:225–239
de Fur PL (2004) Use and role of invertebrate models in endocrine disruptor research and testing. ILAR J 45:484–493
Duft M, Schulte-Oehlmann U, Weltje L, Tillmann M, Oehlmann J (2003) Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mudsnail Potamopyrus antipodarum. Aquat Toxicol 64:437–449
Fukuhori N, Kitano M, Kimura H (2005) Toxic effects of bisphenol A on sexual and asexual reproduction in Hydra oligactis. Arch Environ Contam Toxicol 48:495–500
Hahn T, Schenk K, Schultz R (2002) Environmental chemicals with known endocrine potential affect yolk protein content in the aquatic insect Chironomus riparius. Environ Pollut 120:525–528
Hill M, Stabile C, Steffen LK, Hill A (2002) Toxic effects of endocrine disruptors on freshwater sponges: common developmental abnormalities. Environ Pollut 117:295–300
Hirano M, Ishibashi H, Matsumura N, Watanabe N, Watanabe A, Onikura N, Kishi K, Arizono K (2004) Acute toxicity responses of two crustaceans, Americamysis bahia and Daphnia magna, to endocrine disrupters. J Health Sci 50:97–100
Jobling S, Casey D, Rodgers-Gray T, Oehlmann J, Schulte-Oehlmann U, Pawlowski S, Baunbeck T, Turner AP, Tyler CR (2003) Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquat Toxicol 65:205–220
Kang J, Katayama Y, Kondo F (2006) Biodegradation or metabolism of bisphenol A: from microorganisms to mammals. Toxicology 217:81–90
Keshan B, Ray AK (1998) Action of estradiol-17B on the synthetic activity of the silk gland in Bombyx mori L. J Insect Physiol 44:491–498
Keshan B, Ray AK (2001) The presence of estradiol-17β and its specific binding sites in posterior silk gland of Bombyx mori. Gen Comp Endocrinol 123:23–30
Khan AR, Ahamed N, Saha BN (1998) Effects of vertebrate sex-hormones, mestranol and norethindrone on jute hairy caterpillar, Spilarctia obliqua. Bangladesh J Zool 26:1–6
Kirkbride-Smith AE, Bell HA, Edwards JP (2001) Effects of three vertebrate hormones on the growth, development, and reproduction of the tomato moth, Lacanobia oleracea L. Environ Toxicol Chem 20:1838–1845
Lahnsteiner F, Berger B, Kletzl M, Weismann T (2005) Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario. Aquat Toxicol 75:213–224
Levy G, Lutz I, Kruger A, Kloas W (2004) Bisphenol A induces feminization in Xenopus laevis tadpoles. Environ Res 94:102–111
Lindholst C, Pedersen S, Bjerregaard P (2001) Uptake, metabolism and excretion of bisphenol A in the rainbow trout. Aquat Toxicol 55:75–84
MacLusky NJ, Hajszan T, Leranth C (2005) The environmental estrogen bisphenol-A inhibits estrogen-induced hippocampal synaptogenesis. Environ Health Perspect 113:675–679
Mechoulam R, Brueggemeier RW, Denlinger DL (1984) Estrogens in insects. Experientia 40:942–944
Nieto-Fernandez FE, Ianuzzi F, Ruiz A, Nodimele L (2004) Estradiol-stimulated nitric oxide release in nervous tissue, vasculature, and gonads of the giant cockroach, Blaberus craniifer. Acta Biol Hung 55:143–148
Oehlmann J, Schulte-Oehlmann U, Tillmann M, Markert B (2000) Effects of endocrine disruptors on prosobrach snails (Mollusca: Gastropoda) in the laboratory. Part I: Bisphenol A and octylphenol as xeno-estrogens. Ecotoxicology 9:383–397
Ogiso M, Ohnishi E (1986) Does estradiol play a role in ovarian maturation or embryonic development of the silkworm? Gen Comp Endocrinol 61:82–86
Ohnishi E, Ogiso M, Wakabayashi H, Fujimoto Y, Ikekawa N (1985) Identification of estradiol in the ovaries of the silkworm, Bombyx mori. Gen Comp Endocrinol 60:35–38
Porte C, Janer G, Lorusso LC, Ortiz-Zarragoitia M, Cajaraville MP, Fossi MC, Canesi L (2006) Endocrine disruptors in marine organisms: Approaches and perspectives. Comp Biochem Physiol C 143:303–315
Quinn B, Gagne F, Costello M, McKenzie C, Wilson J, Mothersill C (2004) The endocrine disrupting effect of municipal effluent on the zebra mussel (Dreissena polyphema). Aquat Toxicol 66:279–292
Rankin SM, Palmer JO, Larocque Risser AL (1995a) Life history characteristics of the ring-legged earwig (Dermaptera: Labiduridae): emphasis on ovarian development. Ann Entomol Soc Am 88:887–893
Rankin SM, Palmer JO, Yagi KJ, Scott GL, Tobe SS (1995b) Biosynthesis and release of juvenile hormone during the reproductive cycle of the ring-legged earwig. Comp Biochem Physiol C 110:241–251
Rankin SM, Innocenti MA, Eicher CA, Furst D (2004) The effect of ventral nerve cord severance and male castration on female mating behavior, clutch size, and maternal care in the ring-legged earwig. Comp Biochem Physiol C 139:533–541
Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS (2007) In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224
Roepke TA, Snyder MJ, Cherr GN (2005) Estradiol and endocrine disrupting compounds adversely affect development of sea urchin embryos at environmentally relevant concentrations. Aquat Toxicol 71:155–173
Roy S, De J, Kundu S, Biswas A, Pramanik M, Ray AK (2007) Estradiol-17 β: tracing its metabolic significance in female fatbody of fifth instar larvae of silkworm, Bombyx mori L. (race : Nistari). Life Sci 80:446–453
Schirling M, Jungmann D, Ladewig V, Ludwichowski K-U, Nagel R, Kőhler H-R, Triebskorn R (2006) Bisphenol A in artificial indoor streams: II. Stress response and gonad histology in Gammarus fossarum (Amphipoda). Ecotoxicology 15:143–156
Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y (2004) Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocrinol J 51:165–169
Timms BG, Howdeshell K, Barton L, Richter CA, vom Saal FS (2005) Estrogenic chemicals in plastic and oral contraceptives disrupt development of the mouse prostate and urethra. PNAS (US) 102:7014–7019
Tollefsen KE (2001) Interaction of estrogen mimics singly and in combination with plasma sex steroid-binding proteins in rainbow trout. Aquat Toxicol 56:215–225
vom Saal FS, Hughes C (2005) An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 113:926–933
Wang YW, Olmstead AW, Li H, LeBlanc GA (2005) The screening of chemicals for juvenoid-related endocrine activity using the water flea Daphnia magna. Aquat Toxicol 74:193–204
Waring RH, Harris RM (2005) Endocrine disrupters: a human risk? Mol Cell Endocrinol 244:2–9
Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS (2003) Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 111:994–1006
Acknowledgments
This work was supported by the Shanbrom Fund of Allegheny College. We are also indebted to Nicholas D. Mains and Mark C. Sutton for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rankin, S.M., Grosjean, E.M. Effects of bisphenol A in the ring-legged earwig, Euborellia annulipes . Ecotoxicology 19, 635–642 (2010). https://doi.org/10.1007/s10646-009-0435-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0435-x