Abstract
Effective restoration of tidal wetlands for fish communities requires clear goals and mechanistic understanding of the ecosystem drivers which affect fish distribution and abundance. We examined fish community responses to abiotic habitat features in two adjacent but dissimilar freshwater tidal wetlands in the Sacramento-San Joaquin Delta, CA, USA, each of which represents a potential restoration configuration. The first wetland was characterized by a broad, intertidal basin with relatively high hydrodynamic exchange with surrounding waterways. The second wetland was characterized by a dendritic network of shallow subtidal channels with relatively low hydrodynamic exchange. Fish community composition significantly differed between the two wetlands, based on permutational analysis of variance. Fish abundance within and among the two wetlands was also highly affected by specific geomorphic and hydrodynamic characteristics: distance from connection with the main external waterway, bed elevation, and water surface elevation. The physical configuration of a restored tidal wetland, in conjunction with the way tides move across the restored landscape, has strong implications for local fishes. Manipulating these elements to create a landscape mosaic of habitat configurations can be an effective tool for targeting desired restoration outcomes, such as specific fish communities or target fish densities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tidal wetlands are among the world’s most biologically productive systems (Mitsch and Gosselink 2000), often providing important habitat and food resources for numerous fish species (Boesch and Turner 1984; Barbier et al. 2011). However, tidal wetlands worldwide have been heavily degraded as a result of historic and contemporary human activities (Gedan et al. 2009). Threats to tidal wetlands include direct (e.g., land use change, invasive species, altered sediment loads, and pollution) and indirect (e.g., climate change) effects (Kennish 2002; Kirwan and Megonigal 2013). In response to these threats, tidal wetlands are increasingly the focus of management plans and restoration efforts (Zedler 2000; Lepage et al. 2022).
Across tidal wetland ecosystems, the dynamic physical environment structures biological outcomes. For example, in estuaries, fish communities are broadly organized around salinity gradients (Bulger et al. 1993; Greenwood 2007; Feyrer et al. 2015). These salinity gradients fluctuate on seasonal and tidal timescales affecting fish distribution (Islam et al. 2006; Feyrer et al. 2021). Within these salinity zones, fish distribution in tidal wetlands is driven by interactions between physical, chemical, and biological components of habitat (Peterson and Ross 1991), including bathymetry and substrate type (Becker and Suthers 2014), hydrodynamics (Bever et al. 2016; Young et al. 2021), vegetation (Bloomfield and Gillanders 2005; Gilby et al. 2018), food availability (Whitfield 1988; Tableau et al. 2019), and predation risk (Becker and Suthers 2014; Jones et al. 2021). Thus, fish species composition in tidal wetlands will likely depend on the restored wetland’s location relative to the freshwater-marine gradient and the specific attributes of the local environment. In restored tidal wetland ecosystems, understanding specific relationships between physical habitat and fishes could provide key insight for long-term management and restoration efforts.
In California’s Sacramento-San Joaquin Delta, 90% of the historical tidal wetlands have been lost due to human modifications (Whipple et al. 2012; Brophy et al. 2019). In remaining wetlands, varying scales of physical habitat, hydrodynamics, and submerged aquatic vegetation are key drivers of fish distribution (Brown and Michniuk 2007; Feyrer et al. 2015; Young et al. 2018; Huntsman et al. 2023). In brackish regions, channel configuration and marsh plain elevation drive the abundance and distribution of native and non-native fishes; native fishes are generally most abundant in natural dead-end channels with tidally activated marsh plains (Visintainer et al. 2006; Colombano et al. 2020). In freshwater regions, less is known about the effects of specific wetland features on fish community. Generally, habitat alteration has been negatively associated with the abundance of native fishes (Feyrer and Healey 2003; Nobriga et al. 2005; Brown and Michniuk 2007; Moyle et al. 2010, 2012). However, spatiotemporal variability in hydrodynamics, submerged aquatic vegetation, and physical habitat obfuscates generalizations that would be relevant to predicting fish community responses to specific restoration actions (Grimaldo et al. 2012; Whitley and Bollens 2014; Feyrer et al. 2017; Young et al. 2018, 2021).
We examined fish community responses to abiotic habitat features in the Sacramento-San Joaquin Delta in two adjacent but dissimilar freshwater tidal wetlands to determine if geomorphology and hydrodynamic features had measurable effects on fish community responses. We sampled fishes in these wetlands for 2 years to identify the fish communities they support and elucidate relationships between specific geomorphic characteristics and fish abundance.
Methods
Study area
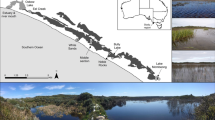
The Cache Slough complex in the northern Sacramento-San Joaquin Delta (Fig. 1) consists of a network of tidal channels and wetlands with high primary productivity and turbidity relative to other freshwater regions of the Sacramento-San Joaquin Delta (Downing et al. 2016; Stumpner et al. 2020). This region has a diverse fish community that includes a relatively high proportion of native species (Young et al. 2015, 2020; Huntsman et al. 2023). We studied two freshwater tidal wetlands, Little Holland Tract and Liberty Island Conservation Bank (Fig. 1), selected because they are near one another (within 1.5 km) yet exhibit unique physical characteristics and represent two potential restoration configurations.
Map of the study area showing the location of the study site within California (a, b) and the legal boundary of the Sacramento-San Joaquin Delta (b). c The location of each gill net/otter trawl and the USGS streamgage 11455167 (Little Holland Tract, intertidal basin; Liberty Island Conservation Bank, dendritic wetland). Samples were collected to assess fish community composition in both wetlands from 2017 to 2018
Little Holland Tract (hereafter referred to as “intertidal basin”) is a former agricultural tract that was “restored” to a tidal wetland when it flooded due to levee failure in 1983 (Bates and Lund 2013). It is now a broad, shallow, intertidal embayment with a surface area of 292 ha fringed with emergent marsh plain and vegetation (primarily Schoenoplectus). Mean bed elevation of the intertidal basin relative to sea level is − 0.70 m (standard deviation = 1.42 m; NAVD88; Snyder et al. 2016), with sampled depths ranging from 0.5 to 3.1 m. On average, 78% of the intertidal basin’s water volume exchanges with surrounding channels during each complete tidal cycle (Snyder et al. 2016), resulting in a substantial area of the intertidal basin completely dewatering at low tides and exposing the bed to air.
In contrast, Liberty Island Conservation Bank (hereafter referred to as “dendritic wetland”) is an engineered tidal wetland restoration project that was constructed in 2010 and has a surface area of 3 ha (Lehman et al. 2010; Wildlands Inc 2019). The dendritic wetland consists of a network of shallow, subtidal dendritic channels embedded in intertidal marsh plain. Mean bed elevation of the dendritic wetland relative to sea level is − 0.68 m (standard deviation = 0.87 m; NAVD88) (Snyder et al. 2016; Fregoso et al. 2020). There are no published quantitative measurements of tidal exchange; based on water elevation data and field observations, the primary channel in the dendritic wetland remains inundated at low tides. For those familiar with the region, Liberty Island Conservation Bank is distinct from Liberty Island, another nearby flooded agricultural island.
Fish sampling
We used otter trawls and gill nets to sample juvenile to adult life stages of fishes across all tidal conditions during daylight hours. Otter trawls targeted relatively small (mean standard length of catch ± standard deviation: 77 mm ± 54 mm) fishes, and gill nets targeted relatively large (mean standard length of catch ± standard deviation: 238 mm ± 75 mm) fishes. Otter trawls measured 5.3 m in length, had a mouth opening of 1.5 m × 4.3 m, and had 35-mm stretch mesh in the main body and 6-mm stretch mesh in the cod end. We towed the otter trawl at a constant speed (~ 3.5 km/h) targeting up to 10 min (mean ± standard deviation: 5 min ± 3 min) based on submerged obstructions. Gill nets were 1.8 m in height × 45.7 m in length with five equal length panels of 38 mm, 51 mm, 64 mm, 76 mm, and 89 mm stretched monofilament mesh. We deployed multi-mesh gill nets to minimize potential size and species-specific biases that often correspond to traditional gill net sampling with one mesh size (Shoup and Ryswyk 2016). These multi-mesh gill nets are relatively unbiased when sampling most fish species and size classes commonly encountered within the study area, except for small young-of-the-year life stages (Wulff et al. 2022). We targeted gill net set durations of 60 min (mean ± standard deviation: 58 min ± 20 min) to minimize stress and mortality of entangled fish. Gill nets were set to ensure that more than half the net was in 1.5 m or deeper water. We selected sampling periods (days/weeks within a season) to maximize the range of tidal conditions that could be sampled in daylight hours. We were also constrained in sampling location by depth, as certain locations were inaccessible at low tide.
For each net sample, we used a hand-held multiparameter sonde (YSI EXO2, Yellow Springs Instruments Inc.) to collect associated water quality variables: water temperature (°C), specific conductivity (μS cm−1 at 25 °C), turbidity (FNU), dissolved oxygen concentration (mg/L), pH, and fluorescent dissolved organic matter (QSU).
We completed six individual seasonal sampling events: winter 2017, spring 2017, summer 2017, fall 2017, winter 2018, and spring 2018. The exact sites sampled were determined randomly with the use of GIS software (Environmental Systems Research Institute 2022) and located in the field with GPS. All captured fishes were identified to species, measured for standard length (SL), and released alive. Data collected for this study (e.g., sample metadata, environmental data, and fish counts) are publicly available in Farruggia et al. (2019).
Data analysis
We used permutational analysis of variance (PERMANOVA) to compare fish community composition in each wetland. PERMANOVA conducts permutational tests on multiple variables to determine whether mean differences among groups are likely to have occurred by chance. A separate PERMANOVA was conducted for each sampling gear, and each model included wetland as a fixed effect and sampling event as a random variable to account for seasonal differences. Extremely rare species (less than five individuals captured total) were excluded. Analyses were done in Program R (R Core Team 2022) using the vegan package (Oksanen et al. 2022) and performed using the Bray-Curtis distance matrix with 999 permutations.
We used generalized additive mixed models (GAMMs) to model relationships between fish counts and specific geomorphic characteristics. GAMMs are extensions of generalized linear models applicable when relationships are not linear, and GAMMs use smoothed splines to characterize relationships among variables (Wood 2017). We modeled sampled fish counts as a function of three specific geomorphic/hydrodynamic characteristics: elevation (m) of the bed with respect to mean sea level, elevation (m) of the water surface with respect to mean sea level, and distance (m) of the sample from the nearest connection to the main waterway. Together, bed and water elevations address variability in dynamic habitat volume caused by tides; bed elevation is constant in time but variable in space, and water elevation is variable in space and time (Rozas and Reed 1993). Thus, we considered that bed and water elevations would each contribute to the overall effect of instantaneous volume of available habitat, which in tidal wetlands is inherently a combination of both features and is a known driver of fish distribution (Kneib and Wagner 1994; Silver et al. 2017; Colombano et al. 2020). Bed elevation associated with each sample was obtained from digital elevation models of the study areas (Snyder et al. 2016; Fregoso et al. 2020). Water elevation was obtained for each net using the median time of the sample and the associated reading from USGS streamgage 11455167 located in the study area (Fig. 1c; U.S. Geological Survey, 2023). Distance to the main waterway was measured by GIS on aerial imagery.
Each sampling gear was modeled separately. Sampling event (i.e., season-year combination) was included as a categorical random effect, allowing the model intercept to vary across each effect level, and wetland (intertidal basin or dendritic wetland) was included as categorical random effect that allowed the model slope to vary across each wetland. Sampling effort (i.e., the duration in minutes of a gill net set or an otter trawl tow) was included as an offset to standardize the fish counts across samples. The basic model structure was as follows:
The zero-inflation component (𝑃𝑖) was modeled with a logit link and intercept only due to the high number of empty nets. Modeling was done using the package brms (Bürkner 2017) in Program R (R Core Team 2022) and rSTAN (Stan Development Team 2022). Models were implemented with default values of four chains with iterations = 5000 and warmup = 1000. Combinations of continuous geomorphic and hydrodynamic characteristics were assessed with both varying intercept and varying intercept-varying slope models and compared using k-fold cross validation, with k = 10. Model prediction accuracy was estimated using the expected log pointwise predictive density (ELPD) based on the k-fold cross-validation performed in the package brms in Program R. Model weights were calculated using pseudo-Bayesian Model Averaging (pBMA) stabilized with a Bayesian bootstrap (n = 1000) using the package loo (Vehtari et al. 2017) in Program R.
We used the model with the highest weight to demonstrate relationships among fish counts and geomorphic/hydrodynamic characteristics and then used an ensemble of all models with model weights greater than 5% to generate fish density predictions for the two wetlands. We used the predictions to generate smoothed surface maps to visualize spatial distribution of fish densities at a snapshot in time using data from a single season-year combination (summer 2017). Because the figure is static, only variation in the two spatial geomorphic characteristics could be visualized (i.e., distance from a main waterway connection and bed elevation) and the temporally variable characteristic (water surface elevation) was kept constant at the mean value for the sampling period. We used raster datasets of bed elevation (Snyder et al. 2016; Fregoso et al. 2020) and distance from main waterway to generate zonal statistics for a grid of 30 m2 cells for each wetland, thus creating a spatially explicit grid of model covariates. We then used this grid to generate multiple (n = 1000) model predictions for a stage equivalent to the mean stage for all samples. These model predictions were then averaged for each cell and summarized to compare the density of fishes in each wetland. Predictions were limited to the range of geomorphic characteristics sampled (i.e., values above or below sampled ranges were omitted).
Results
We collected 700 fishes in 89 otter trawl tows and 1009 fishes in 132 gill net sets (Table 1) with 989 fishes in the intertidal basin (22 species) and 720 in the dendritic wetland (21 species). We collected a total of 26 species, nine of which were native. The dominant species in otter trawl samples were threadfin shad Dorosoma petenense (28% of total otter trawl catch; non-native, Günther 1867), American shad Alosa sapidissima (18%; non-native, Wilson 1811), black crappie Pomoxis nigromaculatus (10%; non-native, Lesueur 1829), striped bass Morone saxatilis (5%; non-native, Walbaum 1792), and white catfish Ameiurus catus (5%; non-native, Linnaeus 1758). The dominant species in gill net samples were white catfish (41% of total gill net catch; non-native), Sacramento splittail Pogonichthys macrolepidotus (25%; native, Ayres 1854), striped bass (12%; non-native), Sacramento sucker Catostomus occidentalis (6%; native, Ayres 1854), and Sacramento pikeminnow Ptychocheilus grandis (4%; native, Ayres 1854).
Water quality was similar between the two wetlands for most measured parameters (temperature, turbidity, dissolved oxygen, and pH). The wetlands only qualitatively differed in specific conductance (μS cm−1) and fluorescent dissolved organic matter (QSU), both of which were higher in the dendritic wetland (mean ± standard deviation: dendritic wetland 423 ± 177 μS cm−1, 41 ± 17 QSU; intertidal basin 287 ± 156 μS cm−1, 26 ± 14 QSU). Due to the similarities in water quality between the two wetlands, none of the parameters was included in the modeling or analysis.
PERMANOVA results indicated a statistically significant difference in fish community composition between the two wetlands for each sampling gear type (otter trawl: pseudo-F1,69 = 5.84, P-value < 0.001; gill net: pseudo-F1, 83 = 2.44, P-value < 0.023). There were key species differing between the two tidal wetlands in otter trawl samples. Catch per unit effort (CPUE) of american shad (intertidal basin = 1.91 fish/5min, dendritic wetland = 0.30 fish/5min) and mississippi silverside Menidia audens (Hay 1882) (intertidal basin = 0.52, dendritic wetland = 0.00) were both higher in the intertidal basin; CPUE of white catfish (intertidal basin = 0.05, dendritic wetland = 0.95), threadfin shad (intertidal basin = 0.49, dendritic wetland = 5.48), and black crappie (intertidal basin = 0.03, dendritic wetland = 2.27) were higher in the dendritic wetland (Table 1). There were also key species differing between the two tidal wetlands in gill net samples. CPUE of white catfish (intertidal basin = 3.80 fish/hr, dendritic wetland = 2.35 fish/hr), and Sacramento splittail (intertidal basin = 2.42, dendritic wetland = 1.22) were higher in the intertidal basin; CPUE of black crappie (intertidal basin = 0.06, dendritic wetland = 0.44), tule perch Hysterocarpus traskii (Gibbons 1854) (intertidal basin = 0.10, dendritic wetland = 0.36), and Sacramento pikeminnow (intertidal basin = 0.09, dendritic wetland = 0.80) were higher in the dendritic wetland) (Table 1).
The best-fitting (highest weight) GAMM model for each sampling gear indicated that all three geomorphic/hydrodynamic variables (bed elevation, water surface elevation, and distance from a main waterway connection) influenced fish counts (Table 2 and Fig. 2). For otter trawls, the best-fitting model included all three variables and a varying intercept associated with event; the best-fitting model did not include wetland as a categorical random effect. However, the second-best model (with a model weight of 30.8%) did include a varying slope associated with wetland. Based on the best-fitting otter trawl model, in both wetlands, fish counts were highest at intermediate bed elevations and low water elevations; fish counts were highest at greater distances from a main waterway connection but declined again at the farthest distances modeled. For gill nets, a model that included all three variables with a variable slope associated with wetland and varying intercept associated with event was the best-fitting model. For gill nets in the intertidal basin, fish counts were highest at low values of bed elevation, high values of water elevation, and intermediate distance from a main waterway connection. For gill nets in the dendritic wetland, fish counts did not change with bed elevation or water elevation but increased with distance from a main waterway connection.
Conditional effects for each parameter included in the best-fitting GAMM models as defined by model weights using the sample data from both wetlands collected in 2017. Predicted total fish counts are presented as functions of bed elevation (BedElev), water surface elevation (WaterElev), and the distance (Distance) to the closest connection to surrounding waterways (see Table 2). The gray regions around the curves are the predicted 95% credible intervals. Each parameter was separated by gear type (otter trawl (A), gill net (B)). Zero on the X-axis represents the mean value of the parameter, and each integer is one standard deviation from that mean (i.e., 0 for Distance is the mean Distance with 1 being farther away from the breach and − 1 being closer to the breach). There was no difference in the conditional effects for otter trawl between the intertidal basin (Little Holland Tract) and the dendritic wetland (Liberty Island Conservation Bank), which is why only one smooth line was drawn for each parameter
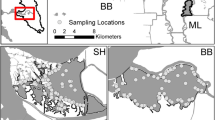
Surface maps of predicted fish densities highlight spatial variability in habitat suitability across the two wetlands at a snapshot in time and constant water surface elevation (Fig. 3). Predicted densities of fishes sampled by otter trawl in the intertidal basin were generally higher in the interior of the wetland (CPUE 2-4 fish/5min) and lower near the primary main waterway connections (CPUE 1-2 fish/5min); for fishes sampled by gill net in the intertidal basin, predicted densities were highest nearest the primary main waterway connections at the northern-most (CPUE 5-20 fish/hr) and southern-most (CPUE 10-25 fish/hr) ends of the wetland and decreased towards the center, approaching 0 fish/hr CPUE (Fig. 3). Predicted densities of fishes sampled by otter trawls in the dendritic wetland where predicted densities were lowest (CPUE ca. 1 fish/5min) at the northern-most (nearest the opening) and southern-most (farthest from the opening) ends of the wetland and highest in the center (CPUE 2-3 fish/5min); The pattern was different for fishes sampled by gill net in the dendritic wetland, where predicted densities were relatively uniform (CPUE ca. 5 fish/hr) with a peak (CPUE 10-25 fish/hr) at the southern-most end of the wetland that is farthest from the main waterway connection.
Maps of predicted fish density within the study areas (outlined in black) and a separate enlarged panel for the dendritic wetland (see inset). Predicted densities were generated based on an ensemble of all models with model weights greater than 5% and set for mean tide for each sampling gear in each wetland for summer 2017. Blank areas within the study region represent areas which were inaccessible to sampling, and predictions of fish density are based on out-of-sample prediction and so were excluded. a Otter trawl predicted densities (small fish). b Gill net predicted densities (large fish)
Discussion
Geomorphologic and hydrodynamic features affected fish communities and distribution in our study areas. These differences generally correspond with life history characteristics. For example, higher densities of relatively resident littoral fish in the dendritic wetland (e.g., black crappie and tule perch) and higher densities of more mobile fish in the intertidal basin (e.g., Sacramento splittail) demonstrated the different properties of the two wetlands. Due to large intertidal and shallow subtidal areas (Snyder et al. 2016), the majority of the intertidal basin was unsuitable for many species of fish for at least a portion of every day, and the considerable size and few connections to the surrounding channels limit access to the intertidal basin on any given tidal cycle. In contrast, the majority of the dendritic wetland remains inundated throughout tidal cycles, allowing fish to more consistently use the area within the wetland. If the desired outcome of restoration is habitat that can support higher fish densities, our results suggest that a focus on accessibility throughout the tidal cycle rather than total wetted area could be a viable approach.
Geomorphic variability drives fish responses at multiple scales
Geomorphic characteristics have been found to influence fish (Delong et al. 2019; Trotter et al. 2021) in both natural (McIvor and Odum 1988; Meyer and Posey 2019) and restored (Williams and Zedler 1999) tidal wetlands. The spatial and temporal scales of our study allowed us to expand on this knowledge by identifying how hydrodynamics (in this case, tides) interact with geomorphology to drive fine-scale fish responses in adjacent wetlands that are otherwise subject to similar regional and ecological constraints. Fine-scale geomorphic and hydrodynamic variability (identified in fish count models) can drive broader-scale fish responses (identified in the community analyses) which could then be generalized across the two wetlands. At the site scale, subtle differences in geomorphology can alter hydrodynamic conditions in ways that can drive fine-scale fish distribution, even between nearby sites. At the landscape scale, our findings emphasize that a mosaic of landforms can provide suitable habitat for a variety of targeted species and life stages (Jin et al. 2014; Silver et al. 2017; Schaberg et al. 2019). These findings have important implications for both site- and landscape-scale restoration actions. Creating a variety of habitats using relatively subtle differences in geomorphic design could promote positive restoration outcomes.
Tidal processes intersect with geomorphic variability to mediate fish response
Physical habitat and hydrodynamics drive water quality conditions in the study area and may also represent proximate drivers of fish responses. Observed differences in specific conductance and dissolved organic matter indicated higher water residence time within the dendritic wetland, consistent with general spatial patterns observed in the region (Downing et al. 2016). Residence time, a major driver of biogeochemical processes, corresponds to geomorphic and hydrologic attributes (Downing et al. 2016), adult fish distribution (Huntsman et al. 2023), and trophic structure (Young et al. 2021). In tidal wetlands, like those in this study, the timing of tides and the distance of the tidal excursion (maximum distance a parcel of water moves during a tidal cycle) interact with fixed geomorphic features to affect physical properties such as mixing, stratification, and water velocity. This relationship between the always-changing tidal interface and fixed geomorphic characteristics can interact to affect total tidal influence, governing the mixing and exchange of key resources such as sediments, algal biomass, and dissolved oxygen (Stumpner et al. 2021).
Previous work has documented the influence of tidal processes on various ecosystem constituents in the region, including biogeochemical cycling (Downing et al. 2016), primary productivity (Stumpner et al. 2020), fish prey distribution (Montgomery 2017; Feyrer et al. 2017; Young et al. 2021), and adult fish distribution (Huntsman et al. 2023). Our analysis underlines the importance of considering both physical habitat structure and hydrodynamics when attempting to understand the ecological processes and functions driving organismal abundance and distribution in complex systems, such as tidal wetlands. This principle extends beyond fish abundance to other ecosystem elements that can be crucial components of fish habitat. For example, the abundance and behavior of zooplankton, an important food resource for many fishes, are also influenced by hydrodynamics, seasonality, and tidal exchange with surrounding habitats (Yelton et al. 2022). Restored tidal wetlands in the San Francisco Estuary support varying zooplankton abundance and diversity through time, with strong seasonal and interannual variability not fully explained by individual habitat characteristics (Hartman et al. 2022). Much of this observed variability could potentially be explained by an application of the above methods to fully identify the influence of geomorphic and hydrodynamic characteristics.
Species-specific responses
Many native fishes in the San Francisco Estuary have declined in recent decades, including Chinook salmon Oncorhynchus tshawytscha (Walbaum 1792) (Yoshiyama et al. 1998; Sommer et al. 2007, and non-native nuisance species have proliferated widely (Brown and Michniuk 2007; Mahardja et al. 2017). We found no difference in native species richness or native density between the two wetlands, but the distributions of a few species warrant further discussion. Juvenile Chinook salmon were observed in both wetlands, consistent with other studies documenting out-migrating salmonid use of tidal wetlands (Shreffler et al. 1990; Davis et al. 2019). Threadfin shad, a non-native species that nonetheless may indicate overall ecosystem health and/or pelagic productivity, dominated the dendritic wetland, potentially indicating variability in water column productivity. In contrast, American shad were much more abundant in the intertidal basin, perhaps indicating increased involuntary movements into the wetland during out-migration due to the high tidal exchange. Mississippi silverside, largely considered an undesirable nuisance species in the San Francisco Estuary due to competitive and predatory effects on native fishes (Williamson et al. 2015; Schreier et al. 2016), were frequently encountered in the intertidal basin but were not encountered in the dendritic wetland. Mississippi silverside colonization of restored habitats is largely seen as an unwelcome potential outcome of restoration efforts in the Delta (Herbold et al. 2014; Sherman et al. 2017; Williamshen et al. 2021). While the goals of restoration will be system specific, our results suggest that restored tidal wetland habitats, regardless of configuration, improve regional diversity and support both native and non-native species, which can serve as a useful starting point in making informed management choices. However, the application of the above methods in each unique system would be necessary to predict the specific effects of geomorphic and hydrologic characteristics on the outcomes of restoration actions in other systems. In our study system specifically, if a desired restoration outcome includes limiting the presence of fish species such as Mississippi silverside, dendritic subtidal wetland habitats could provide a possible solution. If the goal is to support fishes with more mobile life histories, intertidal basins may provide the necessary habitat without the need for complex wetland construction.
Potential confounding factors
In complex systems such as tidal wetlands, there are many aspects of the environment which may influence observations, including diurnal cycles, tidal variability, and habitat area. All observations in this study occurred during daylight hours, which may have affected our assessment of fish community composition and distribution. Fishes often use different habitats across diel cycles, and by only sampling in daylight hours, we may miss fish that can move to or from sampled and unsampled habitats diurnally (Nemerson and Able 2020; Colombano et al. 2021). Tidal variability is known to drive fish distributions in many systems (Boswell et al. 2019; Nemerson and Able 2020; Young et al. 2021; Colombano et al. 2021; Huntsman et al. 2023) and may have potentially influenced our observations. For example, our sampling efficiency in small channels may have changed during the highest tides when access to the inundated intertidal habitats (i.e., mudflat and marsh plain) may have provided fishes a refuge from capture. We addressed this by sampling across the entire range of tidal conditions and explicitly incorporating tide stage in our statistical analyses, but additional variation related to tide may exist. Finally, differences in habitat area between the two wetlands could have also affected our findings. Species richness is often a function of area (sensu Rosenzweig 1995), and the intertidal basin has nearly one hundred times the surface area of the dendritic wetland (292 ha vs. 3 ha) which could influence fish community differences irrespective of other geomorphic differences. However, despite the vast difference in area, species richness across the two habitats was nearly identical and included most resident fish species found in the region. The extent that habitat area may influence community dynamics in the Sacramento-San Joaquin Delta is unclear, but recent restoration, once mature, may provide substantial insight to this question and other elements of regional ecosystem function.
Conclusion
Considering geomorphology and tides is important to understand relationships between fish and the environment, particularly as they pertain to restoration outcomes. Differences in geomorphology between the two wetlands were sufficient to affect tidal hydrodynamics (in the form of tidal exchange) as well as fish communities and distributions. From an ecological perspective, our study results are demonstrative of the idea that habitat features act as environmental filters which drive emergent properties of community assembly (McGill et al. 2006; Loreau 2010). From a practical perspective, our study results indicate that manipulating the morphology, inundation, and connectivity of wetted habitats can be an effective approach for targeting specific restoration outcomes in systems such as the Sacramento-San Joaquin Delta.
Habitats in this study supported different, complementary suites of native and non-native fishes, and the presence of wetlands representing distinct geomorphic attributes contributes to the region’s diverse species pool. A regional mosaic of different interconnected habitat configurations could provide multiple additional benefits. Variability in wetland structure could potentially fulfill the changing needs of fishes throughout ontogeny, mitigate seasonal or interannual variability which would render one wetland less suitable for a period of time, or fulfill basic ecological functions and services in different ways (e.g., rearing, reproduction, and refuge). Careful consideration should be taken when designing any new restoration action, including understanding how the action fits within the regional habitat mosaic. An interdisciplinary, region-specific understanding of how geomorphic and hydrodynamic characteristics drive ecological outcomes can inform evaluations of existing or planned tidal wetland restoration projects.
Data availability
The datasets used and/or analyzed during the current study are publicly available online: Farruggia MJ, Clause JK, Feyrer FV, Young MJ (2019) Fish abundance and distribution in restored tidal wetlands in the northern Sacramento-San Joaquin Delta, California, 2017-2018. US Geol Surv Data Release. https://doi.org/10.5066/P9F0ZASV.
References
Barbier EB, Hacker SD, Kennedy C et al (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193. https://doi.org/10.1890/10-1510.1
Bates ME, Lund JR (2013) Delta subsidence reversal, levee failure, and aquatic habitat—a cautionary tale. San Franc Estuary Watershed Sci 11:1. https://doi.org/10.15447/sfews.2013v11iss1art1
Becker A, Suthers IM (2014) Predator driven diel variation in abundance and behaviour of fish in deep and shallow habitats of an estuary. Estuar Coast Shelf Sci 144:82–88. https://doi.org/10.1016/j.ecss.2014.04.012
Bever AJ, MacWilliams ML, Herbold B et al (2016) Linking hydrodynamic complexity to Delta smelt (Hypomesus transpacificus) distribution in the San Francisco Estuary, USA. San Franc Estuary Watershed Sci 14:3. https://doi.org/10.15447/sfews.2016v14iss1art3
Bloomfield AL, Gillanders BM (2005) Fish and invertebrate assemblages in seagrass, mangrove, saltmarsh, and nonvegetated habitats. Estuaries 28:63–77. https://doi.org/10.1007/bf02732754
Boesch DF, Turner RE (1984) Dependence of fishery species on salt marshes: the role of food and refuge. Estuaries 7:460. https://doi.org/10.2307/1351627
Boswell KM, Kimball ME, Rieucau G et al (2019) Tidal stage mediates periodic asynchrony between predator and prey nekton in salt marsh creeks. Estuaries Coasts 42:1342–1352. https://doi.org/10.1007/s12237-019-00553-x
Brophy LS, Greene CM, Hare VC et al (2019) Insights into estuary habitat loss in the western United States using a new method for mapping maximum extent of tidal wetlands. PLoS ONE 14(8):e0218558. https://doi.org/10.1371/journal.pone.0218558
Brown LR, Michniuk D (2007) Littoral fish assemblages of the alien-dominated Sacramento – San Joaquin Delta, California, 1980-1983 and 2001-2003. Estuaries Coasts 30:186–200. https://doi.org/10.2307/4494076
Bulger AJ, Hayden BP, Monaco ME et al (1993) Biologically-based estuarine salinity zones derived from a multivariate analysis. Estuaries 16:311–322. https://doi.org/10.2307/1352504
Bürkner P-C (2017) brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 80:1–28. https://doi.org/10.32614/RJ-2018-017
Colombano DD, Donovan JM, Ayers DE et al (2020) Tidal effects on marsh habitat use by three fishes in the San Francisco Estuary. Environ Biol Fish 103:605–623. https://doi.org/10.1007/s10641-020-00973-w
Colombano DD, Handley TB, O’Rear TA et al (2021) Complex tidal marsh dynamics structure fish foraging patterns in the San Francisco Estuary. Estuaries Coasts 44:1604–1618. https://doi.org/10.1007/s12237-021-00896-4
Davis MJ, Woo I, Ellings CS et al (2019) Freshwater tidal forests and estuarine wetlands may confer early life growth advantages for Delta-reared Chinook salmon. Trans Am Fish Soc 148:289–307. https://doi.org/10.1002/tafs.10134
Delong MD, Thoms MC, Sorenson E (2019) Interactive effects of hydrogeomorphology on fish community structure in a large floodplain river. Ecosphere 10:5. https://doi.org/10.1002/ecs2.2731
Downing BD, Bergamaschi BA, Kendall C et al (2016) Using continuous underway isotope measurements to map water residence time in hydrodynamically complex tidal environments. Environ Sci Technol 50:13387–13396. https://doi.org/10.1021/acs.est.6b05745
Environmental Systems Research Institute (ESRI) (2022) ArcGIS Desktop: 10.8.1. Environmental Systems Research Institute, Redlands, CA
Farruggia MJ, Clause JK, Feyrer FV, Young MJ (2019) Fish abundance and distribution in restored tidal wetlands in the northern Sacramento-San Joaquin Delta, California, 2017-2018. US Geol Surv Data Release. https://doi.org/10.5066/P9F0ZASV
Feyrer F, Cloern JE, Brown LR et al (2015) Estuarine fish communities respond to climate variability over both river and ocean basins. Glob Change Biol 21:3608–3619. https://doi.org/10.1111/gcb.12969
Feyrer F, Healey MP (2003) Fish community structure and environmental correlates in the highly altered southern Sacramento-San Joaquin Delta. Environ Biol Fish 66:123–132. https://doi.org/10.1023/A:1023670404997
Feyrer F, Slater SB, Portz DE et al (2017) Pelagic nekton abundance and distribution in the Northern Sacramento–San Joaquin Delta, California. Trans Am Fish Soc 146:128–135. https://doi.org/10.1080/00028487.2016.1243577
Feyrer F, Young MJ, Huntsman BM, Brown LR (2021) Disentangling stationary and dynamic estuarine fish habitat to inform conservation: species-specific responses to physical habitat and water quality in San Francisco Estuary. Mar Coast Fish 13:548–563. https://doi.org/10.1002/mcf2.10183
Fregoso TA, Stevens AW, Wang R-F et al (2020) Bathymetry, topography, and acoustic backscatter data, and a digital elevation model (DEM) of the Cache Slough Complex and Sacramento River Deep Water Ship Channel. US Geol Surv Data Release, Sacramento-San Joaquin Delta, California. https://doi.org/10.5066/P9AQSRVH
Gedan KB, Silliman BR, Bertness MD (2009) Centuries of human-driven change in salt marsh ecosystems. Annu Rev Mar Sci 1:117–141. https://doi.org/10.1146/annurev.marine.010908.163930
Gilby BL, Olds AD, Connolly RM et al (2018) Seagrass meadows shape fish assemblages across estuarine seascapes. Mar Ecol Prog Ser 588:179–189. https://doi.org/10.3354/meps12394
Greenwood MFD (2007) Nekton community change along estuarine salinity gradients: can salinity zones be defined? Estuaries Coasts 30:537–542. https://doi.org/10.1007/BF03036519
Grimaldo L, Miller RE, Peregrin CM, Hymanson Z (2012) Fish assemblages in reference and restored tidal freshwater marshes of the San Francisco Estuary. San Franc Estuary Watershed Sci 10:1. https://doi.org/10.15447/sfews.2012v10iss1art2
Hartman R, Barros A, Avila M et al (2022) I’m not that shallow – different zooplankton abundance but similar community composition between habitats in the San Francisco Estuary. San Franc Estuary Watershed Sci 20:3. https://doi.org/10.15447/sfews.2022v20iss3art1
Herbold B, Baltz DM, Brown L et al (2014) The role of tidal marsh restoration in fish management in the San Francisco Estuary. San Franc Estuary Watershed Sci 12:1. https://doi.org/10.15447/sfews.2014v12iss1art1
Huntsman BM, Young MJ, Feyrer FV et al (2023) Hydrodynamics and habitat interact to structure fish communities within terminal channels of a tidal freshwater delta. Ecosphere 14:e4339. https://doi.org/10.1002/ecs2.4339
Wildlands Inc. (2019) Liberty Island Conservation Bank. https://www.wildlandsinc.com/banks/liberty-island-conservation-bank-salm/. Accessed 5 Jan 2021
Islam MS, Hibino M, Tanaka M (2006) Distribution and diets of larval and juvenile fishes: influence of salinity gradient and turbidity maximum in a temperate estuary in upper Ariake Bay, Japan. Estuar Coast Shelf Sci 68:62–74. https://doi.org/10.1016/j.ecss.2006.01.010
Jin B, Xu W, Guo L et al (2014) The impact of geomorphology of marsh creeks on fish assemblage in Changjiang River estuary. Chin J Oceanol Limnol 32:469–479. https://doi.org/10.1007/s00343-014-3002-0
Jones TR, Henderson CJ, Olds AD et al (2021) The mouths of estuaries are key transition zones that concentrate the ecological effects of predators. Estuaries Coasts 44:1557–1567. https://doi.org/10.1007/s12237-020-00862-6
Kennish MJ (2002) Environmental threats and environmental future of estuaries. Environ Conserv 29:78–107. https://doi.org/10.1017/S0376892902000061
Kirwan ML, Megonigal JP (2013) Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504:53–60. https://doi.org/10.1038/nature12856
Kneib RT, Wagner SL (1994) Nekton use of vegetated marsh habitats at different stages of tidal inundation. Mar Ecol Prog Ser 106:227–238. https://doi.org/10.3354/meps106227
Lehman PW, Mayr S, Mecum L, Enright C (2010) The freshwater tidal wetland Liberty Island, CA was both a source and sink of inorganic and organic material to the San Francisco Estuary. Aquat Ecol 44:359–372. https://doi.org/10.1007/s10452-009-9295-y
Lepage M, Capderrey C, Elliott M, Meire P (2022) Estuarine degradation and rehabilitation. In: Fish and Fisheries in Estuaries. John Wiley & Sons, Ltd, pp 458–552
Loreau M (2010) Linking biodiversity and ecosystems: towards a unifying ecological theory. Philos Trans Biol Sci 365:49–60. https://doi.org/10.1098/rstb.2009.0155
Mahardja B, Farruggia MJ, Schreier B, Sommer T (2017) Evidence of a shift in the littoral fish community of the Sacramento-San Joaquin Delta. PLoS ONE 12(1):e0170683. https://doi.org/10.1371/journal.pone.0170683
McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21:178–185. https://doi.org/10.1016/j.tree.2006.02.002
McIvor CC, Odum WE (1988) Food, predation risk, and microhabitat selection in a marsh fish assemblage. Ecology 69:1341–1351. https://doi.org/10.2307/1941632
Meyer DL, Posey MH (2019) Salt marsh habitat size and location do matter: the influence of salt marsh size and landscape setting on nekton and estuarine finfish community structure. Estuaries Coasts 42:1353–1373. https://doi.org/10.1007/s12237-019-00555-9
Mitsch WJ, Gosselink JG (2000) The value of wetlands: importance of scale and landscape setting. Ecol Econ 35:25–33. https://doi.org/10.1016/S0921-8009(00)00165-8
Montgomery JR (2017) Foodweb dynamics in shallow tidal sloughs of the San Francisco Estuary. University of California, Davis, M.S.
Moyle PB, Bennett WA, Durand JR et al (2012) Where the wild things aren’t: making the Delta a better place for native species. Public Policy Institute of California
Moyle PB, Bennett WA, Fleenor WE, Lund JR (2010) Habitat variability and complexity in the upper San Francisco Estuary. San Franc Estuary Watershed Sci 8:24. https://doi.org/10.15447/sfews.2010v8iss3art1
Nemerson DM, Able KW (2020) Diel and tidal influences on the abundance and food habits of four young-of-the-year fish in Delaware Bay, USA, marsh creeks. Environ Biol Fish 103:251–268. https://doi.org/10.1007/s10641-020-00956-x
Nobriga ML, Feyrer F, Baxter RD, Chotkowski M (2005) Fish community ecology in an altered river delta: spatial patterns in species composition, life history strategies, and biomass. Estuaries 28:776–785. https://doi.org/10.1007/BF02732915
Oksanen J, Blanchet FG, Friendly M et al (2022) vegan: Community Ecology Package_. R package version 2.6-4. https://CRAN.R-project.org/package=vegan
Peterson MS, Ross ST (1991) Dynamics of littoral fishes and decapods along a coastal river-estuarine gradient. Estuar Coast Shelf Sci 33:467–483. https://doi.org/10.1016/0272-7714(91)90085-P
R Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Roenzweig ML (1995) Species Diversity in Space and Time. Cambridge University Press
Rozas L, Reed D (1993) Nekton use of marsh-surface habitats in Louisiana (USA) deltaic salt marshes undergoing submergence. Mar Ecol Prog Ser 96:147–157. https://doi.org/10.3354/meps096147
Schaberg SJ, Patterson JT, Hill JE et al (2019) Fish community composition and diversity at restored estuarine habitats in Tampa Bay, Florida, United States. Restor Ecol 27:54–62. https://doi.org/10.1111/rec.12712
Schreier BM, Baerwald MR, Conrad JL et al (2016) Examination of predation on early life stage Delta smelt in the San Francisco Estuary using DNA diet analysis. Trans Am Fish Soc 145:723–733. https://doi.org/10.1080/00028487.2016.1152299
Sherman S, Hartman R, Contreras D (2017) Effects of tidal wetland restoration on fish: a suite of conceptual models. California Department of Water Resources, Sacramento
Shoup DE, Ryswyk RG (2016) Length selectivity and size-bias correction for the North American standard gill net. North Am J Fish Manag 36:485–496. https://doi.org/10.1080/02755947.2016.1141809
Shreffler DK, Simenstad CA, Thom RM (1990) Foraging by juvenile salmon in a restored estuarine wetland. Can J Fish Aquat Sci 47:2079–2084. https://doi.org/10.2307/1352693
Silver BP, Hudson JM, Lohr SC, Whitesel TA (2017) Short-term response of a coastal wetland fish assemblage to tidal regime restoration in Oregon. J Fish Wildl Manag 8:193–208. https://doi.org/10.3996/112016-JFWM-083
Snyder AG, Stevens AW, Carlson E, Lacy JR (2016) Digital elevation model of Little Holland Tract, Sacramento-San Joaquin Delta, California, 2015. US Geol Surv Data Release. https://doi.org/10.5066/F7RX9954
Sommer T, Armor C, Baxter R et al (2007) The collapse of pelagic fishes in the Upper San Francisco Estuary. Fisheries 32:270–277. https://doi.org/10.1577/1548-8446(2007)32[270:TCOPFI]2.0.CO;2
Stan Development Team (2022) RStan: the R interface to Stan. R package version 2.32.3. https://mc-stan.org/
Stumpner EB, Bergamaschi BA, Kraus TEC et al (2020) Spatial variability of phytoplankton in a shallow tidal freshwater system reveals complex controls on abundance and community structure. Sci Total Environ 700:134392. https://doi.org/10.1016/j.scitotenv.2019.134392
Stumpner PR, Burau JR, Forrest AL (2021) A Lagrangian-to-Eulerian metric to identify estuarine pelagic habitats. Estuaries Coasts 44:1231–1249. https://doi.org/10.1007/s12237-020-00861-7
Tableau A, Le Bris H, Saulnier E et al (2019) Novel approach for testing the food limitation hypothesis in estuarine and coastal fish nurseries. Mar Ecol Prog Ser 629:117–131. https://doi.org/10.3354/meps13090
Trotter AA, Ritch JL, Nagid E et al (2021) Using geomorphology to better define habitat associations of a large-bodied fish, common snook Centropomus undecimalis, in coastal rivers of Florida. Estuaries Coasts 44:627–642. https://doi.org/10.1007/s12237-020-00801-5
U.S. Geological Survey (2023) 2023 U.S. Geological Survey. 1145516
Vehtari A, Gelman A, Gabry J (2017) loo: Efficient leave-one-out cross-validation and WAIC for Bayesian models, R package version 2.6.0. https://mc-stan.org/loo/
Visintainer T, Bollens S, Simenstad C (2006) Community composition and diet of fishes as a function of tidal channel geomorphology. Mar Ecol Prog Ser 321:227–243. https://doi.org/10.3354/meps321227
Whipple AA, Grossinger RM, Rankin D et al (2012) Sacramento-San Joaquin Delta historical ecology investigation: exploring pattern and process. Rep SFEI-ASCs Hist Ecol Program Publ 672:408
Whitfield AK (1988) The fish community of the Swartvlei Estuary and the influence of food availability on resource utilization. Estuaries 11:160–170. https://doi.org/10.2307/1351968
Whitley SN, Bollens SM (2014) Fish assemblages across a vegetation gradient in a restoring tidal freshwater wetland: diets and potential for resource competition. Environ Biol Fish 97:659–674. https://doi.org/10.1007/s10641-013-0168-9
Williams GD, Zedler JB (1999) Fish assemblage composition in constructed and natural tidal marshes of San Diego Bay: relative influence of channel morphology and restoration history. Estuaries 22:702. https://doi.org/10.2307/1353057
Williamshen BO, O’Rear TA, Riley MK et al (2021) Tidal restoration of a managed wetland in California favors non-native fishes. Restor Ecol 29:e13392. https://doi.org/10.1111/rec.13392
Williamson BO, O’Rear TA, De Carion D et al (2015) Fishes of the Nurse-Denverton Slough Complex: managed wetlands and tidal waterways in Suisun Marsh. Interag Ecol Program Newsl 28:29–35
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. Chapman and Hall/CRC, New York
Wulff ML, Feyrer FV, Young MJ (2022) Gill net selectivity for fifteen fish species of the Upper San Francisco Estuary. San Franc Estuary Watershed Sci 20:2. https://doi.org/10.15447/sfews.2022v20iss2art4
Yelton R, Slaughter AM, Kimmerer WJ (2022) Diel behaviors of zooplankton interact with tidal patterns to drive spatial subsidies in the Northern San Francisco Estuary. Estuaries Coasts 45:1728–1748. https://doi.org/10.1007/s12237-021-01036-8
Yoshiyama RM, Fisher FW, Moyle PB (1998) Historical abundance and decline of Chinook salmon in the Central Valley region of California. North Am J Fish Manag 18:487–521. https://doi.org/10.1577/1548-8675(1998)018<0487:haadoc>2.0.co;2
Young M, Howe E, O’Rear T et al (2020) Food web fuel differs across habitats and seasons of a tidal freshwater estuary. Estuaries Coasts 286–301. https://doi.org/10.1007/s12237-020-00762-9
Young MJ, Feyrer F, Stumpner PR et al (2021) Hydrodynamics drive pelagic communities and food web structure in a tidal environment. Int Rev Hydrobiol 106:69–85. https://doi.org/10.1002/iroh.202002063
Young MJ, Feyrer FV, Colombano DD et al (2018) Fish-habitat relationships along the estuarine gradient of the Sacramento-San Joaquin Delta, California: implications for habitat restoration. Estuaries Coasts 41:2389–2409. https://doi.org/10.1007/s12237-018-0417-4
Young MJ, Perales KM, Durand JR, Moyle PB (2015) Fish distribution in the Cache Slough Complex of the Sacramento-San Joaquin Delta during drought. Interag Ecol Program Newsl 28:23–28
Zedler JB (2000) Progress in wetland restoration ecology. Trends Ecol Evol 15:402–407. https://doi.org/10.1016/S0169-5347(00)01959-5
Acknowledgements
We express our appreciation to E. Van Nieuwenhuyse for supporting this study. D. Ayers, E. Clark, E. Enos, B. Fessenden, E. Fong, E. Gusto, J. Kathan, V. Violette, O. Patton, R. Spankowski, and D. Valentine assisted with field work. We would like to thank L. Brown and C. Smith as well as the anonymous reviewers for their valuable feedback on this manuscript.
Funding
Funding was provided by the Bureau of Reclamation (IA# R15PG00085).
Author information
Authors and Affiliations
Contributions
J.K.C. and M.J.F. contributed equally to this work and are co-first authors.
F.F. and M.J.Y. conceived and designed the research; all authors performed the experiments; J.K.C., M.J.Y. analyzed the data; all authors contributed to writing the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Field sampling was authorized by California Department of Fish and Wildlife Scientific Collection Permit SC-3602, National Marine Fisheries Service Research Permit #19121, memorandums of understanding for the take of threatened and endangered species issued by the California Department of Fish and Wildlife, and take authority obtained through the Interagency Ecological Program (IEP).
Competing interests
The authors declare no competing interests.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the view of the member agencies of the IEP. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Clause, J.K., Farruggia, M.J., Feyrer, F. et al. Wetland geomorphology and tidal hydrodynamics drive fine-scale fish community composition and abundance. Environ Biol Fish 107, 33–46 (2024). https://doi.org/10.1007/s10641-023-01507-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01507-w