Abstract
A mountain mass from which multiple river basins originate is a possible determinant of genetic structure of freshwater fishes. In this study, subspecies composition and genetic structure were examined for Japanese white-spotted char comprising of two subspecies, Salvelinus leucomaenis imbrius (Jordan and McGregor), “Gogi,” and S. l. pluvius (Hilgendorf), “Nikkoiwana,” collected in the 19 river basins originating from 6 mountain masses in the Chugoku Region, Japan, based on mitochondrial DNA sequences. In the western mountain masses, only Gogi was collected, while in the eastern mountain mass, only Nikkoiwana was collected. A total of 27 haplotypes were detected, among which only seven were shared by two subspecies and three were detected only in Nikkoiwana whereas 17 were detected only in Gogi. As many as 11 haplotypes were distributed only in the Oyorogi Mountain mass in the middle region. In the network, one group was exclusively constructed by the samples from the Oyorogi Mountain mass. Furthermore, in a tree of river basins based on average genetic distance of char, the Gono River basin originating from the Asa Mountain mass, situated in the western region, participated in a clade constructed by the rivers originating from the westernmost Sukegatake and the adjacent Asa Mountain masses, whereas the Gono River basin originating from the Oyorogi Mountain mass participated in a different clade constructed by the rivers originating from the Oyorogi to the easternmost Hyonosen Mountain masses. These results suggest that a mountain mass is a determinative factor to subspecies composition and genetic structure of char, with an implication of invasion of fish taking advantage of some geological events or conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the Chugoku region of the Honshu Island in Japan, two subspecies of the white-spotted char, Salvelinus leucomaenis (Pallas) (called “Iwana”), S. l. pluvius (Hilgendorf) (called “Nikkoiwana”), and S. l. imbrius (Jordan et McGregor) (called “Gogi”), are distributed (Hosoya 2000). Gogi is distributed in the western part while Nikkoiwana is distributed in the eastern part of this region with unclear distribution limits (Imanishi 1967; Kobeshinbun 1974; Kimura 1989). Gogi is distinguishable from Nikkoiwana by clear white spots on the dorsal surface reaching to the tip of the snout, although this feature seems rather ambiguous (Miyaji et al. 1975; Hosoya 2000). Unfortunately, however, there is a concern that populations of native Nikkoiwana are shrinking because of a large-scale artificial stocking of cultured char in the central region of Japan into the eastern Chugoku Region (Tansui Tsuriba Henshubu 2016).
Yamamoto et al. (2004) reported 14 haplotypes from Nikkoiwana and Gogi based on the DNA sequences of cytochrome b region of mitochondrial DNA and detected only 2 Gogi-specific haplotypes in contrast to 7 Nikkoiwana-specific haplotypes. In our previous studies, 8 haplotypes were newly found in the Chugoku Region (Kawai et al. 2020), among which 7 were Gogi-specific. Besides, the spot types (Kawai et al. 2000) were categorized into 3 groups based on haplotype composition similarity, and it was suggested that Gogi and Nikkoiwana are genetically separated partially, and wide spot types are likely to have genetically been isolated from narrow types.
A river basin can determine the distribution area and genetic diversity of a freshwater fish species. A mountain mass from which multiple river basins originate is also a possible determinant of the genetic structure. Judging from the results in our previous study on genetic relationships of char distributed in the border areas between 3 watersheds of the eastern Chugoku Region, genetic distance was considered to be determined more strongly by geographical distance than by river basin, suggesting an involvement of some geologic events in the range expansion of char (Kawai et al. 2006). River capture is a representative of these geologic events (Hattori and Tanaka 1999). Oshima (1961) described a possibility of invasion of char into adjacent river basins by taking advantage of highland marshes. Katayama and Fujioka (1966) have also suggested a possibility of invasion of Gogi from the Takatsu River Basin in the Sea of Japan side to the Nishiki River Basin in the Seto Inland Sea side, taking advantage of river capture. More recently, Kikko et al. (2008) explained the distribution of a major haplotype of Nikkoiwana in the northern inlet rivers of Lake Biwa by invasion from adjacent inlet rivers of the Sea of Japan, taking advantage of watershed exchanges in the glacial periods of the Pleistocene. If these geological factors are more determinative to genetic composition of the population than river basins, genetic separation seems clearer among mountain masses than among river basins.
In this study, we tried to clarify the significance of mountain masses as a factor determining the subspecies and genetic composition of char distributed in the western region of the Honshu Island in Japan. For this purpose, we collected the char samples including Gogi and Nikkoiwana and examined the DNA sequences of the mitochondrial cytochrome b region. Then, we categorized the samples by subspecies, river basin, and mountain mass and examined the separation of subspecies and haplotypes by these factors. Finally, we tried to explain the relationships between subspecies or haplotype composition and mountain masses from a viewpoint of some geologic events or conditions.
Materials and methods
Samples
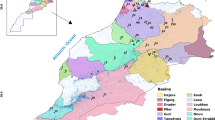
Char samples were collected at 95 sites of a total of 18 river basins from the Takatsu River in the Shimane Prefecture to the Maruyama River in the Hyogo Prefecture in the Sea of Japan side and from the Nishiki River in the Yamaguchi Prefecture to the Chigusa River in the Hyogo Prefecture in the Seto Inland Sea side (Fig. 1). Subspecies of char were identified according to the description by Hosoya (2000).
Sampling was performed by fishing using earthworm as main bait at as much upper reach as possible for the collection of native fish only. Samples were transported to the laboratory alive using a potable aeration system. After killing by bleeding, samples were measured for body sizes, a fin clip was cut, and the liver was dissected out and stored in an Eppendorf tube at − 20 ℃ until use.
Environmental factors
Each collection site was categorized into the groups by river basin and mountain mass, and mountain masses were divided by the contour line lower than about 500 m above sea level (Fig. 1).
PCR
Template DNA was prepared from the fin or liver samples using DNeasy Tissue Kit (Quiagen, Tokyo, Japan), according to the manufacturer’s instruction.
The posterior part of cytochrome b region of mitochondrial DNA (557 bp) was amplified by PCR with a mixture of a template DNA (50 ng) and primers H15915 (5′-ACCTCCGATCTYCGGATTACAAGAC-3′; Aoyama et al. 2000) and L15285 (5′-CCCTAACCGGVTTCTTYGC-3′; Inoue et al. 2000) by using the TaKaRa PCR Amplification kit (TaKaRa, Ohtsu, Japan) in a thermal cycler (Mastercycler personal; Eppendorf, Hamburg, Germany) using the following protocol: preheating at 94 ℃ for 11 min, followed by 30 cycles of denaturation at 94 ℃ for 30 s, annealing at 55 ℃ for 30 s, extension at 72 ℃ for 1 min, and a final extension at 72 ℃ for 7 min. PCR products were purified using NucleoSpin Gel and PCR cleanup (Takara, Ohtsu, Japan).
Sequencing was performed directly with the Genetic Analyzer 3130xl (Applied Biosystem, CA, USA) in the Genetic Research Center of Hiroshima University and was in part undertaken by FASMAC Co. Ltd. (Kanagawa, Japan) or Eurofin Genomics Co. Ltd. (Tokyo, Japan). A fragment of 480 bp in cytochrome b region was used as the consensus sequence.
Haplotyping, haplotype diversity, genetic distance, and nucleotide diversity
Haplotyping and haplotype diversity were performed with DnaSP ver. 6 (Rozas et al. 2017). Genetic distance was determined as Kimura-2 parameter using MEGA 6 (Tamura et al. 2013). Nucleotide diversity was determined as π-value using MEGA 6.
Network analysis
Haplotype network was constructed by NETWORK 5.0.1.1.
Neutrality test
Neutrality was examined by Tajima’s D-value and Fu and Li’s D* test using DnaSP ver. 6.
Dendrogram
Alignment was performed by Clustal W (Thompson et al. 1994), and a genetic tree of the samples from a mountain mass was constructed by the Neighbor-Joining method using Kimura-2 parameter as a distance by MEGA 6.
A tree of river basins was constructed as follows. The samples of a river basin were categorized into 2 groups such as “Gono-Asa” and “Gono-Oyorogi” based on the mountain mass from which each branch originates, and a river basin from a mountain mass was clustered on the basis of Kimura-2 parameter as an average genetic distance by the Mountford method (Kimoto 1976).
Results
A total of 228 samples were collected. Total and body length were in the range of 6.8–24.0 and 5.8–21.2 cm, respectively. Body weight was in the range of 2.9–156.0 g (Table 1).
Subspecies composition by river basins
Subspecies composition by river basin is shown in Table 2. In the westernmost Takatsu to Iinashi Rivers in the Sea of Japan side, only Gogi was collected, while in the eastern Kishida to the easternmost Maruyama, only Nikkoiwana was collected. For the middle region, both subspecies were collected in the Hino, Tenjin, and Sendai River basins, and Nikkoiwana and Gogi were collected in the Kinoe and Katsuta River basins, respectively. In the Seto Inland Sea side, only Gogi was collected in the westernmost Nishiki and adjacent Ohta River basins, while only Nikkoiwana was collected in the easternmost Chigusa to Asahi River basins.
Subspecies composition by mountain mass
Subspecies composition by mountain mass is shown in Fig. 2. In the Sukegatake, Asa, and Oyorogi mountain masses, only Gogi was distributed, whereas in the Hyonosen mountain mass, only Nikkoiwana was distributed. In the Daisen and Nagisan mountain masses, both subspecies were distributed, and both subspecies were co-dominant in the Daisen Mountain mass.
Distribution patterns of Gogi and Nikkoiwana by mountain mass. Other explanations are the same as in Fig. 1
Haplotyping
A total of 27 haplotypes were recorded (Table 3). Among these, haplotypes 1, 4, 15, 21, and 22 corresponded to Hap-20, 7, 17, 16, and 9, respectively, by Yamamoto et al. (2004), and haplotypes 3, 11, 13, 16, and 23 corresponded to haplotypes 1, 5, 15, 13, and 3, respectively, by our previous study (Kawai et al. 2020). The remaining 17 haplotypes were recorded for the first time in DDBJ (accession nos. LC636454, LC636455, LC636456, LC636457, LC636458, LC636459, LC636460, LC636461, LC636462, LC636463, LC636464, LC636465, LC636466, LC636467, LC636468, LC636469, and LC636470 for haplotypes 2, 5, 6, 7, 8, 9, 10, 12, 14, 17, 18, 19, 20, 24, 25, 26, and 27, respectively).
Haplotype composition by subspecies
One hundred eighty six individuals were identified as Gogi whereas 42 individuals were identified as Nikkoiwana, suggesting the dominance of Gogi in the Chugoku Region (Table 3). Haplotypes 1, 4, 5, 7, 15, 22, and 23 were shared by both subspecies. Haplotypes 17, 20, and 21 were only Nikkoiwana whereas other 17 haplotypes were only Gogi. Average genetic distance was 0.005 in Kimura-2 parameter for both Gogi and Nikkoiwana. Average nucleotide diversity was 0.005 for both Gogi and Nikkoiwana. Tajima’s D-value was − 1.544 (p > 0.05) and − 1.187 (p > 0.1) for Gogi and Nikkoiwana, respectively. Fu and Li’s D* test statistics were − 4.586 (p < 0.02) and − 1.501 (p > 0.1) for Gogi and Nikkoiwana, respectively.
Haplotype composition by river basin
Haplotypes 1, 4, 5, and 7 were detected in a wide range (Table 2). As many as 7 haplotypes, 2, 3, 8, 10, 22, 23, and 27, were detected only in the Hino River. Haplotypes 12, 25, and 26; 18 and 24; 13; 14; 6; 17, 19, 20, and 21; and 16 were detected only in the Gonogawa, Hii, Iinashi, Katsuta, Tenjin, Sendai, and Ohta Rivers, respectively. Thus, as many as 20 out of 27 haplotypes were detected in only one of the 18 river basins.
Haplotype composition by mountain mass
Haplotype 4 was distributed in all mountain masses excepting Sukegatake mountain mass, and haplotype 1 was also widely distributed from Oyorogi to Hyonosen Mountain masses (Table 4). Haplotypes 5, 7, and 15 were also distributed in a wide range. On the other hand, haplotypes 11 and 22 were distributed only in Asa and Oyorogi and Oyorogi and Daisen Mountain masses, respectively. Haplotypes 12 and 16; 6, 14, and 23; 19 and 20; and 17 and 21 were distributed only in Asa, Daisen, Nagisan, and Hyonosen mountain masses, respectively. As many as 11 haplotypes, 2, 3, 8, 9, 10, 13, 18, 24, 25, 26, and 27, were distributed only in the Oyorogi Mountain mass.
Haplotype network by subspecies
By the network analysis, haplotypes 5, 15, 16, and 22 were estimated to construct a “torso” (Fig. 3). Haplotype 1 was the most dominant. Three haplotype groups were recognized. Group 1 (haplotypes 2, 3, 8, 10, 13, and 27) was composed by Gogi only. Group 2 (haplotypes 4 and 20) was composed by Gogi and Nikkoiwana. Group 3 (haplotypes 1, 6, 9, 14, 18, 19, 23, 24, and 25) was largely composed by Gogi with 2 haplotypes of both subspecies. There were only 4 missing haplotypes.
Haplotype network by mountain masses
Only G1 was exclusively constructed by the samples from Oyorogi Mountain mass (Fig. 4). G2 was constructed by the samples from the adjacent mountain masses, Asa to Hyonosen. G3 was also constructed by the samples from the adjacent mountain masses, Oyorogi to Hyonosen Mountain masses.
Relationship between number of samples in mountain mass and average genetic distance
There were no significant relationships between the number of samples collected in a mountain mass and the average genetic distance for both Gogi and Nikkoiwana (Fig. 5).
Average genetic distances within and among mountain masses
Average genetic distance within a mountain mass was the highest for the Daisen and Hyonosen mountain masses and it was the lowest for the Sukegatake mountain mass (Table 5). Average genetic distance between mountain masses was the highest between the Sukegatake and Oyorogi or Daisen Mountain masses, and it was the lowest between the Sukegatake and Asa, and Nagisan and Oyorogi, Daisen or Hyonosen mountain masses.
Haplotype diversity in a mountain mass
Haplotype diversity was by far the highest in the Daisen Mountain mass and the lowest in the Sukegatake Mountain mass (Table 6). It was also very low in the Nagisan Mountain mass.
Genetic relationships of haplotypes in the Oyorogi Mountain mass
Sixteen haplotypes detected in the Oyorogi Mountain mass were divided into 2 large clades (Fig. 6). The smaller clade of haplotypes 2, 3, 8, 10, 13, and 27, all detected only in this mountain mass, was constructed by those consisting of only Gogi whereas the larger clade was constructed by those consisting of only Gogi as well as those consisting of both subspecies.
Genetic relationships between river basins by mountain mass
The samples of only 7 river basins originating from adjacent two mountain masses were selected. The samples of a river basin were categorized into 2 groups based on the mountain mass from which each branch originates, and a river basin from a mountain mass was clustered on the basis of average genetic distance. All the samples collected from both the Takatsu and Nishiki Rivers originating from both the Sukegatake and Asa Mountain masses constructed a clade (Fig. 7). In addition, those from the branches of the Gono River originating from the Asa Mountain mass also participated in this clade. The samples collected from the Tenjin, Sendai, and Yoshii Rivers originating from the Daisen, Nagisen, and Hyonosen Mountain masses also constructed a large clade. In addition, those from the Gono River originating from the Oyorogi Mountain mass participated in this clade. Those from the Hino River originating from the Daisen Mountain mass also participated in this large clade, while those from the Hino River originating from the Oyorogi Mountain mass made a clade by itself.
Discussion
In this study, char samples were categorized into some groups by subspecies, river basin, and mountain mass from which the river originates, and subspecies and genetic composition were examined in relation to river basin and mountain mass. In the western region, only Gogi was collected while in the eastern region, only Nikkoiwana was collected. In the middle region of the Sea of Japan side, both subspecies were collected in the Hino, Tenjin and Sendai River basins. Besides, Gogi and Nikkoiwana were co-dominant in the Daisen Mountain mass (Table 2, Fig. 2). This suggests the Daisen Mountain mass, including the highest mountain, Mt. Daisen (1729 a.s.l.), in the Chugoku Region as a strong barrier to range expansion to freshwater fishes. Thus, Gogi possibly invaded from the continent via the Chosen Strait and eastwardly expanded the distribution during ocean retreats like other China lineage freshwater fishes, and Nikkoiwana possibly invaded from the continent via the Tatar Strait and westwardly expanded like other Siberia lineage freshwater fishes (Aoyagi 1957), resulting into the coexistence of both in Daisen and Nagisan mountain masses.
Among the haplotypes constructing a “torso” in the network by subspecies, haplotypes 5, 15, and 22 were shared by Gogi and Nikkoiwana whereas only haplotype 16 was detected only in Gogi (Fig. 3 and Table 3). Haplotype 15 corresponded to Hap-17 in Yamamoto et al. (2004), detected in the Hokuriku Region, in the middle of Japan, and Chugoku Region. In addition, haplotype 4 in G2, connected to “torso” via a missing haplotype, corresponded to Hap-7 by Yamamoto et al. (2004), detected in the Tohoku Region, in the northern Japan, to the Hokuriku Regions. Besides, haplotype 4 was distributed in all mountain masses except the westernmost Sukegatake mountain mass (Table 4). These results suggest a possibility of south- and west-ward range expansions of the ancestral char to the Chugoku Region during the repeated glacial periods as estimated by Aoyagi (1957) and Yamamoto et al. (2004). On the other hand, haplotype 7, directly connected to “torso,” as well as haplotypes 4 and 5, were also detected in the westernmost Takatsu River, in this study (Table 2), suggesting a possibility of an eastward range expansion of the ancestral char like China lineage freshwater fishes (Aoyagi 1957). However, the highest average genetic distance observed between the westernmost Sukegatake and middle Oyorogi or Daisen Mountain masses (0.008), not between the Sukegatake and the easternmost Hyonosen (0.007), supports neither the eastward nor westward expansion (Table 5). Further study should be undertaken incorporating an exact chronological estimation of ancestral char invasion.
Both average genetic distance and average nucleotide diversity were the same (0.005) for both Gogi and Nikkoiwana although the number of samples was more than 4 times higher in Gogi than in Nikkoiwana. Seven haplotypes were shared by both subspecies, and only three haplotypes were detected only in Nikkoiwana whereas other 17 haplotypes were detected only in Gogi (Table 3). In the network by subspecies, haplotype group 1, composed only by Gogi, was clearly separated from the “torso,” suggesting a certain level of differentiation of a group of Gogi during a long reproductive isolation in the headwater environments (Fig. 3). Indeed, a typical Gogi with short and wide head spots has been considered as an adaptation to confinement in creeks flowing in a plateau-like topography widely distributed in the Chugoku Mountain Chains (Kawai et al. 2000). Besides, a deeply differentiated haplotype 14, detected only in a short river, the Katsuta River, was estimated to have differentiated during interglacial periods in a deeply cutting valley in the Daisen Mountain mass with a high volcanic activity in the Pleistocene (Kurasawa and Tsukumi 2003). However, further studies with additional samples of native Nikkoiwana should be undertaken, although it seems extremely difficult to increase the number of Nikkoiwana samples, considering a large scale of artificial stocking of Nikkoiwana, bred in far distantly located areas in Japan, into main habitats of char in the eastern Chugoku Region (Tansui Tsuriba Henshubu 2016).
Sixteen haplotypes detected in the Oyorogi Mountain mass were largely divided into 2 clades (Fig. 6). The smaller clade of 6 haplotypes, all detected only in this mountain mass, was constructed by those consisting of only Gogi, whereas the larger clade was constructed by those consisting of only Gogi as well as those consisting of both subspecies. Besides, the larger clade seemed a little more deeply differentiated from the ancestral lineage than the smaller one. This may suggest multiple chances of invasion of ancestral lineages into this mountain mass, possibly explained by multiple glacial periods when char populations had a chance to expand their ranges by seaward migration, in an interval of 0.04–0.1 million years in recent 3 million years (Nishimura 1974).
In the network analyses, only one group was exclusively constructed by Gogi or the samples from the Oyorogi Mountain mass (Figs. 3 and 4). In the Gono River basin, furthermore, the samples collected from the branches originating from the Asa Mountain mass participated in a clade by those collected from the branches of the Takatsu and Nishiki Rivers originating from the Sukegatake and Asa Mountain masses whereas those originating from the Oyorogi Mountain mass participated in a different clade by those of the Tenjin, Yoshii, and Sendai Rivers originating from the Daisen, Nagisen, and Hyonosen Mountain masses (Fig. 7). Similarly, in the Hino River basin, the samples from the branches originating from the Daisen Mountain mass participated in the Tenjin-Yoshii-Sendai clade while those originating from the Oyorogi Mountain mass made a clade by itself. These mean a stronger influence of mountain mass on genetic structure than river basin.
What does it mean when a clade of char is made by a mountain mass? For cold-water salmonid fishes, the existence of “corridors” at low altitudes might have been a strong barrier to range expansion in long interglacial periods. Thus, such a clade is estimated to have been formed by a genetic exchange between the populations in different river basins originating from a mountain mass, taking advantage of some geological events or topographical situations. This may be supported by the distribution patterns of two subspecies clearly limited by mountain masses in this study (Fig. 2). Indeed, Kikko et al. (2008) have also suggested a dispersion of white-spotted char into the northern inlet rivers of Lake Biwa from adjacent inlet rivers of the Sea of Japan by watershed exchanges in the glacial periods of the Pleistocene. Furthermore, Katayama and Fujioka (1966) described a possibility of invasion of Gogi from the Takatsu to Nishiki River basin by river capture. Oshima (1961) has also suggested that a char possibly invaded into adjacent river basins via highland marshes from which multiple streams originate particularly in snow-melting early spring. Although the Chugoku Mountain Chains are considered to have been formed by a wide range upheaval in the late Miocene to Pliocene (Obata 1991), further historical studies should be performed in detail on these geological events.
As many as 7 haplotypes were detected only in the Hino River basin whose branches originate from both the Oyorogi and Daisen Mountain masses (Fig. 1; Table 2). Among these, 5 haplotypes, 2, 3, 8, 10, and 27, were detected only from the Oyorogi Mountain mass, whereas only haplotype 23 was detected only from the Daisen Mountain mass, and only haplotype 22 was detected both from the Oyorogi and Daisen Mountain masses (Table 4). This means that the Oyorogi Mountain mass might have provided a “stage” of deep differentiation for some reasons. This deep differentiation is considered to be provided by the situation of the largest Oyorogi Mountain mass among the 6 masses, clearly separated from the western adjacent Asa Mountain masses by a “corridor,” a leading valley, formed by the main stream of an antecedent huge basin, the Gono River (Ohga et al. 2011) (lower than 200 m a.s.l. in the western margin of the Oyorogi Mountain mass) and from the eastern adjacent Daisen Mountain mass by another “corridor” formed by the main stream of a large basin, the Hino River. These “corridors” might have been a strong barrier to genetic exchange of char populations in interglacial periods.
Average genetic distance within a mountain mass was the highest for the Daisen and Hyonosen Mountain masses, and it was the lowest for the Sukegatake mountain mass (Table 5). Similarly, haplotype diversity was also the highest in the Daisen Mountain mass and the lowest in the Sukegatake Mountain mass (Table 6). Besides, there were no significant relationships between the number of samples in mountain mass and average genetic distance (Fig. 5A and B). On the other hand, average genetic distance between mountain masses was relatively high in the distantly located combinations: the Sukegatake and Nagisan or Hyonosen Mountain mass, although it was the highest in rather closely located combinations, the Sukegatake and Oyorogi or Daisen Mountain mass, suggesting a rather weak effect of geographic distance on chance of genetic exchange.
Altitude can also be a determinant to genetic structure of char. Stepwise upheaval was estimated to have occurred in the Chugoku Region, and at least 4 planes, Azumayama (1000–1300 m a.s.l.), Ningyo Pass (700–800 m a.s.l.), Kibi (100–500 m a.s.l.) and Setouchi (< 100 m a.s.l.) Planes, and “Kibi Plateau,” were estimated to have been formed by sandstone and mudstone accumulated by transgression in Miocene (Obata 1991). In our preliminary study, some haplotypes were detected only in an altitude range of 400–599 m. In addition, adults of a freshwater chironomid species, Cricotopus sylvestris (Fabricius), widely distributed in stagnant waters such as lakes, ponds, and rice paddies in Japan, were shown to make a genetic clade by the individuals collected in a certain plane or adjacent planes, based on RAPD (random amplified polymorphic DNA) analysis in our previous study (Kawai et al. 2009).
In this study, char samples were identified according to the criteria by Hosoya (2000), although the identification depends on somewhat ambiguous features, e.g., clearness of spots on the snout surface. Thus, the distribution limits of Gogi and Nikkoiwana are also controversial (Imanishi 1967; Kimura 1989; Taki et al. 2005). On the other hand, 3 haplotypes (17, 20, and 21) were detected only for Nikkoiwana whereas 17 haplotypes (2, 3, 6, 8, 9, 10, 11, 12, 13, 14, 16, 18, 19, 24, 25, 26, and 27) were detected only for Gogi in this study (Table 2). Among these, haplotype 21 has been proven to be Nikkoiwana-specific by Yamamoto et al. (2004), and haplotypes 3, 11, 13 and 16 have been also proven to be Gogi-specific by our previous study (Kawai et al. 2020). Therefore, these supposedly subspecies-specific haplotypes can possibly be used for the estimation of distribution range of subspecies or analysis of subspecies composition of fish communities. However, these subspecies specificities are necessary to be confirmed by new and more reliable methods incorporating microsatellite analysis, concatenated sequence data set, and whole-genome sequencing in the near future.
Data availability
The data that support these findings and conclusions are available upon reasonable request from the corresponding author.
References
Aoyagi H (1957) A survey of freshwater fishes in Japanese archipelagoes. Daishukan Shoten, Tokyo, pp 272 (in Japanese)
Aoyama J, Watanabe S, Ishikawa S, Nishida M, Tsukamoto K (2000) Are morphological characters distinctive enough to discriminate between two species of freshwater eels, Anguilla selebesensis and A. interiolis? Icthyol Res 47:157–161
Hattori I, Tanaka K (1999) Regional environment in Fukui found in topographic maps. 2. Succession of river flow and river capture: Nature and environment in the Sea of Japan Area. Res Bul Res Edu Cent Reg Environ, Fukui Univ 6:143–154 (in Japanese)
Hosoya K (2000) Salmonidae. In: Nakabo T (ed) Nihonsan Gyorui Kensaku. Tokai University Press, Tokyo, pp 256–261 (in Japanese)
Imanishi K (1967) Chars, the genus Salvelinus, its distribution in Japan. Shizen, Chuo-koron, Tokyo, pp 3–46 (in Japanese)
Inoue JG, Miya M, Tsukamoto K, Nishida M (2000) Complete mitochondrial DNA sequence of the Japanese sardine Sardinops melanostictus. Fish Sci 66:924–932
Katayama M, Fujioka Y (1966) Fishes in the Jakuchi Mountains. Nishi-chugokusanchi Kokuteikouen Kouhochi Gakujutsuchousa Houkoku, Yamaguchi Pref, pp 1–16 (in Japanese)
Kawai K, Masuhara A, Imabayashi H (2000) Relationships between the spot patterns of the head of the Japanese char Salvelinus leucomaenis, distributed in the Chugoku region, and water quality. Fish Sci 66:901–907
Kawai K, Katayama T, Imabayashi H (2006) Genetic relationships between chars distributed around the watershed borders in the eastern Chugoku Mountains, Japan, on the basis of RAPD analysis. J Grad Sch Biosp Sci Hiroshima Univ 45:1–8
Kawai K, Kashu K, Saito H (2020) An association between head-spot types and genetic types in char distributed in the Chugoku region, Japan. Environ Biol Fish 103:339–347
Kawai K, Okamoto H, Imabayashi H (2009) A fundamental study on range expansion of chironomids. In: Wang X, Liu W (eds) Contemporary chironomid studies –proceeding of the 17th. International Symposium on Chironomidae, Nankai University, 6–9 July, Tianjin, China
Kikko T, Kuwahara M, Iguchi K, Kurumi S, Yamamoto S, Kai Y, Nakayama K (2008) Mitochondrial DNA population structure of the white-spotted charr (Salvelinus leucomaenis) in the Lake Biwa water system. Zool Sci 25:146–153
Kimoto S (1976) Research methods for animal community I-diversity and species composition. Kyoritsu Press, Tokyo, p 192 (in Japanese)
Kimura S (1989) Salvelinus leucomaenis f. imbrius. In: Kawanabe H, Mizuno N (eds) Japanese Freshwater Fishes. Yama To Keikoku-sha, Tokyo, pp 128–131 (in Japanese)
Kobeshinbun (1974) River fish. In: Kobeshinbun (ed) Hyogotanken Shizen-hen. Kobe, Kobeshinbunsha, pp 339–343 (in Japanese)
Kurasawa H, Tsukumi M (2003) Daisen volcano. In: Physical Geography Encyclopedia New Edition Editorial Board (ed) Physical geography encyclopedia new edition. Heibonsha, Tokyo, pp 747 (in Japanese)
Miyaji D, Kawanabe H, Mizuno N (1975) Genshoku Nihon Tansuigyorui Zukan. Hoikusha, Osaka, p 275 (in Japanese)
Nishimura S (1974) The history of the Sea of Japan. Tsukiji Shokan, Tokyo, p 228 (in Japanese)
Obata H (1991) Geographical features of the Chugoku Region. Kokin Shoin, Tokyo, p 262 (in Japanese)
Ohga Y, Moriwaki T, Masui Y, Fukuoka S (2011) Evaluation of river cross-section for harmonization between flood control and river environment in the upper reach of Gounokawa River. River Technology Papers 17:1–6 (in Japanese)
Oshima M (1961) Studies on Japanese Chars. Chojushuho 18:3–70 (in Japanese)
Rozas J, Ferrer-Mata A, Sanchez-Del Barrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sanchez-Gracia A (2017) DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol Biol Evol 34:3299–3302
Taki Y, Kohno H, Sakamoto K, Hosoya K (2005) Illustrated fishes in color revised edition. Hokuryukan, Tokyo, p 971 (in Japanese)
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tansui Tsuriba Henshubu (2016) Introduction to Freshwater Fishing Areas, http://kawatsuri.com. (in Japanese)
Thompson JD, Higgins DG, Gibson TG (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Yamamoto S, Morita K, Kitano S, Watanabe K, Koizumi I, Maekawa K, Takamura K (2004) Phylogeography of white-spotted charr (Salvelinus leucomaenis) inferred from mitochondrial DNA sequence. Zool Sci 21:229–240
Acknowledgements
We are grateful to the collaborators in our laboratory for their kind help in char collection and continuous encouragement.
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Contributions
KK: study conception and design, sample collection, analysis and interpretation of results, and manuscript preparation; MM: sample collection, analysis, and interpretation of results; KF and STada: sample collection and analysis; STani: sample collection and interpretation of results; HS: interpretation of results and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests. All applicable university guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kawai, K., Matsuo, M., Fujiwara, K. et al. Mountain masses as a determinant to genetic structure of char distributed in the Chugoku region, Japan. Environ Biol Fish 107, 75–90 (2024). https://doi.org/10.1007/s10641-024-01511-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-024-01511-8