Abstract
The Media Luna spring, Mexico, is the main reservoir of the endemic and endangered fish Ataeniobius toweri. In the last decades, the ecosystem has been modified by tourism, and the habitat has changed for this species. Therefore, for better conservation management of the natural fish population, it is necessary to understand its abundance status and suitable habitat conditions, in ecological and spatial scenarios, on a temporal scale. In the present study, we modeled A. toweri’s ecological responses and spatial distribution for adult and juvenile life stages, in three summer periods (years 1999, 2009, and 2019). As habitat variables, we used water depth and underwater coverage. Ecological response curves were obtained from a Generalized Linear Model; distribution models were obtained with DOMAIN. In the modeling evaluation, for the Linear Regression Model, we obtained true statistical skills metric > 0.30 and, for DOMAIN, an area under the curve (AUC) > 0.70 with an AUC ratio > 1.00. In general, as the summer periods progressed, we found the highest probability of occurrence (P > 0.20) and distribution (P > 0.60) in areas with conditions of large coverage of underwater vegetation, in the first 1.5 m of depth, and near the shores of the spring. Also, the variations of relative abundance were always observed at sites with these habitat conditions. Thus, we concluded that our models had the performance to discern between suitable and unsuitable habitat conditions for A. toweri, and that areas with little or no anthropogenic pressure are more important for this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater systems in arid and semi-arid zones present a unique richness of fishes, with different abundance, habitat selection, and distribution (Ceballos et al. 2018), that depend on their spatial ecology (Winemiller et al. 2008). In these ecosystems, the ichthyofauna is usually geographically isolated, often restricted to a single spring (Contreras-Balderas and Lozano-Vilano 1993; Palacio-Núñez et al. 2010a), presenting adaptations to the ecological conditions of the habitat (Albrecht and Gotelli 2001; Poff et al. 2003; Wisz et al. 2012). This leads to particular geographic patterns in response to the physical, chemical, and biological variables of the site (Contreras-B and Lozano-V 1994; Olden et al. 2002). However, natural and unnatural hydrological disturbances (e.g., drought, tourism pressure, underwater vegetation composition) change the ecosystem conditions in space and time and the dynamic patterns of the fishes (Ruetz III et al. 2005). Therefore, it is of utmost importance to understand the ecological and spatial dynamics of species populations (Engen et al. 2018).
In the arid and semi-arid zones of Mexico, springs with variable habitats have been recorded harboring a singular richness of endemic fishes with specific ecological affinities and spatial patterns that depend on habitat conditions (Contreras-Balderas and Lozano-Vilano 1993; CONABIO 2020). A clear example is the Rioverde plain, located in the northeastern portion of the country, in which there are scattered springs, with a richness of endemism. The Media Luna spring stands out for its larger water surface and popularity (Miller et al. 2005). This spring has a special landscape, with crystalline thermal water that attracts thousands of tourists each year. Thus, attention must be drawn both to the possible structural changes of this freshwater ecosystem and the pressure suffered by fish populations (Galván-Meza et al. 2018), as has been indicated for other sites (e.g., Hickley et al. 2004). This need for attention has motivated studies of the spatial ecology and conservation of the endemic ichthyofauna at this site (e.g., De la Vega Salazar 2009; Miller et al. 2005; Palacio-Núñez et al. 2010a).
In particular, the Media Luna spring is the habitat of four exclusive endemic fishes of the Rioverde plain (Ceballos et al. 2018) and is the main reservoir of the endemic goodeid, Ataeniobius toweri (Meek, 1904) (Palacio-Núñez et al. 2010b). This species is listed as endangered (IUCN 2021) because its population has been declining in the present century (Contreras-MacBeath et al. 2014; Lyons et al. 2019). In terms of its affinity for in-spring habitat, this species swims in open water above dense underwater vegetation or among stems (Palacio-Núñez et al. 2010b). Its greatest activity is recorded at a depth gradient of less than 1.0 m (Miller et al. 2005). However, it has been observed at depths slightly greater than 2.0 m (pers. obs.). Despite this information, there is a lack of understanding of the degree of affinity for habitat conditions and the sites of greatest occurrence of A. toweri. Also, the affinity for particular habitat conditions and sites can vary by life stages (Brosse and Lek 2002; Zhang et al. 2020), such as adults and juveniles, for this and other fish species (e.g., Brandt 1980).
The ecological response curves and spatial distribution models are suitable for correlating species occurrence with habitat variables (i.e., Miller 2010; Mateo et al. 2011). They are also useful for predicting spatial patterns and selection of habitat conditions (Benito and Peñas 2007; Lobo et al. 2015); especially when grouping by life stages. This, together with estimating relative abundance indexes from the records and analyzing different periods, makes it possible to study the variability of detection of the species (Martin-Garcia et al. 2022).
The studies that take into account wide-ranging periods to record species occurrence and habitat variables are fundamental to explaining the ecological and geographic scenario of the species (Ruetz III et al. 2005; Elith and Leathwick 2009). This makes it possible to establish exhaustive biomonitoring of site variables and species of interest, particularly endemic species, to generate ecological-spatial models (Joy and Death 2002; Maloney et al. 2013) that closely match the ecological reality of the species. This supports leading conservation management decisions based on scientific evidence (Pullin et al. 2004).
With this approach, we present a contribution to the ecological-spatial knowledge of A. toweri in the Media Luna spring in Mexico. We aimed to estimate its relative abundance and to model A. toweri’s ecological response curves and spatial distribution by life stage, in the summers of 1999, 2009, and 2019, in the Media Luna spring, Mexico. Based on the literature reviewed (e.g., Miller et al. 2005; Palacio-Núñez et al. 2010b), we hypothesized that A. toweri exhibits temporal variation in abundance, habitat selection, and distribution patterns under structural and compositional changes within its natural habitat. Therefore, we evaluated the response variable of occurrence to determine the temporal variation of this endemic species. We used water depth and underwater cover as predictor variables for modeling. This approach was necessary to understand the suitable habitat conditions, geographic patterns, and population status for this species, in specific ecological and spatial scenarios, on a temporal scale.
Methods
Study site
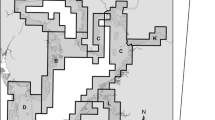
The Media Luna spring is located in the semiarid plain of Rioverde, municipality of Rioverde, in the state of San Luis Potosí, northeastern Mexico. This spring rises from a main lagoon, where six craters are located at different depths (36.2 m is the maximum recorded; Fig. 1), from which an average of 4.35 m3 s−1 of very crystalline thermal water flows (Galván-Meza et al. 2018). Three main canals continue from this lagoon, where the depth does not usually exceed 2.5 m. In this natural system, there is a wide extension of underwater vegetation coverage, dominated by the water lily Nymphaea ampla (Salisb.) DC. 1806. Due to the ecological singularity of the spring and the concern about damage caused by increased tourism, it was decreed as a natural protected area under the modality of a State Park on June 07, 2003 (Periódico Oficial del Estado Libre y Soberano de San Luis Potosí 2003).
Geographic location of the Media Luna spring, Mexico. a shows the distribution of Ataeniobius toweri inside the Rioverde plain (Koeck 2019) and the location of the study site; there are marks for the main lagoon, where the six craters are located, and the three main canals (above square). The digital bathymetric model is used as a base map for the water spring surface; it was based on Palacio-Núñez et al. (2010b). b shows a general view of the study site location at the country and state levels
Occurrence records and relative abundance
We assessed the temporal variation in the relative abundance, habitat selection, and spatial distribution of A. toweri over 20 years, from 1999, when the first sampling was carried out, until 2019. For this purpose, we selected three study replicates (years 1999, 2009, and 2019), separated by 10 years. This avoids assumptions of uniform animal distribution and biased temporal variation (Ruetz III et al. 2005). In addition, we selected only records from summer periods, because we studied variations when tourism increased at the site, which we consider to influence the natural habitat of this endemic fish.
The records of A. toweri were obtained from two sources. For the summer of 1999, data were obtained from the literature (Palacio-Núñez et al. 2010b), and for 2009 and 2019, data was obtained by our research group (Rössel-Ramírez et al. 2023a). These records were categorized into adult and juvenile life stages. For correct identification of life stage, based on body size, the dataset was verified with information from previous studies on the life history of A. toweri (e.g., Miller et al. 2005; Palacio-Núñez et al. 2010b; Ceballos et al. 2018). It should be mentioned that all data in each period was recorded from 66 underwater transects, evenly distributed along the spring surface (e.g., Palacio-Núñez et al. 2010b). For the positioning of these transects, the system was sectorized (14 sectors; Fig. S1), as proposed by Palacio-Núñez et al. (2010b). The criteria for sector boundaries were based on underwater coverage within the water depth, as well as the anthropic impact degree (Table S1). Since 1999, sectors 1 (S1) and 10 to 14 (S10 to S14) showed little or no underwater vegetation, mainly due to tourism activity, such as diving or swimming. These activities damage or eliminate the vegetation, in addition to dispersing native ichthyofauna (Maloney et al. 2013; Galván-Meza et al. 2018). In contrast, sectors 2 to 9 (S2 to S9) had a higher density of macrophyte coverage of N. ampla, which grows as emergent, sub-emergent, and floating vegetation (Palacio-Núñez et al. 2010b; pers. obs.).
Using a standardized method, in 1999, 3 or 6 transects were designed for each sector, as a function of the length and width of the canal; each transect replicate occurred at random habitat conditions and was placed from the shore to the center of the canal. In the following two summer periods, the number of transects was the same and the location coordinates were kept unchanged to carry out this spatio-temporal study. In each transect (dimensions: 2 m width × 10 m length), all occurrence records were obtained by direct observation, which included photographic documentation since 2009. Thus, no specimens of this species were handled, damaged, or captured (e.g., Prchalová et al. 2009).
Subsequently, using databases for summer periods, we first performed some filtering, following the method of García-Roselló et al. (2014), to identify spatial outliers or incomplete data (which were not found) or to exclude records with duplicate coordinates. With the remaining records, we also performed spatial filtering with the QGIS® software (i.e., excluding records outside of the study area or with spatial unlikelihood) and environmental filtering (i.e., normality test) in Rstudio®. The number of records in the final database for 1999 consisted of 407 adults and 189 juveniles; in 2009, there were 28 adults and 11 juveniles in the database, and in 2019, 80 adults and 71 juveniles (Fig. S2). From these records, we estimated the relative abundance (RA) to describe the temporal variation of the population by summer period and life stage in each sector. We plotted these estimations in Rstudio® using the ggplot2 package (Wickham et al. 2019).
Predictor variables selection
As predictor variables, we used water depth (WDp) and underwater coverage (UC) from previous literature reviews on freshwater fish distribution and habitat selection (e.g., Rozas and Odum 1988; Joy and Death 2002; Prchalová et al. 2009) and from our field observations. Particularly, WDp has a significant effect on the richness, abundance, and distribution of fish populations (Prchalová et al. 2009). In addition, UC conditions influence food and shelter for the fish (Rozas and Odum 1988; Brosse and Lek 2002), mainly in early life stages. Both variables are also related to water temperature (Brandt 1980; Eby et al. 2003), dissolved oxygen, and primary biomass productivity (Brosse et al. 1999; Del Río et al. 2019).
Given the information from both WDp and UC, we obtained a digital bathymetric model for WDp and a raster with the classification of UC in the three summer periods (Rössel-Ramírez et al. 2023b). Each raster was processed and rasterized with different spatial information by the summer period. The WDp variable was interpolated by the Inverse Distance Weighted method of Bartier and Keller (1996), with a P distance coefficient of 2.0. All raster layers had a range from 0.0 to 36.2 m depth. UC was reclassified using a supervised method, according to NASA’s accuracy methodology for coverage classification (Kalluri et al. 2003). This reclassification included the categories of UC proposed by Palacio-Núñez et al. (2010b) and Rössel-Ramírez et al. (2023b) for the Media Luna spring, based on the presence of macrophyte vegetation or bare soil. The vegetation coverage, formed by N. ampla, was classified as (1) small carpet (Sc), plants with a height below 0.3 m and heart-shaped leaves; (2) big carpet (Bc), plants with stems of variable length and ovoid leaves that do not reach the water surface; and (3) mature shape (Ms), with floating leaves and characteristic white flowering. Also, the isolated remnants of vegetation were (1) big carpet patch (Bcp) and (2) mature shape patch (Msp). The bare soil was classified as (1) natural bare soil (Nbs), (2) bare soil by tourism (Bst), and (3) bare soil by depth (Bsd).
For both variables, we downloaded the raster layers with the same geographic projection in WGS 84/Pseudo-Mercator (EPSG: 3857) and the same extent for each summer period. Also, we retained the Ground Sample Distance of 0.1 m (e.g., Joy and Death 2002; Guisan et al. 2007).
Relative contribution and multicollinearity of predictor variables
Using the statistical software R® and Rstudio® (Rstudio team 2020), we extracted the pixel values of each variable according to the location of the occurrence points (i.e., response variable) and generated a new database with this information. Then, we estimated the contribution percentage for both predictors in each summer period using a Gradient Boost Machine model with the gbm package (Freund and Schapire 1997; Friedman 2002). This model allows us to estimate the relative influence of explanatory variables, as a function of the response variables (Dedman et al. 2017). Before running the model, the variables and the filtered occurrence records were loaded into Rstudio®. Then, we followed this parameterization: (1) Gaussian distribution with a cross iteration of 5000 repetitions, (2) random training (80%) and test (20%) groups from the occurrence records, and (3) occurrence dataset as response variable (e.g., Friedman 2002; Hijmans 2012).
In the three summer periods, both variables had a contribution percentage ≥ 2% (Fig. 2), so both were selected for the model (e.g., Awan et al. 2021). It should be mentioned that, in general, we obtained a value over 80% for WDp, while for UC, it was between 2 and 20%. Subsequently, using the same database, to avoid redundancy and high correlation between the variables, the collinearity was evaluated using Pearson’s correlation coefficient (Guisan and Hofer 2003) in Rstudio®. In this process, we considered a bivariate correlation below ± 0.70 for the two variables, following the criteria of Awan et al. (2021). Thus, WDp and UC were independent and were included in the models for the three summer periods (Table 1).
Partial dependence blocks of the contribution percentage of the variables water depth (WDp) and underwater cover (UC) by study period (summers of years 1999, 2009, and 2019) and life stage (adult and juvenile), for their first selection for the models of ecological response and spatial distribution of Ataeniobius toweri in the Media Luna spring, Mexico
Ecological response curves
We estimated the occurrence probability (OP) of A. toweri adult and juvenile as a function of changing conditions. Therefore, we ran a Generalized Regression Model (GLM) by the summer period to evaluate the quadratic and linear response by individual and aggregated variables. This approach is widely used to avoid overfitting in the prediction of the ecological response during logistic regression (Fielding and Bell 1997; Allouche et al. 2006). The following packages were used in Rstudio®: raster (Hijmans and Elith 2017), sp (Pebesma 2012), RColorBrewer (Neuwirth 2014), pander (Farlane 2013), and rgdal (Bivand et al. 2015). Similarly, to Gradient Boost Machine models, we loaded the correspondent predictor variables and trained the models with the corresponding occurrence records by life stage and study period. Also, based on the literature (e.g., Barbet-Massin et al. 2012), we randomly generated and included 500 background points to evaluate the correct prediction of habitat selection in each stage on the entire surface of the Media Luna spring. Subsequently, both occurrence and background records were grouped into a training set (80%) and a test set (20%).
We ran the GLM models, using the R codes provided by Rössel-Ramírez et al. (2023c), which were two individual linear models (one for WDp and one for UC) and one to explore the interaction between predictor variables. The statistical prediction dataset of the individual models was used to plot the ecological response curves that estimated the OP according to the conditions of the continuous variable WDp and the categorical variable UC. We presented this probability in a polynomial line of quadratic order for WDp and probability bars for UC. In addition, in each graph, we also included the mean–variance relationship (i.e., residual variance) and the training points group for occurrence and background from the prediction dataset. For WDp, we clipped the graphs from 0.0 to 3.5 m of depth, because in all cases an OP with P > 0.01 was predicted at this depth range. Subsequently, we evaluated the performance of the models using the GLM for both variables, from a 2 × 2 contingency table with the observed versus projected data. To minimize the percentage of type I (omission) or II (commission) errors, we followed the method of Allouche et al. (2006): we selected a threshold cutoff of 0.5 and divided the points corresponding to both presence and background into 80% training and 20% testing datasets. The metrics estimated were overall performance (OAC) and true statistical skills (TSS); we decided to use TSS because it gives equal importance to the prevalence of the predictions in the presence of random performance corrections (Fielding and Bell 1997; Allouche et al. 2006).
Spatial distribution models
In the geographic scenario, Carpenter et al. (1993), Naoki et al. (2006), and Pandit et al. (2017) propose DOMAIN as a suitable distribution model for threatened and endemic species, such as A. toweri. For this, we ran DOMAIN distribution models to estimate the distribution probability in the Media Luna spring, by fish life stage and summer period. We used the R code provided by Rössel-Ramírez et al. (2023c). In addition to the packages used for GLM, we added dismo and maptools (Hijmans and Elith 2017), Rcpp (Eddelbuettel and Francois 2011), lattice (Sarkar 2008), caret (Kuhn et al. 2020), pROC (Robin et al. 2011,) and sdm (Naimi and Araujo 2016) R packages. We also loaded the two predictor variables and the occurrence records in the statistical software and trained the modeling with the occurrence records and 500 background points (e.g., Barbet-Massin et al. 2012); each database was divided into a training (80%) and test (20%) group. After this parameterization, only the stack with WDp and UC and the training group set were included in the DOMAIN function. The final predictive raster, which showed the distribution probability of A. toweri adult and juvenile by summer period, was loaded into QGIS® software to plot the spatial distribution maps.
Finally, we evaluated the prediction performance of these models using the partial receiver operating characteristic (ROC) curve (Fielding and Bell 1997) and the area under the curve (AUC) of the ROC curve (Fawcett 2006). For both assessments, we included the two test groups (i.e., occurrence, and background), the statistical data of the DOMAIN model metric, and the stack with the variables. The values of the partial ROC were evaluated, following the criteria of Deshpande (2020), who argues that as long as the bandwidth ranges between 0.2 and 0.6, between true positive and true negative groups, the model has excellent predictive performance. For the ROC curve, we used the Pearce and Ferrier (2000) independent method for calculating AUC, where a value > 0.5 indicates better prediction performance, away from a random classifier.
Results
Spatio-temporal variation of RA
For both stages of A. toweri (i.e., adults and juveniles), there was a different proportion of RA between S3 and S9, in the three summer periods (Fig. 3). In 1999, we also calculated relative abundance for both stages (juvenile RA = 3.70%; adult RA = 0.74%) in S10 and also for adults in S12 (RA = 0.49%); no relative abundance was calculated in the rest of the sectors. In 2009, the juveniles in S2 had a RA of 9.09%. In 2019, the juvenile’s RA was 2.82% and the adult’s RA was 11.25%. For both life stages, the highest abundance was observed in the summer of 1999, and the lowest in the summer of 2009. In 2019, the RA increased to over 10% in some sectors (i.e., adults: S2, S3, S8, S9, and juveniles: S3, S6, S7, S9). Particularly, between 1999 and 2019, for the adult stage, RA increased in S9 from 4.91 to 45%. In juveniles in S3, RA increased from 5.29 to 23.94%. In general, the trend with the polynomial line was similar in the different life stages and summer periods.
Relative abundance (%) of Ataeniobius toweri in the Media Luna spring, Mexico, in the summer periods of years 1999 (red), 2009 (green), and 2019 (blue), in each sector (S1-S14), by life stage. The second-degree polynomial trend line (black line with dashes) shows the temporal variation of abundance in each case. Also, it shows the standard deviation bars with the range of variability associated with the mean value
Modeling evaluation and validation
In general, in the GLM models for A. toweri (Table S2), the overall predictive performance (OAC) was > 0.60, except for the adult stage in 2009 with OAC = 0.51. Concerning the TSS metrics, the values were over 0.40 in all cases. On the other hand, in the evaluation of the DOMAIN models, we estimated a higher proportion of true negative cases compared to true positive cases in the partial ROC curves, for both life stages in the three summer periods (Fig. S3). Each curve’s density had a bandwidth < 0.1 concerning the predicted value in the models and an AUC ratio > 1.0; only the juvenile stage in 2009 had a bandwidth of 0.17. For the ROC curve, in the adult stage, the AUC values were 0.72 (1999), 0.79 (2009), and 0.86 (2019); in the juvenile stage, the AUC values were 0.75 (1999), 0.85 (2009), and 0.76 (2019). These AUC values on the ROC curve meant higher sensitivity and low specificity (i.e., high power of discrimination) in all models (Fig. S4).
Suitable habitat selection
For both life stages, the highest OP, estimated as a function of the WDp range, was from 0.3 to 1.0 m depth in the three summer periods (Fig. 4). In all three periods, for the adult stage, the OP (P > 0.01) was from ~ 1.6 m, although it was highest between 0.6 and 1.5 m depth. In 1999, the maximum OP was P = 0.65, while it was below P = 0.20 in 2009 (P = 0.13) and 2019 (P = 0.19). For the juvenile stage, in 1999 and 2009, the OP increased from ~ 1.0 m (1999, P = 0.08; 2009, P = 0.01) to 0.3 m depth, with maximum P = 0.67 and P = 0.08, respectively. In 2019, the OP was estimated from ~ 3.4 m (P = 0.02) to exceed 0.3 m (P > 0.21) following a linear trend, where the probability increased at shallower depths.
Ecological response curves for Ataeniobius toweri in adult and juvenile stages, as a function of water depth (WDp), in three summer periods (in the years 1999, 2009, and 2019) in the Media Luna spring, Mexico. The polynomial line of order two shows the probability of occurrence, as a function of the WDp range, from 0.0 to 3.5 m depth. Also, it shows the confidence interval between the upper and lower values of the fit prediction along the probability line. It included the three fitted values of probability and water depth, and the training set of the presence and background points are shown in gray color
In the case of UC categories (Fig. 5), in 1999, we estimated the highest OP (P > 0.40) for the adult stage in Bc, Ms, and Nbs; the trend gradually decreased in the rest of the coverages. In the next two summer periods, the first three coverages with high probability (2009, P > 0.01; 2019, P > 0.10) were the same as in 1999. In 2009, the maximum OP was estimated in Bc (P ≈ 0.07) and in 2019 in Ms (P ≈ 0.16). Similarly, in these two periods, the OP decreased in Bst, Bcp, Sc, Bsd, and Msp conditions (2009, P < 0.01; 2019, P < 0.10). For the juvenile stage, only in 1999, an OP trend was maintained with P > 0.20 between coverages, with the highest probability (P ≈ 0.48) in Bc. In 2009, the highest OP was in Bc (P ≈ 0.04) and the probability was null in the rest of the coverages. In 2019, the highest OP (P > 0.10) was estimated also in Bc (P ≈ 0.15), as well as in Ms (P ≈ 0.16) and Nbs (P ≈ 0.14). Similarly, to the adult stage, the OP decreased in Bst, Bcp, Sc, Bsd, and Msp conditions (P < 0.10).
Ecological response curves for Ataeniobius toweri in adult and juvenile stages, as a function of underwater coverage (UC), in three summer periods (in the years 1999, 2009, and 2019) in the Media Luna spring, Mexico. The bars show the probability of occurrence between the following eight UC categories: natural bare soil (Nbs), bare soil by tourism (Bst), bare soil by depth (Bsd), small carpet (Sc), big carpet (Bc), mature shape (Ms), big carpet patch (Bcp), and mature shape patch (Msp). The confidence interval between the upper and lower values of the fit prediction along the probability bars is also shown. It includes the mean-fitted values of probability per UC. The training set of the presence and background points are shown in gray color
Spatial distribution models
In the three summer periods, for the adult stage of A. toweri, the models had a P > 0.70, between S2 and S9 (Fig. 6). Only in 1999, its distribution was extended to S10 and included the maximum probability (P ≈ 0.77). For the summer of 1999, the maximum probability of distribution (P ≈ 0.55) of the juvenile stage was also from S2 to S10. In contrast, in 2009 and 2019, the highest probability (P ≥ 0.80) was between S2 and S9 (Fig. 7). In general, for both life stages, the range between S2 and S9 kept the highest probability of distribution throughout the three summer periods.
Spatial distribution models for adult Ataeniobius toweri in the three summer periods (years 1999, 2009, and 2019) in the Media Luna spring, Mexico. The models were generated based on the variables of water depth and underwater coverage. Regarding the probability of distribution, in the three cases, it was higher in sectors between S2 and S9, preferentially towards the shores of the natural system
Spatial distribution models of juvenile Ataeniobius toweri in the three summer periods (years 1999, 2009, and 2019) in the Media Luna spring, Mexico. The models were generated based on the variables of water depth and underwater coverage. The probability of distribution, in the three cases, was higher in sectors between S2 and S9, preferentially towards the shores of the natural system
Discussion
In the Media Luna spring, in the three summer periods, the different proportions of relative abundance (RA) and highest probability of distribution (P ≥ 0.50) of adult and juvenile A. toweri were in sectors 2 to 9 (Figs. 3, 6, and 7). In these sectors, the water depth (WDp) below 2.5 m (Fig. 4) and the underwater coverage (UC) corresponded mainly to the big carpet (Bc) and mature shape (Ms) (Fig. 5), where the highest probability of occurrence was observed for both juvenile and adult stages. It is worth mentioning that, as documented by Palacio-Núñez et al. (2010b) (and according to our observations), in all the sectors the depth was below ~ 2.9 m, except in S1. In addition, between S2 to S9, UC had extensive underwater vegetation coverage, both in the form Bc and Ms. In contrast, from S10 to S14, the vegetation corresponded to isolated remnants of big carpet patches (Bcp) and mature shape patches (Msp), or completely bare soil by tourism (Bst).
Considering the wide existing gradient of WDp (from 0.0 to 36.2 m depth), fish show higher occurrence and spatial dynamics in shallower areas and towards the water’s shore (McClain and Etter 2005; Prchalová et al. 2009). In addition, according to our results, both stages had the highest OP between 0.1 and 1.5 m depth, in all summer periods. Furthermore, based on our field data, juveniles and adults were always observed at this depth range; occasionally, in the deeper portions of the canals, the adult stage only overtopped the cover given by Bc, which moved it away from the bottom of the system.
Regarding UC, as summer periods progressed, we observed a decrease in the extent of underwater vegetation cover, especially in 2009, to which we attribute the increase of RA from 1999 to 2019, in S9 for adults and S3 for juveniles. In these sectors, Bc and Ms coverages have been conserved with a high density (pers. obs.). Even so, the highest OP was always in Bc and Ms coverages, which are used for fish food, fitness, and shelter (Brandt 1980; Rozas and Odum 1988; Brosse and Lek 2002). Therefore, taking into account WDp and UC, we considered that the selected habitat conditions were conserved for adults and juveniles between the three study periods. However, the temporal variation in OP was related to changes in the composition of the UC, caused by anthropogenic interference in the site (Galván-Meza et al. 2018) between summer periods, which implies a change in other underwater environmental variables (e.g., temperature or water chemistry; Rozas and Odum 1988; Brosse and Lek 2002).
We confirmed a good prediction performance of the OAC metric (Fielding and Bell 1997) and the TSS prediction (Allouche et al. 2006) for our GLM models and response curves, which also reflected a good quality of fieldwork (Lobo et al. 2015). Thus, the OP modeled for the selection of habitat conditions, using both WDp and UC as predictors, was suitable for A. toweri at both stages. These findings are important for directing conservation management for the threatened endemic species and their habitat (Sánchez-Cordero et al. 2005), as is the case of A. toweri.
We also had a good prediction performance of the DOMAIN models, being able to discriminate between the true presence and absence of A. toweri in at least 72% of the cases, for juvenile and adult stages. Based on DOMAIN predictions, the probability of spatial distribution of both stages of A. toweri remained highest in sectors S2 to S9, where suitable conditions of WDp and UC were present (based on ecological response curves). In these sectors, the distribution was more marked towards the water’s shore with a depth of less than 1.5 m. Furthermore, according to Palacio-Núñez et al. (2010b) and our observations at these sites, the UC was dense at Bc and Ms. In this geographic scenario, there was a clear continuous distribution of both stages in 1999 and 2019, in the sectors S2 to S9. Only in 2009, probably due to the scarcity of records, the predictions indicated an adjusted distribution. There was also a probability of distribution in sectors S11 to S14 and in S1 for both stages (except for adult in 2009); the prediction (P < 0.50) in each of these sectors also showed very marked stripes towards the water’s shore. We considered that the modifications suffered by the natural system, particularly in UC, were responsible for projecting these variations in distribution between periods.
It should be mentioned that the distribution models generated with DOMAIN had a good predictive performance considering the ROC/AUC curve (Fielding and Bell 1997) and partial ROC curve (Manzanilla-Quiñones 2020). This corroborated the statistical significance of the modeling to explain the spatial distribution patterns of A. toweri, both for adult and juvenile stages.
Regarding the partial ROC curves, we obtained a higher proportion of true negative than true positive cases, suggesting the existence of the spatial ecology of a threatened species (Fielding and Bell 1997), as is the case of A. toweri (Koeck 2019). Regarding the ROC curve, Deshpande (2020) indicates that an AUC between 0.70 and 0.80 reflects better presence-variable correlation, which was the case for our results for both stages. In general, our models had the power to discern between areas with a low, medium, or high probability of A. toweri distribution, as a function of the selected variables, through the three summer periods.
Both the ecological response and spatial distribution models, together with the RA plots, were representative of the temporal variation in the spatial ecology of adult and juvenile A. toweri, during the three periods. All these modeling results were consistent with our field data, and we consider them to be adequate. On the other hand, DOMAIN was efficient for modeling the distribution and habitat affinity of this endemic and specialist fish, which is in agreement with Lyons et al. (2019). This ecological and spatial information increases knowledge about the spatial ecology of A. toweri and thus enables us to identify suitable habitat conditions and population status to make pertinent recommendations for the conservation of its natural population (e.g., Ferrier 2002; Joy and Death 2002; Benito and Peñas 2007) in the Media Luna spring. In addition, we considered that the incorporation of variables to local and temporal scales in the modeling was crucial to know the natural system and its modifications, as it happened with UC. These modifications can affect the population sensitivity of this and other endemic and threatened species (e.g., Ferrier 2002; Benito and Peñas 2007). Therefore, particularly for springs and wetlands, especially in arid and semi-arid areas, we highlight the need to obtain prior knowledge about the biotic and abiotic conditions of the study site. This was the case of Media Luna, which led the process of generating predictor variables from in situ information.
On the other hand, for ichthyofaunal recording in freshwater systems, our sampling designs, particularly underwater sampling, must be adapted to each situation; species and habitat information must be included in each record (Ruetz III et al. 2005; Prchalová et al. 2009), which can ensure a better understanding of population variation and life stage distribution (Engen et al. 2002; Gamelon et al. 2016).
Finally, we associated this RA, as well as the OP and distribution probability with tourism pressure that affects the vegetation and alters the habitat of A. toweri. As a consequence, in sectors S11 to S14 and S1, the OP was low, scarce, or null in conditions of remnants of vegetation (Bcp and Msp) and Bst. In contrast, we argued that A. toweri was found mainly between sectors S2 and S9, due to its affinity for high vegetation cover. Therefore, a natural habitat with a high density of emergent, sub-emergent, and floating vegetation cover, as well as a shallow depth, is important for the conservation of A. toweri in general. This avoids causing abrupt temporal variations in the occurrence and distribution of this and other endemic fish populations. Based on these results, we proposed an urgent prioritization of sectors S2 to S9 as areas subject to conservation, and better management, to avoid the extinction of the species that inhabit it.
Research data policy and data availability
The datasets generated and/or analyzed during the current study are available in the repositories:
Rössel-Ramírez DW, Palacio-Núñez J, Espinosa S, Martínez-Montoya JF (2023a) Spatial dataset for ecological response models and spatial distribution of Ataeniobius toweri (Cyprinodontiformes: Goodeidae) in the Media Luna spring, Mexico [Data set]. Zenodo. https://doi.org/10.5281/zenodo.7605376
Rössel-Ramírez DW, Palacio-Núñez J, Espinosa S, Martínez-Montoya JF (2023b) Raster layers of underwater coverage and water depth of the Media Luna spring, Mexico, generated from data of three summer periods (in the years 1999, 2009 and 2019). Zenodo. https://doi.org/10.5281/zenodo.7603890
Rössel-Ramírez DW, Palacio-Núñez J, Espinosa S, Martínez-Montoya JF (2023c) Codes in R for spatial statistics analysis, ecological response models and spatial distribution models [Data set]. Zenodo. https://doi.org/10.5281/zenodo.7603557
References
Albrecht M, Gotelli NJ (2001) Spatial and temporal niche partitioning in grassland ants. Oecologia 126(1):134–141. https://doi.org/10.1007/s004420000494
Allouche O, Tsoar A, Kadmon R (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J Appl Ecol 43(6):1223–1232. https://doi.org/10.1111/j.1365-2664.2006.01214.x
Awan MN, Saqib Z, Buner F, Lee DC, Pervez A (2021) Using ensemble modeling to predict breeding habitat of the red-listed Western Tragopan (Tragopan melanocephalus) in the Western Himalayas of Pakistan. Glob Ecol Conserv 31:e01864. https://doi.org/10.1016/j.gecco.2021.e01864
Barbet-Massin M, Jiguet F, Albert CH, Thuiller W (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods Ecol Evol 3(2):327–338. https://doi.org/10.1111/j.2041-210x.2011.00172.x
Bartier PM, Keller CP (1996) Multivariate interpolation to incorporate thematic surface data using inverse distance weighting (IDW). Comput Geosci 22(7):795–799
Benito B, Peñas J (2007) Aplicación de modelos de distribución de especies a la conservación de la biodiversidad en el sureste de la Península Ibérica. Geofocus 7:100–119
Bivand R, Keitt T, Rowlingson B, Pebesma E, Sumner M, Hijmans R et al (2015) Package ‘rgdal’. Bindings for the Geospatial Data Abstraction Library. https://cran.r-project.org/web/packages/rgdal/index.html. Accessed 15 October 2019
Brandt SB (1980) Spatial segregation of adult and young-of-the-year alewives across a thermocline in Lake Michigan. Trans Am Fish Soc 109(5):469–478. https://doi.org/10.1577/1548-8659(1980)109%3c469:SSOAAY%3e2.0.CO;2
Brosse S, Lek S (2002) Relationships between environmental characteristics and the density of age-0 Eurasian perch Perca fluviatilis in the littoral zone of a lake: a nonlinear approach. Trans Am Fish Soc 131(6):1033–1043. https://doi.org/10.1577/1548-8659(2002)131%3c1033:RBECAT%3e2.0.CO;2
Brosse S, Lek S, Dauba F (1999) Predicting fish distribution in a mesotrophic lake by hydroacoustic survey and artificial neural networks. Limnol Oceaongr 44(5):1293–1303. https://doi.org/10.4319/lo.1999.44.5.1293
Carpenter G, Gillison AN, Winter J (1993) DOMAIN: a flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers Conserv 2(6):667–680
Ceballos G, Pardo ED, Estévez LM, Pérez HE (2018) Los peces dulceacuícolas de México en peligro de extinción. Fondo de Cultura Económica, Cd. de México
Comisión Nacional para el Conocimiento y Uso de la Biodiversidad [CONABIO] (2020) México megadiverso. https://www.biodiversidad.gob.mx/pais/quees. Accessed 08 June 2020
Contreras-B S, Lozano-V ML (1994) Water, endangered fishes, and development perspectives in arid lands of Mexico. Cons Biol 8(2):379–387. https://doi.org/10.1046/j.1523-1739.1994.08020379.x
Contreras-Balderas S, Lozano-Vilano MDL (1993) Ictiodiversidad, peces amenazados y disponibilidad de agua para el desarrollo en zonas áridas del norte de México. Publicaciones Biológicas FCB/UANL 1:40–49
Contreras-MacBeath T, Rodríguez MB, Sorani V, Goldspink C, Reid GM (2014) Richness and endemism of the freshwater fishes of Mexico. J Threat Taxa 6(2):5421–5433. https://doi.org/10.11609/JoTT.o3633.5421-33
De la Vega Salazar MY (2009) Situación de los peces dulceacuícolas en México. Ciencias 072. UNAM, Cd. De México. https://www.revistas.unam.mx/index.php/cns/article/view/11911. Accessed 09 September 2020
Dedman S, Officer R, Clarke M, Reid DG, Brophy D (2017) Gbm. auto: a software tool to simplify spatial modelling and Marine Protected Area planning. PLoS One 12(12):e0188955. https://doi.org/10.1371/journal.pone.0188955
Del Río JL, Malvárez G, Navas F (2019) Estimación de la retención de aportes sedimentarios a los sistemas litorales provocada por los embalses. El caso de La Concepción (Marbella). In: Durán R, Guillén J, Simarro G (eds) X Jornadas de Geomorfología Litoral. Libro de ponencias. Castelldefels, Barcelona, pp 185–188 https://www.researchgate.net/publication/335924618. Accessed 08 September 2020
Deshpande R (2020) ROC curve and AUC in machine learning and R pROC package. https://medium.com/swlh/roc-curve-and-auc-detailed-understanding-and-r-proc-package-86d1430a3191. Accessed 08 September 2020
Eby LA, Fagan WF, Minckley WL (2003) Variability and dynamics of a desert stream community. Ecol Appl 13(6):1566–1579. https://doi.org/10.1890/02-5211
Eddelbuettel D, Francois R (2011) Rcpp: seamless R and C++ integration. J Stat Softw 40(8):1–18. URL http://www.jstatsoft.org/v40/i08/andavailableasvignette("Rcpp-introduction"). Accessed 10 September 2020
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40(1):677–697. https://doi.org/10.1146/annurev.ecolsys.110308.120159
Engen S, Lande R, Sæther BE (2002) The spatial scale of population fluctuations and quasi-extinction risk. Am Nat 160(4):439–451. https://doi.org/10.1086/342072
Engen S, Cao FJ, Sæther BE (2018) The effect of harvesting on the spatial synchrony of population fluctuations. Theor Popul Biol 123:28–34. https://doi.org/10.1016/j.tpb.2018.05.001
Farlane JM (2013) Pandoc user’s guide. http://johnmacfarlane.net/pandoc/README.html
Fawcett T (2006) An introduction to ROC analysis. Pattern Recognit Lett 27(8):861–874. https://doi.org/10.1016/j.patrec.2005.10.010
Ferrier S (2002) Mapping spatial pattern in biodiversity for regional conservation planning: where to from here? Syst Biol 51(2):331–363. https://doi.org/10.1080/10635150252899806
Fielding AH, Bell JF (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv J 38–49
Freund Y, Schapire RE (1997) A decision-theoretic generalization of on-line learning and an application to boosting. J Comput Syst Sci 55(1):119–139. https://doi.org/10.1006/jcss.1997.1504
Friedman JH (2002) Stochastic gradient boosting. Comput Stat Data Anal 38(4):367–378. https://doi.org/10.1016/S0167-9473(01)00065-2
Galván-Meza CJ, Flores Castillo E, Espericueta Bocanegra ED, Gutiérrez Pérez MG (2018) Análisis del impacto multidisciplinar del turismo dentro del ejido El Jabalí – Media Luna – San Luis Potosí. Medio Ambiente. Sustentabilidad y Vulnerabilidad Soc 5:419–434
Gamelon M, Grøtan V, Engen S, Bjørkvoll E, Visser ME, Sæther BE (2016) Density dependence in an age-structured population of great tits: identifying the critical age classes. Ecol 97(9):2479–2490. https://doi.org/10.1002/ecy.1442
García-Roselló E, Guisande C, Heine J, Pelayo-Villamil P, Manjarrés-Hernández A, Vilas G et al (2014) Using ModestR to download, import and clean species distribution records. Methods Ecol Evol 5(7):708–713. https://doi.org/10.1111/2041-210X.12209
Guisan A, Hofer U (2003) Predicting reptile distributions at the mesoscale: relation to climate and topography. J Biogeogr 30(8):1233–1243. https://doi.org/10.1046/j.1365-2699.2003.00914.x
Guisan A, Graham CH, Elith J, Huettmann F, NCEAS Species Distribution Modelling Group (2007) Sensitivity of predictive species distribution models to change in grain size. Divers Distrib 13(3):332–340. https://doi.org/10.1111/j.1472-4642.2007.00342.x
Hickley P, Muchiri M, Boar R, Britton R, Adams C, Gichuru N, Harper D (2004) Habitat degradation and subsequent fishery collapse in Lakes Naivasha and Baringo, Kenya. Int J Ecohyd Hydrob 4(4):503–517. http://karuspace.karu.ac.ke/handle/20.500.12092/1918
Hijmans RJ (2012) Cross-validation of species distribution models: removing spatial sorting bias and calibration with a null-model. Ecol 93:679–688. https://doi.org/10.1890/11-0826.1
Hijmans RJ, Elith J (2017) Species distribution modeling with R. R CRAN Project. https://doi.org/10.1016/B978-0-12-384719-5.00318-X. Accessed 11 May 2020
International Union for Conservation Nature [IUCN]. (Version 2021.3). The IUCN red list of threatened species. https://www.iucnredlist.org/. Accessed 25 June 2021
Joy MK, Death RG (2002) Predictive modelling of freshwater fish as a biomonitoring tool in New Zealand. Freshw Biol 47(11):2261–2275. https://doi.org/10.1046/j.1365-2427.2002.00954.x
Kalluri S, Gilruth P, Bergman R (2003) The potential of remote sensing data for decision makers at the state, local and tribal level: experiences from NASA’s Synergy program. Environ Sci Policy 6(6):487–500. https://doi.org/10.1016/j.envsci.2003.08.002
Koeck M (2019) Ataeniobius toweri. The IUCN red list of threatened species 2019: e.T2271A2782910. https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T2271A2782910. Accessed 25 November 2020
Kuhn M, Wing J, Weston S, Williams A, Keefer C, Engelhardt A et al (2020) Package ‘caret’: classification and regression training, R-package version 6.0-p. https://github.com/topepo/caret/. Accessed 26 May 2021
Lobo JM, Herrero A, Zavala MA (2015) ¿Debemos fiarnos de los modelos de distribución de especies? Los Bosques y la Biodiversidad Frente al Cambio Climático: Impactos, Vulnerabilidad y Adaptación en España. Ministerio de Agricultura, Alimentación y Medio Ambiente. Madrid, pp 407–417
Lyons J, Piller KR, Artigas-Azas JM, Dominguez-Dominguez O, Gesundheit P, Köck M et al (2019) Distribution and current conservation status of the Mexican Goodeidae (Actinopterygii, Cyprinodontiformes). ZooKeys 885:115–158. https://doi.org/10.3897/zookeys.885.38152
Maloney KO, Weller DE, Michaelson DE, Ciccotto PJ (2013) Species distribution models of freshwater stream fishes in Maryland and their implications for management. Environ Model Assess 18(1):1–12. https://doi.org/10.1007/s10666-012-9325-3
Manzanilla-Quiñones U (2020) Validación de modelos de distribución con ayuda de la plataforma Niche Toolbox de CONABIO. https://www.researchgate.net/publication/341820933. Accessed 15 May 2021
Martin-Garcia S, Rodriguez-Recio M, Peragón I, Bueno I, Virgós E (2022) Comparing relative abundance models from different indices, a study case on the red fox. Ecol Indic 137:108778. https://doi.org/10.1016/j.ecolind.2022.108778
Mateo RG, Felicísimo ÁM, Muñoz J (2011) Modelos de distribución de especies: una revisión sintética. Rev Chil Hist Nat 84(2):217–240. https://doi.org/10.4067/S0716-078X2011000200008
McClain CR, Etter RJ (2005) Mid-domain models as predictors of species diversity patterns: bathymetric diversity gradients in the deep sea. Oikos 109(3):555–566. https://doi.org/10.1111/j.0030-1299.2005.13529.x
Miller J (2010) Species distribution modeling. Geogr Compass 4(6):490–509. https://doi.org/10.1111/j.1749-8198.2010.00351.x
Miller RR, Minckley WL, Norris SM, Gach MH (2005) Freshwater fishes of Mexico (No. QL 629. M54 2005). University of Chicago Press. Chicago
Naimi B, Araujo MB (2016) sdm: a reproducible and extensible R platform for species distribution modelling. Ecography 39:368–375. https://doi.org/10.1111/ecog.01881
Naoki K, Gómez MI, López RP, Meneses RI, Vargas J (2006) Comparación de modelos de distribución de especies para predecir la distribución potencial de vida silvestre en Bolivia. Ecología En Bolivia 41(1):65–78
Neuwirth E (2014) RColorBrewer: ColorBrewer palettes. R package version 1.1–2. The R Foundation
Olden JD, Jackson DA, Peres-Neto PR (2002) Predictive models of fish species distributions: a note on proper validation and chance predictions. Trans Am Fish Soc 131(2):329–336. https://doi.org/10.1577/1548-8659(2002)131%3c0329:PMOFSD%3e2.0.CO;2
Palacio-Núñez J, Verdú JR, Numa C, Jiménez-García D, Olmos-Oropeza G, Galante E (2010) Freshwater fishes spatial patterns in isolated water springs in North-eastern Mexico. Rev Biol Trop 58(1):413–426
Palacio-Núñez J, Olmos-Oropeza G, Verdú JR, Galante E, Rosas-Rosas OC, Martínez-Montoya JF, Enríquez J (2010) Traslape espacial de la comunidad de peces dulceacuícolas diurnos en el sistema de humedal Media Luna, Rioverde, SLP, México. Hidrobiológica 20(1):21–30
Pandit SN, Maitland BM, Pandit LK, Poesch MS, Enders EC (2017) Climate change risks, extinction debt, and conservation implications for a threatened freshwater fish: Carmine shiner (Notropis percobromus). Sci Total Environ 598:1–11. https://doi.org/10.1016/j.scitotenv.2017.03.228
Pearce J, Ferrier S (2000) Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Modell 133:225–245. https://doi.org/10.1016/S0304-3800(00)00322-7
Pebesma E (2012) Map overlay and spatial aggregation in sp. Technical Report. Sp Vignette 1:141–161
Periódico Oficial del Estado Libre y Soberano de San Luis Potosí [POE-SLP] (2003) Declaración de Área Natural Protegida bajo la modalidad de “Parque Estatal” denominado “Manantial de la Media Luna”. http://201.144.107.246/InfPubEstatal2/_SECRETAR%C3%8DA%20DE%20ECOLOG%C3%8DA%20Y%20GESTI%C3%93N%20AMBIENTAL/Art%C3%ADculo%2018.%20fracc.%20II/Normatividad/Decretos/Decreto%20Media%20Luna.pdf. Accessed 07 October 2019
Poff NL, Allan JD, Palmer MA, Hart DD, Richter BD, Arthington AH et al (2003) River flows and water wars: emerging science for environmental decision making. Front Ecol Environ 1(6):298–306. https://doi.org/10.1890/1540-9295(2003)001[0298:RFAWWE]2.0.CO;2
Prchalová M, Kubečka J, Čech M, Frouzová J, Draštík V, Hohausová E et al (2009) The effect of depth, distance from dam and habitat on spatial distribution of fish in an artificial reservoir. Ecol Freshw Fish 18(2):247–260. https://doi.org/10.1111/j.1600-0633.2008.00342.x
Pullin AS, Knight TM, Stone DA, Charman K (2004) Do conservation managers use scientific evidence to support their decision-making? Biol Conserv 119(2):245–252. https://doi.org/10.1016/j.biocon.2003.11.007
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform 12:77. https://doi.org/10.1186/1471-2105-12-77
Rössel-Ramírez DW, Palacio-Núñez J, Espinosa S, Martínez-Montoya JF (2023a) Codes in R for spatial statistics analysis, ecological response models and spatial distribution models. Zenodo. https://doi.org/10.5281/zenodo.7603557
Rössel-Ramírez DW, Palacio-Núñez J, Espinosa S, Martínez-Montoya JF (2023b) Raster layers of underwater coverage and water depth of the Media Luna spring, Mexico, generated from data of three summer periods (in the years 1999, 2009 and 2019). Zenodo. https://doi.org/10.5281/zenodo.7603890
Rössel-Ramírez DW, Palacio-Núñez J, Espinosa S, Martínez-Montoya JF (2023c) Spatial dataset for ecological response models and spatial distribution of Ataeniobius toweri (Cyprinodontiformes: Goodeidae) in the Media Luna spring, Mexico . Zenodo. 10.5281/zenodo.7605376
Rozas LP, Odum WE (1988) Occupation of submerged aquatic vegetation by fishes: testing the roles of food and refuge. Oecologia 77(1):101–106. https://www.jstor.org/stable/4218746
RStudio Team (2020). RStudio: integrated development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/
Ruetz CR III, Trexler JC, Jordan F, Loftus WF, Perry SA (2005) Population dynamics of wetland fishes: spatio-temporal patterns synchronized by hydrological disturbance? J Anim Ecol 74(2):322–332. https://doi.org/10.1111/j.1365-2656.2005.00926.x
Sánchez-Cordero V, Cirelli V, Munguial M, Sarkar S (2005) Place prioritization for biodiversity content using species ecological niche modeling. Biodivers Inform 2. https://doi.org/10.17161/bi.v2i0.9
Sarkar D (2008) Lattice: multivariate data visualization with R, Springer. http://lmdvr.r-forge.r-project.org/
Wickham H, Chang W, Henry L, Pedersen TL, Takahashi K, Wilke C, Woo K (2019) ggplot2: create elegant data visualisations using the grammar of graphics. https://CRAN.R-project.org/package= ggplot2. R package version 2(1):2
Winemiller KO, Agostinho AA, Caramaschi ÉP (2008) Fish ecology in tropical streams. In: Dudgeon D (ed). Tropical Stream Ecology. Academic Press. San Diego, pp. 107–126. https://doi.org/10.1016/B978-012088449-0.50007-8
Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF et al (2012) The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol Rev 88(1):15–30. https://doi.org/10.1111/j.1469-185x.2012.00235.x
Zhang Z, Mammola S, Zhang H (2020) Does weighting presence records improve the performance of species distribution models? A test using fish larval stages in the Yangtze Estuary. Sci Total Environ 741:140393. https://doi.org/10.1016/j.scitotenv.2020.140393
Acknowledgements
We thank Genaro Olmos Oropeza, who contributed to the fieldwork in all three sampling periods, and Jesús Enríquez for his participation in the first sampling period.
Author information
Authors and Affiliations
Contributions
The initial project, from 1998, was conceived, designed, and directed by Jorge Palacio-Núñez, who also performed the collection of field data in all periods and interpreted the results. He participated as director of Rössel-Ramírez’s thesis. Juan Felipe Martínez-Montoya collaborated in the field data collection from the second period (2009), as well as in adjustments to the initial cartography and the incorporation of remote sensing analysis and photo interpretation. David Walter Rössel-Ramírez did his professional thesis on this project. He participated in the field work and in the recording and ordering of the data for the summer of 2019; he incorporated the use of the echo sounder and also updated the cartographic information with satellite images for the three study periods. He was also in charge of sorting and filtering the databases and processing the raster variables for each period. In addition, he built the code in Rstudio® for statistical and spatial analyses. He also participated in the preparation of all the parts of the manuscript, which were derived from his professional thesis. Santiago Espinosa participated as co-director of the thesis. He provided significant expertise in the spatial analysis and systematization of the study. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All figures were created with the QGIS® program. Figures that include additional photos or drawings (1, 2, 9, and 10) were assembled in PowerPoint® and subsequently attached in QGIS®. Also, all the graphics were created through Rstudio® and R®.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rössel-Ramírez, D.W., Palacio-Núñez, J., Espinosa, S. et al. Temporal variation in the relative abundance, suitable habitat selection, and distribution of Ataeniobius toweri (Meek, 1904) (Goodeidae), by life stages, in the Media Luna spring, Mexico. Environ Biol Fish 107, 173–188 (2024). https://doi.org/10.1007/s10641-024-01520-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-024-01520-7