Abstract
Predation, and the risk of predation, shape the ecology, behavior, and evolution of many species. Small fishes navigate a perilous landscape of risk in the shallow waters of the littoral zone. Moreover, in small lakes, the predator community can be dynamic due to stochastic colonization and extirpation events. These conditions select for the ability by small fish to acquire recognition of novel predators through associative learning. Chemical cues associated with predation events, such as damage-released chemical cues from conspecifics, and the odor of predators, inform prey of the presence of risk, and facilitate acquired recognition of novel predator odor. Deming Lake, MN, is a small meromictic lake with intermittent connections to neighboring lakes in the watershed. Annual sampling of the littoral fish community between the years 2000 and 2023 reveals a history of colonization and extirpation by relatively large-bodied species such as yellow perch Perca flavescens and pumpkinseed sunfish Lepomis gibbosus. Ice cover data, combined with limnological depth profiles of dissolved oxygen, confirm that dissolved oxygen is limiting during the winter and consistent with the hypothesis that extirpation of large-bodied species in Deming Lake is likely due to winter anoxia. These data set the stage for an experimental demonstration of acquired recognition of the odor of allopatric rock bass Ambloplites rupestris by bass-naïve northern redbelly dace Chrosomus eos from Deming Lake. Rock bass have been absent from Deming Lake since at least the year 2000. Predator-recognition learning allows redbelly dace, and many other small-bodied fishes that face variable predator species over ontogenetic, spatial, and temporal scales, a mechanism to adapt quickly to indicators of predation risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence and absence of predator species vary naturally over ecological and evolutionary time scales, and more recently as a direct result of anthropogenic accidental and intentional introductions (Cox and Lima 2006; Carthey and Blumstein 2018; Anton et al. 2020). A variety of biotic and abiotic factors influence fish community structure in small lakes (Bouvier et al. 2009; Jackson et al. 2001). Species richness reflects an equilibrium between two opposing rates: the rate of colonization and the rate of extirpation. Colonization is correlated with the degree of connectedness to other water bodies, while rate of local extirpation is determined by biotic interactions with predator and competitors, and tolerance for physiochemical factors such as pH, temperature, and dissolved oxygen (Tonn and Magnuson 1982; Magnuson et al. 1998; Miranda 2005; Bouvier et al. 2009; Jackson et al. 2001; Guimarães et al. 2014). In a survey of 18 lakes in northern Wisconsin, lake connectedness and winter dissolved oxygen levels were the two best predictors of fish community composition (Tonn and Magnuson 1982). Assemblages characterized by Centrarchids and Esox were found in lakes with a high degree of connectedness and high levels of dissolved oxygen in the winter, whereas lakes classified as Umbra-cyprinid lakes were relatively isolated with low connectedness and experienced very low levels of dissolved oxygen during winter. A similar pattern occurs in alluvial lakes adjacent to the Mississippi River where Centrarchids occur only in relatively large, deep, well-oxygenated lakes that have frequent connections to the main river channel (Miranda 2005). A third example can be found in coastal lakes in Brazil where species richness is a balance between rate of colonization, determined by interconnectedness, and species loss, determined by tolerance to salinity (Guimarães et al. 2014).
For lakes with low connectivity, the probability of colonization is lower than the probability of extirpation; i.e., once a species successfully colonizes a lake, it persists for some time before abiotic factors cause that species to be lost from the community, followed by a long period of absence before another colonization event occurs (Magnuson et al. 1998). Northern temperate lakes with low or intermittent connectivity have dissolved oxygen levels during the winter that are below the lethal limit for large-bodied piscivorous species due to winterkill (Tonn and Magnuson 1982; Bouvier et al. 2009). When piscivorous species colonize the lake, they do not persist for more than one or two winters. Consequently, small northern lakes with low connectivity and low dissolved oxygen levels during the winter experience a variable and stochastic predator community. Populations of small-bodied prey species must cope with variation in predator presence/absence to persist in these lakes.
Predation is a powerful agent of natural selection, and the appearance and disappearance of predator species require prey to adapt, if they can, to coexist with this variation (Carthey and Blumstein 2018). Predator–prey interactions in aquatic habitats are often mediated by chemical cues because water is an excellent solvent and several classes of soluble chemical compounds are released as passive by-products of predation events that inform prey of the presence of risk (Wisenden and Chivers 2006; Ferrari et al. 2010; Wisenden 2015). Predators are recognized by their signature body odor (kairomones) and chemical indicators of the predator’s diet emanating from their digestive system. When predators grasp prey, damaged prey tissues passively release chemical alarm cues that inform nearby prey that a conspecific has been attacked. Because alarm cues are released in the context of predation, predation is the most likely selective pressure that led to the evolution of olfactory recognition of conspecific alarm cues. Moreover, alarm cues are used as a releaser of associative learning to associate predation risk with novel stimuli that are temporally and spatially correlated with the predation event, such as the appearance or odor of the predator (Magurran 1989; Suboski 1990; Kelley and Magurran 2003; Brown 2003; Chivers and Smith 1994). Associative learning allows fish to rapidly acquire recognition of predator species that vary over temporal and spatial dimensions of fish ecology. For example, an entire population of fathead minnows Pimephales promelas naïve to northern pike Esox lucius acquired recognition of pike odor in less than 14 days after release of 10 pike into the pond (Chivers and Smith 1995).
Here, we present data from Deming Lake, MN, USA, a small kettle lake with intermittent connection to other wetlands in the Lake Itasca watershed, which is the source of the Mississippi River. Deming Lake is meromictic with dissolved oxygen levels below the lethal limit for large piscivorous fish during the ca. 5 months of ice cover each winter (Tonn and Magnuson 1982; Magnuson et al. 1998). We present over 30 years of data for duration of ice cover on Deming Lake, followed by a summary of catch data from the past 25 years to show that the community dynamics of Deming Lake fluctuate both in relative abundance and in species presence and absence, reflecting colonization and extirpation events. Given these ecological conditions, we predicted that small fishes in Deming Lake should possess the ability to rapidly acquire recognition of novel predators that colonize the lake. Therefore, we conducted a conditioning experiment on one species in the fish community of Deming Lake, northern redbelly dace Chrosomus eos, to test for pre-existing recognition of allopatric rock bass odor and the ability of dace to acquire recognition of this novel predator after the opportunity to associate bass odor with conspecific alarm cues. Combined, these data demonstrate the effect of variable colonization and extirpation events on the predator community, low winter dissolved oxygen as the mechanism by which extirpation occurs, and rapid predator recognition learning that has evolved in response to a variable predator community.
Methods
Study site
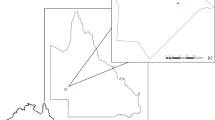
Deming Lake is located within the boundaries of Itasca State Park, Minnesota, USA (47.170183, − 95.168064), with a surface area of approximately 5.5 ha, and maximum depth of 17 m. It is a kettle lake created when a large chunk of ice was deposited within glacial till during glacial retreat approximately 10,000 years ago. As is typical of kettle lakes, Deming Lake is relatively deep compared to its surface area. The lake is surrounded by steep hills, which support tall stands of red and white pine that shelter the lake surface from wind action. The combination of limited exposure to wind, and small surface area relative to its depth, results in only partial mixing of the water column during fall and spring turnover. Thus, Deming Lake is meromictic, with an anoxic monimolimnion “dead zone” at its greatest depth that never mixes with the rest of the water column. Due to this unusual characteristic, Deming Lake and nearby kettle lakes in the park have received attention from limnologists.
Dissolved oxygen profiles and duration of ice-cover for Deming Lake
Depth profiles of dissolved oxygen and other physiochemical parameters were assessed with seasonal-scale frequency from four lakes in the Itasca State Park from 2006 to 2009 and from 2019 to 2022, including Deming Lake using deployable probes at meter or sub-meter resolution at the deepest location in each lake (see Swanner et al. 2022 for description of methods).
Ice-in/ice-out data were acquired from records taken by a succession of three station biologists at the University of Minnesota Itasca Biological Field Station and Laboratory spanning the 33-year interval between the winters of 1989–1990 and 2022–2023, namely, Jon Ross, Lesley Knoll and Dan Brumm. Ice-in was defined as the day when the lake surface was 99% or more covered in ice. Similarly, ice-out was defined as when the lake surface had < 1% ice remaining.
Variation in fish community composition in Deming Lake
For most of the summer seasons since the year 2000, minnow trap experiments have been conducted on Deming Lake. Sampling was conducted using unbaited Gee’s minnow traps (cylindrical traps 43 cm long, 22 cm in diameter with inverted cone entrances at each end) as part of annual Curriculum-based Undergraduate Research Experiences (CUREs) for a field course in Animal Behavior (EEB 3811W) at the Itasca Biological Station and Laboratories, University of Minnesota, held from mid-May to mid-June each year. The CUREs took place on a single day each year; therefore, catch data represent an annual snapshot of the fish community. Traps were set 10 m apart about 1–2 m from shore. Traps were set in statistical blocks representing one or two replicates of the experimental and control treatments for that year’s CURE experiment. After setting the first block of four to six traps, we waited 5 min before setting the next block of traps. We continued setting blocks of traps around the perimeter of the lake until all traps were set. We pulled and checked traps when each block had reached 120 min of fishing time. Teams of students assigned to each treatment had 5 min to process the catch before the next block of traps was ready to be pulled. In this way, fishing time across treatment groups was standardized at exactly 2 h. Fish caught in each trap were sorted to species and counted and returned immediately to the lake. Additional data such as sex, length, and mass were not recorded. The number of traps employed each year varied from 36 to 64, depending on the experiment being conducted, but the by-product of these experimental studies is a longitudinal census of the fish community of Deming Lake. Additional sampling by seine nets (data not shown) revealed golden shiners, black bullhead catfish, and Iowa darters that rarely recruit to minnow traps during the CURE. No piscivorous species have been observed in Deming Lake since 2000 other than yellow perch in 2002.
Predator-recognition learning by northern redbelly dace
Northern redbelly dace Chrosomus eos are small (~ 4.8 cm total length) freshwater fish common to bogs, creeks, ponds, and small lakes in the upper Midwest of the USA and across much of Canada (Scott and Crossman 1973; Fishbase.org) where they feed on zooplankton (Naud and Magnan 1988) and aquatic insects (Johnson and Johnson 1984) but can switch to algae and benthic invertebrates if piscivorous fish preclude use of the pelagic zone (Cochran et al. 1988). They respond behaviorally to conspecific alarm cues (Brown et al. 2020) through area avoidance (Wisenden and Barbour 2005), and increased shoal cohesion (Dupuch et al. 2004, 2009). Northern redbelly dace used in this experiment were collected by minnow trap from Deming Lake. At the time of this study, Deming Lake contained redbelly dace, fathead minnows Pimephales promelas, brook stickleback Culaea inconstans, blacknose shiners Notropis heterolepis, golden shiners Notemigonus crysoleucus, pumpkinseed sunfish Lepomis gibbosus, Iowa darters Etheostoma exile, and black bullhead catfish Ameiurus melas. Specifically, rock bass Ambloplites rupestris have not been recorded in Deming Lake from trapping records, seining efforts, or direct observation since 2000; therefore, to the best of our knowledge, northern redbelly dace used in this study would not have had any opportunity to acquire recognition of rock bass odor.
Preparation of test stimuli
To collect rock bass kairomone, two rock bass (14.0 cm and 15.4 cm in total length) were collected by seine net from Lake Itasca and placed in a 75-L aquarium filled with ~ 38L of lake water from Lake Itasca, with aeration but no filtration for 24 h, to infuse the tank water with their kairomones. Bass diet was not controlled. Tank water was used as “rock bass odor” for this experiment, and stored at 4 °C until needed. The bass were released back to Lake Itasca.
To make alarm cue, five adult northern redbelly dace were euthanized with an overdose of MS222 and cervical dislocation. Skin fillets were removed from both sides of each fish (total area approximately = 10 cm2) and homogenized into a paste with a mortar and pestle, then diluted to a total volume of 240 mL with well water to make 24, 10-mL doses of alarm cue, each representing approximately 0.4 cm2 of fish skin. Alarm cue solution was stored at 4 °C until needed.
Experimental protocol
Redbelly dace were placed in groups of three into rectangular transparent plastic containers (34 × 29 × 12 cm deep, vol = 5.7 L). The long side wall was marked with a 3- × 3-cm grid with 2 rows × 9 columns for scoring shoal cohesion. Shoaling is a common antipredator response of minnows in response to predation risk (Magurran 1990; Magurran and Pitcher 1987; Dupuch et al. 2004, 2009). Shoal cohesion was quantified as the area (i.e., number of grid squares) of the minimum polygon needed to fully enclose the heads of all three dace. Three fish in a 2 × 9 grid can create shoal index values that range from 1 to 10. Heads were chosen instead of the full body to increase quantitative resolution of the cohesion index. Test cue was injected into the tub, and shoal cohesion was recorded every 15 s over a 5-min period to produce an average shoal cohesion score for each 5-min observation period.
Experiment 1: Demonstration of shoaling response to predation risk
This experiment established that a shoaling response to alarm cues by redbelly dace was measurable in the test apparatus. The control treatment group (n = 10 shoals of three redbelly dace) was exposed to 10 mL of water, and the experimental treatment group (n = 10 shoals of three redbelly dace) was presented with 10 mL of alarm cue. Each fish was used only once and not reused in further experiments.
Experiment 2: Testing bass-naïve redbelly dace for response to bass odor
This experiment tested redbelly dace from Deming Lake for pre-existing recognition and response to the odor of allopatric rock bass from Lake Itasca. Control trials received 20 mL of blank well water, and test trials received 10 mL of water and 10 mL of predator odor. Ten trials were performed for each treatment type, involving 60 redbelly dace. Each fish was used only once and not reused in further experiments.
Experiment 3: Acquisition of predator recognition through associative learning
This experiment occurred in two parts; a conditioning trial followed by a testing trial. To condition redbelly dace to associate risk with rock bass odor, the control treatment group (n = 10 shoals of three dace) was exposed to 10 mL of water and 10 mL of alarm cue, and the experimental treatment group (n = 10 shoals of three dace) received 10 mL of rock bass odor and 10 mL of alarm cue. Shoaling behavior was scored as described above.
The testing trials re-exposed dace from the conditioning trials to bass odor. Conditioned fish from each treatment group were combined in separate stock tanks and allowed to mix freely, one tank for control fish and one for experimental fish, that received fresh flow-through water from Lake Itasca. After 90 min, fish from each stock tank were transferred in groups of three fish to fresh containers filled with Lake Itasca water. One fish from each treatment group escaped their respective stock tank through the drain hole; thus, sample size for the final test was n = 9 shoals of three dace for the control treatment group and n = 9 shoals of three dace for the experimental treatment group. All fish were exposed to 10 mL of rock bass odor, and shoaling behavior was scored as described above. One novel aspect of the design of the third experiment, compared to other experiments demonstrating predator recognition learning, is that it controlled for the potential effect of sensitization (e.g., Kenney 2020). Conditioning treatments in previous studies (e.g., Brown et al. 2011a) are (predator odor + water) versus (predator odor + alarm cue), followed by a test of responsiveness to predator odor. This is an imperfect experimental design because a positive response to predator odor by fish conditioned with (predator odor + alarm cue) could result from either associative learning or from sensitization to respond aversively to any new stimulus following exposure to alarm cue. In the current study, the conditioning treatments were (alarm cue + water) versus (alarm cue + predator odor), thus subsequent response to predator odor by fish conditioned with (alarm cue + predator odor) but not fish conditioned with (alarm cue + water) controls for the potential effect of sensitization and affirms that redbelly dace acquire recognition of novel predator odors through associative learning.
Results
Seasonal patterns of ice cover and dissolved oxygen in Deming Lake
Ice cover data for Deming Lake collected for 30 of the years between 1989 and 2022 show an average date of ice-in on Nov 15 and ice-out date on Apr 19 for a mean ± 1 SE duration of ice cover of 154.8 ± 2.3 days (Fig. 1). There were no trends over time from 1989 to 2023 in date of ice-in (linear regression: F1,28 = 0.003, P = 0.958), date of ice-out (F1,30 = 0.145, P = 0.706), or duration of ice cover (F1,28 = 0.413, P = 0.526).
Distribution of dissolved oxygen (mg/L) as a function of depth at different times of year in Deming Lake. Data were taken using probes at meter or sub-meter resolution at the deepest location in the lake (data from Swanner et al. 2022)
Depth profiles of dissolved oxygen revealed a positive heterograde during summer months (Fig. 2). Surface levels of dissolved oxygen were about 9 mg/L at the surface, peaked at 12–16 mg/L at a depth of 4 m, then quickly fell to 0 mg/L by 6 m. Depths below 8 m had levels of dissolved oxygen close to 0 mg/L for all samples. The positive heterograde was absent in late fall (October) resulting in a classic clinograde curve. Data taken in the month of January show a reduction in dissolved oxygen levels in surface waters relative to data taken before ice cover, which persists or deteriorates for another 3 + months until the return of ice-free conditions at the end of April.
Longitudinal trends in fish community composition in Deming Lake
Catch data from 17 of the 24 years between 2000 and 2023, totaling 65 277 fish, revealed marked shifts in species composition (Fig. 3). Because Deming Lake is located within a protected State Park, and does not contain fish species of interest to anglers, these fluctuations presumably reflect natural colonization and extirpation events rather than accidental or intentional stocking events by humans. The abrupt appearance of yellow perch in 2002 forced the CURE program to abandon Deming Lake as a study site because the main species of study, the fathead minnow, was too scarce. Predation by yellow perch is the most likely explanation for reduction in the population density of fathead minnows. By 2006 yellow perch, pumpkinseed sunfish and blacknose shiners were gone, presumably due to winterkill by hypoxia. Populations of fathead minnows, redbelly dace, and stickleback rebounded in the following years. The low representation of fathead minnows in 2010 may be an artefact of small sample size. Pumpkinseed sunfish and blacknose shiners reappeared in 2015 and in 2016 pumpkinseed sunfish came to dominate the catch samples. Since 2017, proportions of species compositions have been stable and similar to community structure observed in samples taken in 2000 and 2001.
Percentage of total catch represented by various fish species between 2000 and 2023 in Deming Lake. FHM, fathead minnows Pimephales promelas; RBD, northern redbelly dace Chrosomus eos; GS, golden shiners Notemigonus crysoleucas; BSN, blacknose shiners Notropis heterolepis; SB, brook stickleback Culaea inconstans; PKS, pumpkinseed sunfish Lepomis gibbosus; YP, yellow perch Perca flavescens; Bull, black bullhead catfish Ameiurus melas; Iowa, Iowa darter Etheostoma exile; MM, central mudminnow Umbra limi. Numbers above each indicate the total sample size for that year
Predator recognition training of northern redbelly dace from Deming Lake
There was no evidence of pre-existing recognition of odor of allopatric rock bass by redbelly dace as measured by a shoaling response (t-test, t18 = 0.605, P = 0.553; Fig. 4a). Dace that received conspecific alarm cue showed significantly tighter shoal cohesion than fish that received the water control treatment (t-test, t18 = 4.18, P < 0.001; Fig. 4b). During conditioning trials, dace that received (alarm cue + rock bass odor) showed a shoaling response of equal magnitude to dace given conspecific alarm cue alone (t-test, t18 = 0.796, P = 0.436; Fig. 4c). When conditioned fish were retested with predator odor only, dace previously conditioned with (alarm cue + rock bass odor) shoaled significantly more tightly than fish that had been previously conditioned with alarm cue only (t-test, t16 = 2.93, P = 0.010; Fig. 4d).
Shoaling behavioral responses by shoals of three redbelly dace. a. Mean ± 1 SE shoaling index for redbelly dace presented with water versus odor of allopatric rock bass. b. Mean ± 1 SE shoaling index for redbelly dace presented with water versus conspecific alarm cue. c. Mean ± 1 SE shoaling index for redbelly dace presented with conspecific alarm cue only, or alarm cue + odor of allopatric rock bass. d. Mean ± 1 SE shoaling index in response to rock bass odor alone for redbelly dace previously conditioned with conspecific alarm cue only, or alarm cue + odor of allopatric rock bass. ns, not significant p > 0.05, ** p ≤ 0.01, *** p ≤ 0.001
Discussion
The deepest levels of small, meromictic kettle lakes such as Deming Lake are permanently anoxic. Water with sufficient levels of dissolved oxygen to support piscivorous fish is commonly reduced or eliminated in many small lakes during winter ice-cover (Tonn and Magnuson 1982; Wellborn et al. 1996; Suski and Ridgway 2009) providing refuge habitat for small-bodies species such as redbelly dace, fathead minnows, and brook stickleback that are able to tolerate winter hypoxia. Long-term data sets (Foley et al. 2012; Jane et al. 2021) and climate models (Fang and Stefan 2009; Chapra et al. 2021) link increased global temperatures to increased duration and severity of hypoxic and anoxic conditions in freshwater habitats globally. However, we did not find any evidence of long-term temporal trends in ice-cover data from Deming Lake. Under severe conditions, antipredator behavior is affected by hypoxia (Domenici et al. 2007; Pink and Abrahams 2018; Strand et al. 2022), but the main effect of seasonal hypoxia in Deming Lake is extirpation of large-bodied piscivorous fish species that occasionally colonize the lake through intermittent connections with surrounding lakes. Long-term data sets such as the one for Deming Lake are important for monitoring ecological shifts in oxygen-dependent community dynamics over time, and for demonstrating variation in predator–prey interactions within fish communities of small lakes.
Northern redbelly dace demonstrated the ability to acquire recognition of a novel predator odor by associating the novel stimulus with conspecific alarm cues released from damaged epidermal tissue of conspecifics. This is the first demonstration of recognition learning for redbelly dace and extends the number of species for which this type of learning has been demonstrated. Recognition learning provides a flexible mechanism for adjusting to predator identities that vary spatially across the species’ range, temporally through ontogenetic time, and over ecological time scales within a site. The longitudinal pattern demonstrated for Deming Lake is typical of small freshwater bodies with intermittent connectivity to the surrounding watershed and low levels of dissolved oxygen over the winter (Tonn and Magnuson 1982; Wellborn et al. 1996). Rather than evolving genetic templates for specific predators, most fish have evolved a plastic learning mechanism that can respond and adapt quickly to changes in local predatory fauna. The major changes observed in fish community structure in the case study provided by Deming Lake illustrate that regular shifts in predator pressure are a predictable variable in the ecology and evolution of small freshwater lakes even if the ways in which predator communities may change is unpredictable. There are limits to the capacity of prey to adapt to new predators. Invasive and introduced predators as a result of human activity can overwhelm the capacity for prey to adapt if novel a predator introduces a new archetype and overwhelms the capacity for prey to adapt (Carthey and Blumstein 2018; Anton et al. 2020).
Although one centrarchid species, pumpkinseed sunfish, are regular and current residents in Deming Lake, they are presumably distinct biochemically from rock bass, also in the family Centrarchidae, evidenced by the absence of a response by redbelly dace to rock bass odor until after the dace had had opportunity to associate rock bass odor with conspecific alarm cues. In other studies, prey have been shown to generalize recognition from the model species to which they were conditioned to taxonomically similar predators that presumably have similar kairomone biochemistry (e.g., Ferrari et al. 2008; Brown et al. 2011b). One source of differences between these two species is that pumpkinseed sunfish prey on plankton and mollusks, but not fish (Berchtold et al. 2015). If pumpkinseed sunfish were predators of redbelly dace in Deming Lake, and generalization among Centrarchid species occurred, then we may have seen pre-existing recognition of rock bass odor in the current study. On the other hand, if a model species has been previously learned to be safe, then latent inhibition of learning causes prey to resist associating that stimulus with danger (Ferrari and Chivers 2006). If latent inhibition of learning and generalization between the two species had occurred, then redbelly dace would not quickly acquire association of risk with rock bass odor. Because redbelly dace rapidly acquired recognition of rock bass odor as an indicator of danger in this study, we have no evidence of generalization between pumpkinseed sunfish and rock bass by redbelly dace. Moreover, the two centrarchid genera, Ambloplites (rock bass) and Lepomis (pumpkinseed sunfish), are on disparate sub-branches of Centrarchid evolution that diverged 40 mya (Near and Kim 2021), and they came from different source lakes and have different diets (e.g., Ward et al. 2009), all of which may have contributed to rendering their kairomones sufficiently distinct that redbelly dace did not generalize from one to the other.
There are several scenarios that bring new predator species to prey communities. One is intermittent connection to other water bodies as demonstrated in the current study on Deming Lake. Another mechanism is through annual seasonal flooding in tropical river systems that bring predators from the river channel into contact with prey that live in the flood plain until predators follow receding flood waters back to the river channel (e.g., Winemiller and Jepsen 1998). Many fish species are diadromous, spending part of their life in freshwater and part of their life cycle in the ocean. Diadromous migration brings fish in contact with new predators and prey (Winemiller and Jepsen 1998). Many cyprinids migrate from lakes to spawning grounds in tributaries where they encounter predators that do not occur in lakes (Brönmark et al. 2008). Similarly, migratory movements of predators produce strong presence/absence cycles of predators to prey communities along the migration route (Andrews and Harvey 2013). In each of these scenarios, prey are required to quickly adapt to shifts in the predator community.
In summary, Deming Lake represents a case study of how fish community composition varies over time because of stochastic colonization and extirpation events, likely related to spring flooding that facilitates inter-lake connectivity (colonization) and the effect of winter ice cover on availability of dissolved oxygen (extirpation). Alarm cue-induced recognition learning provides a mechanism that allows one prey species, the redbelly dace, to track temporal changes in the predator community. This data set from one intensively studied lake, and one focal species, serves as an example of how abiotic and biotic components of aquatic ecosystems impact behavior and ecology of prey fish. Understanding processes that determine the environmental biology of fishes is important for effective management and conservation of fish communities (Cooke et al. 2023).
Data availability
Available upon request.
References
Andrews KS, Harvey CJ (2013) Ecosystem-level consequences of movement: seasonal variation in the trophic impact of a top predator. Mar Ecol Prog Ser 473:247–260. https://doi.org/10.3354/meps10095
Anton A, Geraldi NR, Ricciardi A, Dick JTA (2020) Global determinants of prey naiveté to exotic predators. Proc R Soc B Biol Sci 287:20192978. https://doi.org/10.1098/rspb.2019.2978
Berchtold AE, Colborne SF, Longstaffe FJ, Neff BD (2015) Ecomorphological patterns linking morphology and diet across three populations of pumpkinseed sunfish (Lepomis gibbosus). Can J Zool 93:289–297. https://doi.org/10.1139/cjz-2014-0236
Bouvier LD, Cottenie K, Doka SE (2009) Aquatic connectivity and fish metacommunities in wetlands of the lower Great Lakes. Can J Fish Aq Sci 66:933–948. https://doi.org/10.1139/F09-050
Brönmark C, Skov C, Brodersen J, Nilsson PA, Hansson LA (2008) Seasonal migration determined by a trade-off between predator avoidance and growth. PLoS One 3:e1957. https://doi.org/10.1371/journal.pone.0001957
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish 4:227–234. https://doi.org/10.1046/j.1467-2979.2003.00132.x
Brown GE, Ferrari MCO, Chivers DP (2011a) Learning about danger: chemical alarm cues and threat-sensitive assessment of predation risk by fishes. In: Brown C, Laland K, Krause J (eds) Fish Cognition and Behavior. Wiley-Blackwell, Oxford, UK, pp 59–80
Brown GE, Ferrari MC, Malka PH, Russo S, Tressider M, Chivers DP (2011b) Generalization of predators and nonpredators by juvenile rainbow trout: learning what is and is not a threat. Anim Behav 81:1249–1256. https://doi.org/10.1016/j.anbehav.2011.03.013
Brown GE, Demers EE, Goldman JA, Singh A, Chivers DP, Ferrari MCO (2020) Unpredictable risk enhances induced neophobia in northern red-bellied dace. Anim Behav 168:121–127. https://doi.org/10.1016/j.anbehav.2020.08.012
Carthey AJ, Blumstein DT (2018) Predicting predator recognition in a changing world. Trends Ecol Evol 33:106–115. https://doi.org/10.1016/j.tree.2017.10.009
Chapra SC, Camacho LA, McBride GB (2021) Impact of global warming on dissolved oxygen and BOD assimilative capacity of the world’s rivers: modeling analysis. Water 13:2408. https://doi.org/10.3390/w13172408
Chivers DP, Smith RJF (1994) The role of experience and chemical alarm signalling in predator recognition by fathead minnows, Pimephales promelas. J Fish Biol 44:273–285.https://doi.org/10.1111/j.1095-8649.1994.tb01205.x
Chivers DP, Smith RJF (1995) Free—living fathead minnows rapidly learn to recognize pike as predators. J Fish Biol 46:949–954.https://doi.org/10.1111/j.1095-8649.1995.tb01399.x
Cochran PA, Lodge DM, Hodgson JR, Knapik PG (1988) Diets of syntopic finescale dace, Phoxinus neogaeus, and northern redbelly dace, Phoxinus eos: a reflection of trophic morphology. Environ Biol Fish 22:235–240. https://doi.org/10.1080/02705060.1984.9664641
Cooke SJ, Auld HL, Birnie-Gauvin K, Elvidge CK, Piczak ML, Twardek WM, Raby GD, Brownscombe JW, Midwood JD, Lennox RJ, Madliger C, Wilson ADM, Binder TR, Schreck CB, McLaughlin RL, Grant J, Muir AM (2023) On the relevance of animal behavior to the management and conservation of fishes and fisheries. Environ Biol Fish 106:785–810. https://doi.org/10.1007/s10641-022-01255-3
Cox JG, Lima SL (2006) Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. TREE 21:674–680. https://doi.org/10.1016/j.tree.2006.07.011
Domenici P, Lefrançois C, Shingles A (2007) Hypoxia and the antipredator behaviours of fishes. Phil Trans R Soc B 362:2105–2121. https://doi.org/10.1098/rstb.2007.2103
Dupuch A, Magnan P, Dill LM (2004) Sensitivity of northern redbelly dace, Phoxinus eos, to chemical alarm cues. Can J Zool 82:407–415. https://doi.org/10.1139/z04-003
Dupuch A, Magnan P, Bertolo A, Dill LM, Proulx M (2009) Does predation risk influence habitat use by northern redbelly dace Phoxinus eos at different spatial scales? J Fish Biol 74:1371–1382. https://doi.org/10.1111/j.1095-8649.2009.02183.x
Fang X, Stefan HG (2009) Simulation of climate effects on water temperature, dissolved oxygen, and ice and snow covers in lakes of the continuous U.S. under past and future climate scenarios. Limnol Oceanogr 54:2359–2370. https://doi.org/10.4319/lo.2009.54.6_part_2.2359
Ferrari MCO, Chivers DP (2006) The role of latent inhibition in acquired predator recognition by fathead minnows. Can J Zool 84:505–509. https://doi.org/10.1139/z06-027
Ferrari MCO, Messier F, Chivers DP (2008) Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proc Roy Soc b: Biol Sci 275:1811–1816. https://doi.org/10.1098/rspb.2008.0305
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724. https://doi.org/10.1139/Z10-029
Foley B, Jones ID, Maberly SC, Rippey B (2012) Long-term changes in oxygen depletion in a small temperate lake: effects of climate change and eutrophication. Freshw Biol 57:278–289. https://doi.org/10.1111/j.1365-2427.2011.02662.x
Guimarães TFR, Hartz SM, Becker FG (2014) Lake connectivity and fish species richness in southern Brazilian coastal lakes. Hydrobiologia 740:207–217. https://doi.org/10.1007/s10750-014-1954-x
Jackson DA, Peres-Neto PR, Olden JD (2001) What controls who is where in freshwater fish communities — the roles of biotic, abiotic, and spatial factors. Can J Fish Aq Sci 58:157–170. https://doi.org/10.1139/f00-239
Jane SF, Hansen GJA, Kraemer BM, Leavitt PR, Mincer JL, North R, Pilla RM, Statler JT, Williamson CE, Woolway RI, Arvola L, Chandra S, DeGasperi CL, Diemer L, Dunalska J, Erina O, Flaim G, Grossart H-P, Hambright KD, Hein C, Hejzlar J, Janus LJ, Jenny J-P, Jones JR, Knoll LB, Leoni B, Mackay E, Matsuzaki S-IS, McBride C, Müller-Navarra DC, Paterson AM, Pierson D, Rogora M, Rusak JA, Sadro S, Saulnier-Talbot E, Schmid M, Sommaruga R, Thiery W, Verburg P, Weathers KC, Weyhenmeyer GA, Yokota K, Rose KC (2021) Widespread deoxygenation of temperate lakes. Nature 594:66–70. https://doi.org/10.1038/s41586-021-03550-y
Johnson JH, Johnson EZ (1984) Comparative diets of subyearling redbreast sunfish and subyearling northern redbelly dace in an Adirondack Lake. J Freshw Ecol 2:587–591. https://doi.org/10.1080/02705060.1984.9664641
Kelley JL, Magurran AE (2003) Learned predator recognition and antipredator responses in fishes. Fish 4:216–226. https://doi.org/10.1046/j.1467-2979.2003.00126.x
Kenney JW (2020) Associative and nonassociative learning in adult zebrafish. In: Gerlai RT (ed) Behavioral and Neural Genetics of Zebrafish (pp. 187–204). Academic Press. https://doi.org/10.1016/B978-0-12-817528-6.00012-7
Magnuson JJ, Tonn WM, Banerjee A, Toivonen J, Sanchez O, Rask M (1998) Isolation vs. extinction in the assembly of fishes in small northern lakes. Ecology 79:2941–2956. https://doi.org/10.1890/0012-9658(1998)079[2941:IVEITA]2.0.CO;2
Magurran AE (1989) Acquired recognition of predator odour in the European minnow (Phoxinus phoxinus). Ethology 82:216–223. https://doi.org/10.1111/j.1439-0310.1989.tb00501.x
Magurran AE (1990) The adaptive significance of schooling as an anti-predator defence in fish. Ann Zool Fennici 27:51–66. https://www.jstor.org/stable/23736019
Magurran AE, Pitcher TJ (1987) Provenance, shoal size and the sociobiology of predator-evasion behaviour in minnow shoals. Proc Roy Soc Lond Ser B Biol Sci 229:439–465. https://doi.org/10.1098/rspb.1987.0004
Miranda LE (2005) Fish assemblages in oxbow lakes relative to connectivity with the Mississippi River. Trans Am Fish Soc 134:1480–1489. https://doi.org/10.1577/T05-057.1
Naud M, Magnan P (1988) Diel onshore–offshore migrations in northern redbelly dace, Phoxinus eos (Cope), in relation to prey distribution in a small oligotrophic lake. Can J Zool 66:1249–1253. https://doi.org/10.1139/z88-182
Near TJ, Kim D (2021) Phylogeny and time scale of diversification in the fossil-rich sunfishes and black basses (Teleostei: Percomorpha: Centrarchidae). Mol Phylogenet 161:107156. https://doi.org/10.1016/j.ympev.2021.107156
Pink M, Abrahams MV (2018) In shallow water ecosystems the abiotic environment is more important than prey abundance for foraging terns. Environ Biol Fish 101:355–362. https://doi.org/10.1007/s10641-017-0644-8
Scott WB, Crossman EJ (1973) Freshwater fishes of Canada. Fisheries Research Board of Canada, Bulletin no. 84. 966. https://publications.gc.ca/site/eng/9.870340/publication.html
Strand MC, DeVriendt IG, Seigel AR, Merkord CL, Wisenden BD (2022) Hypoxia constrains behavioral responses to chemical alarm cues by fathead minnows Pimephales promelas. Environ Biol Fish 105:1509–1517. https://doi.org/10.1007/s10641-022-01235-7
Suboski MD (1990) Releaser-induced recognition learning. Psychol Rev 97:271. https://doi.org/10.1037/0033-295X.97.2.271
Suski CD, Ridgway MS (2009) Winter biology of centrarchid fishes. In: Cooke SJ, Philipp DP (eds) Centrarchid fishes: biology, diversity, and conservation. Blackwell-Wiley, Ames, IA, pp 264–292
Swanner ED, Lascu I, Harding C, Ledesma G, Leung T, Akam S (2022) Water properties of Arco Lake, Budd Lake, Deming Lake, and Josephine Lake in Itasca State Park from 2006-2009 and 2019-2021. ver 3. Environmental Data Initiative. 10.6073/pasta/a0fef2010734ea5f77ac7f2cf8ad1729 (Accessed 2023-06-24).
Tonn WM, Magnuson JJ (1982) Patterns in the species composition and richness of fish assemblages in northern Wisconsin lakes. Ecology 63:1149–1166. https://doi.org/10.2307/1937251
Ward AJW, Webster MM, Magurran AE, Currie S, Krause J (2009) Species and population differences in social recognition between fishes: a role for ecology? Behav Ecol 20:511–516. https://doi.org/10.1093/beheco/arp025
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Ann Rev Ecol Syst 27:337–363. https://doi.org/10.1146/annurev.ecolsys.27.1.337
Winemiller KO, Jepsen DB (1998) Effects of seasonality and fish movement on tropical river food webs. J Fish Biol 53:267–296. https://doi.org/10.1111/j.1095-8649.1998.tb01032.x
Wisenden BD (2015) Chemical cues that indicate risk of predation. In: Sorensen PW, Wisenden BD (eds) Fish Pheromones and Related Cues, Wiley-Blackwell Press, Ames, IA, 131–148 https://doi.org/10.1002/9781118794739.ch6
Wisenden BD, Barbour KA (2005) Antipredator responses to skin extract of redbelly dace by free-ranging populations of redbelly dace and fathead minnows. Environ Biol Fish 72:227–233. https://doi.org/10.1007/s10641-004-8753-6
Wisenden BD, Chivers DP (2006) The role of public chemical information in antipredator behaviour. In: Ladich F, Collins SP, Moller P, Kapoor BG (eds) Fish Communication. Science Publisher, NH, pp 259–278
Acknowledgements
Fish sampling in Deming Lake in 2023 was conducted under Minnesota Department of Natural Resources special permit number 35313 awarded to Dan Brumm on behalf of the University of Minnesota Itasca Biological Field Station and Laboratory. We are grateful to Dan Brumm, Lindsey Blake, and Jonathan and Emily Schilling for logistical support.
Author information
Authors and Affiliations
Contributions
JCS: experimental design and data collection of the redbelly dace learning experiment; BDW: mentorship, supervised collection of catch data 2000–2023, data analysis, wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Animal collection was conducted under Minnesota Department of Natural Resources special permit number 35313. Experimental protocols used in this experiment were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee protocol number 2103-38900A and comply with the US National Research Council’s Guide for the Care and Use of Laboratory Animals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soukup, J.C., Wisenden, B.D. Predator recognition learning by northern redbelly dace Chrosomus eos from a small kettle lake with a dynamic predator community. Environ Biol Fish 106, 2193–2204 (2023). https://doi.org/10.1007/s10641-023-01500-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01500-3