Abstract
Understanding the spatial ecology of fish is critical for effective conservation and management. Gar (Lepisosteidae spp.) are an ancient lineage of freshwater fishes that have long been thought of as having high site fidelity with limited large-scale movements. We acoustically tagged Longnose Gar (L. osseus) in a large freshwater embayment (Hamilton Harbour) and tracked the movements of 12 individuals within this system and throughout the coastal waters of Lake Ontario for up to 4 years. Three distinct movement patterns were observed: migrants (N = 7), which moved throughout much of western Lake Ontario and had a maximum displacement of 184 km (linear distance); residents (N = 3), which remained in or near the original tagging location (an industrial slip with year-round warmwater outfall); and sporadic migrants (N = 2), which showed both migratory and resident behavioural patterns in different years. Seasonally, gar were most active during the summer and then fall, with more restricted home ranges and movements during winter suggestive of more quiescent behaviour. This study represents the first use of acoustic telemetry to track Longnose Gar movements year-round in a large lake and confirmed that a majority of tracked fishes make large-scale movements in Lake Ontario. Such results reframe the presumption that gar are more resident fishes with restricted space use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Movement is an intrinsic part of the ecology of fishes and thus understanding a species’ spatial ecology aids effective conservation, fisheries management, and ecosystem-based monitoring (Allen and Singh 2016; Cooke et al. 2022). Quantifying and describing the spatial ecology of fishes can yield new knowledge to assist management in a number of ways (Cooke et al. 2016). For example, movement and migration pathways can reveal movement corridors or aggregations that may need protection (Hayden et al. 2014; Brownscombe et al. 2019), emigration or residency within areas can influence population estimates and demographics informing harvest regulations (Taylor et al. 2013; Dudgeon et al. 2015; Gatch et al. 2022), and different spatial behaviours can reveal variations in habitat use or susceptibility to fisheries, to guide habitat management (Daly et al. 2019; Espinoza et al. 2021). Acoustic telemetry is a useful and increasingly popular tool to track and study the spatial ecology of fishes (Matley et al. 2022), with the added benefit that it can also provide information during times of year when it is difficult to sample and study fish (i.e., winter, deeper waters; Marsden et al. 2021). With the potential for near-continuous detections using acoustic telemetry, a more holistic understanding of a species’ movements and spatial ecology is possible (Brownscombe et al. 2022).
A family with relatively limited spatial ecology information are gar (Lepisosteidae spp.). Gar are primitive piscivorous fish that are often associated with warm, shallow, vegetated waters of lakes and large rivers (Scott and Crossman 1998). Longnose Gar (Lepisosteus osseus) are among the more common gar species and are distributed from Mexico north through the eastern USA into Canada, including throughout coastal waters of the lower Laurentian Great Lakes. Longnose Gar are presumed to be highly resident and can make spawning migrations up streams (Scott and Crossman 1998). Specifically in Great Lakes coastal wetlands, they have been characterized as permanent wetland residents (Jude and Pappas 1992) based on the assumption that they have limited spatial extents. While in most of their range little is known about the movement of Longnose Gar, there are a few studies in the eastern USA that document fairly extensive movements. Six marked Longnose Gar in Weaubleau Creek, Missouri, were later recaptured by anglers that same year, up to 48 km away and an average of 27 km away (Johnson and Noltie 1996). Using radio and acoustic telemetry, two Longnose Gar were tracked for just over a year and moved ~ 70 km from their tagging location in Chesapeake Bay tributaries (McGrath et al. 2012). These longer distance gar movements appeared to be seasonal and associated with temperature, photoperiod, and hydrological changes (McGrath 2010); however, the proportion of the population that exhibited these larger movements and their annual repeatability are unknown. Such evidence for larger scale movements by Longnose Gar challenges their characterization as a sedentary, resident species.
Hamilton Harbour is a large (21.5 km2), protected embayment surrounded by urban and industrial land use, that lies at the western end of Lake Ontario. It was listed as a Great Lake Area of Concern (AOC) in 1985 due to historic and ongoing habitat loss, ecosystem degradation, and impairments to fish and wildlife populations (COA 1992). The system still provides habitat for numerous fish species, including Longnose Gar, but population objectives, including those for piscivores, are still not being met (Boston et al. 2016). Observational data collected at an industrial slip (quay), the Ottawa St. Slip, in Hamilton Harbour, show that Longnose Gar are present year round, and are likely attracted to an industrial warm water discharge and abundant forage fish found there (Smith 2006). However, it is unknown whether individual Longnose Gar remain solely in the harbour in these nearshore habitats, use Lake Ontario to transit between wetland habitats, or whether the outer lake plays a larger role in their lifecycle and ecology. Hamilton Harbour has also been the site of a multi-year acoustic telemetry project where a variety of top-predator, non-native, and ecologically important fish species have been tagged and tracked since 2015 to understand their spatial ecology (see Brooks et al. 2017). Similarly, the use of acoustic telemetry in Lake Ontario has been increasing with an expanding receiver array designed to answer larger-scale movement questions (see Larocque et al. 2022). Thus, using acoustic telemetry provides an excellent opportunity to study the spatial ecology of Longnose Gar in Hamilton Harbour and Lake Ontario.
Understanding fish movement patterns can help improve fish community management as a whole. More specifically, understanding the timing and extent of Longnose Gar immigration and emigration out of the harbour can provide insight on drivers of fish movement in this AOC. Generally, Longnose Gar are associated with shallow, vegetated, warm water habitat but can exhibit both short- and long-distance movements in a stream or estuarine populations (Johnson and Noltie 1996; McGrath et al. 2012). The extent to which those movements are observed in coastal wetlands and a large (great) lake are unclear. To that end, the objective of this study was to characterize the movement ecology of adult Longnose Gar in the coastal waters of Lake Ontario over a four year period. More specifically, we used acoustic telemetry to document the seasonal and inter-annual residence, movement speed, distance, and depth use of Longnose Gar captured in Hamilton Harbour and also explored the consistency in movement patterns by individuals.
Methods
Acoustic telemetry array
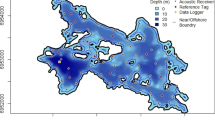
Detection data were available for fish from June 29, 2016, to April 25, 2020. The array in Hamilton Harbour in 2016 consisted of 32 acoustic receivers (VR2W or VR2W-AR 69 kHz, Innovasea, Bedford, Nova Scotia; Fig. 1). Over time the array expanded to a total of 59 receivers by 2020. Similarly in Lake Ontario, the array expanded in the western and central basin from 12 in 2016 to 89 receivers by 2020, with additional receivers in Toronto Harbour and Niagara River region (Fig. 1). See Brooks et al. (2019) and Larocque et al. (2022) for descriptions of receiver moorings in Hamilton Harbour and Lake Ontario, respectively.
Capture and tagging
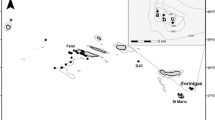
Capture and tagging Longnose Gar followed the methods from Midwood et al. (2018). In brief, 15 Longnose Gar were captured June 29 and 30, 2016, using boat electrofishing (Smith-Root SR 21EH work boat, 7.5 kW Generator Powered Pulsator) in the Ottawa St. Slip (Fig. 2), which is situated along the south shore of Hamilton Harbour at the western tip of Lake Ontario (Fig. 1; 43.27387° N, − 79.80693° W). Prior to surgery, gar were anesthetized using a solution of clove oil emulsified in ethanol (with eugenol as the active ingredient) in a 1:10 ratio at a dose of 0.065 mL/L. The fish was then placed in a v-shaped foam trough (supine), while lake water was pumped over their gills. Fish were measured for mass (g) and total length (mm). To get into the body cavity along the ventral midline, between the pectoral and pelvic fins, a 18-mm cutting disk on a rotary tool was used to cut through the ganoid scales, and a scalpel (no. 21) to breach the body cavity. Surgical scissors were used to increase the length of the incision to ~ 40 mm to allow for the insertion of an acoustic transmitter (hereafter referred to as tag; V13P, 13 mm × 39 mm, 11 g in air, 69 kHz, mean delay = 200 s, max depth reading = 34 m, estimated tag life = 1388 d; InnovaSea, Bedford, Nova Scotia). To allow for two sutures (3–0 monofilament with a reverse cutting needle) to close the incision, four holes were drilled using a 1.5-mm drill bit in the rotary tool, and each suture was threaded through two holes prior to tying off with a surgeon’s knot (3–3-2). Prior to surgery, all surgical tools were disinfected using povidone iodine. Fish recovered from anaesthesia for 10–20 min in a live well pumped continuously with freshwater prior to release. Animal care approval for this study was provided by the Carleton University Animal Care Committee under permit 102935.
Seasonal designations
Temperature profiles were collected using a temperature logger chain that was deployed annually at the centre of Hamilton Harbour from early-spring to late-fall (43.288° N, − 79.845° W). Season was defined by temperature dynamics and thermocline delineation after Larocque et al. (2020): spring (> 5 °C and warming isothermal), summer (established thermocline), fall (first full water column mixing), and winter (temperature is no longer declining and < 5 °C isothermal). Temperature profiles were unavailable in the harbour after 2018, and seasons were based on the mean Julian day of seasonal delineation in the harbour from Larocque et al. (2020): spring — April 25 to June 6, summer — June 7 to October 3, fall — October 4 to November 17, and winter — November 18 to April 24. Although seasonal designations may vary for fish outside of the harbour in Lake Ontario, this was deemed to be a suitable approximation.
Data preparation
All data preparation and analyses were conducted in R version 4.0.2 (R Core Team 2020). Detection data collected from 2016 to 2020 were used for analyses. Data were filtered to remove fish that were presumed dead (or had expelled tags). Fish were inferred to be dead if they continuously exhibited constant depth-use profiles and stayed within the same area of the array (potentially detected on multiple receivers all within the same vicinity; Klinard and Matley 2020). If fish were alive for a period > 1 month prior to suspected mortality, then only the suspect data were removed. Fish with < 1 month of detections were removed from analyses. All instances of depth sensor malfunctions were removed from the dataset. Depth sensor malfunctions would sometimes occur at the end of a tag’s battery life and would incorrectly indicate the fish was at the maximum depth value the tag can sense (34 m). Data that met the criteria for false detections were also excluded from our analyses (Pincock 2012), as were data in which tags were detected on the same receiver earlier than the minimum ping rate of the tags. Overall, detection data from 12 tagged gar were included in the analyses (Table 1).
Due to the close proximity of receivers in Hamilton Harbour, a single ping from a tag could be heard on multiple receivers. Thus, to best associate the fish with a single receiver’s location, we subset detections so only the first receiver to detect that tagged fish were included and any other detections before the minimum lag time to another ping were removed. Otherwise, a tag could potentially be heard at further receivers where the fish was not actually present and skew residency and movement analyses.
Data analyses
Movement groupings
Visualization of gar movements and paths over the study duration revealed three distinct movement patterns (Supplementary Figs. 1A-L). Each gar was assigned to a movement group to further describe and analyse movement patterns. Differences in depth use by movement groups and seasons (and their interaction) were assessed using a general linear mixed model (GLMM) using tag ID and year as random effects and corAR1 temporal correlation structure, using the ‘lme’ function in nlme package (Pinheiro and Bates 2000). Based on autocorrelation function (ACF) plots, mean daily depths showed strong temporal autocorrelation, even with corAR1 incorporated. To remove temporal autocorrelation, data were subset by taking a random depth value each week for each tagged fish and the analyses were run again with a temporal correlation structure as indicated above. A type II analyses of deviance (which uses a Wald chi-square test statistic) was used to determine significance of the predictor variables (e.g., season, group, interaction) in the GLMM. If either group, season, or their interaction were significant, a Tukey post hoc test was conducted using the ‘emmeans’ function in emmeans package (Lenth 2023) to determine differences among the categorical groups of the significant variables.
Harbour residency
Seasonal mean residency within Hamilton Harbour for each movement group was calculated based on each individual’s residency. Residency was calculated as the number of distinct days detected at each receiver location divided by the total distinct days detected within a given season using the ‘residence index’ function in the glatos package (Holbrook et al. 2020). Any detections on receivers outside of the harbour were given a single ‘location’ designated as outside the harbour to determine residency when not in the harbour, and not focused on a location per se. Detections within a season, across years, were combined for easier cross season comparison. Within the harbour, the array coverage is relatively thorough so when a fish is not detected for a day, it is likely in the same area it was last detected, and structure is likely obscuring detections. To account for this, any day a fish was not detected between known detections it was given a single daily detection at the last position it was detected (Last Position Carried Forwards; LPCF), which was then incorporated into the residency analyses. Mean seasonal residency within the harbour for each movement group was determined and plotted.
Lake-wide home range
For migrant Longnose Gar, seasonal core and general home range or 50% and 95% kernel utilization distribution (KUD) were estimated using the ‘kernalUD’ function in the adehabitatHR package (Calenge 2006). When fish were not in the harbour, we did not have confidence that the fish was within the last position it was detected and we did not adjust with LPCF. However, to adjust for variable receiver coverage that resulted in high detectability in Hamilton Harbour and lower detectability in Lake Ontario, we reduced the dataset to daily detections at a receiver to calculate the KUDs. Tagged individuals had their home range plotted as a semi-transparent layer, such that overlapping individual home ranges appear darker on the figures to better understand trends across individuals.
Lake-wide movement patterns
Migration movement patterns via routes, duration, site fidelity, and repeatability were determined based on visualization of individual movements and paths over the study duration. Maximum spatial displacement (linear distance) from the tagging location in the Ottawa St. Slip was determined for each individual. It is important to note that actual movement distances were larger as this did not account for avoiding land or non-direct movements towards the furthest location detected from the tagging site.
Daily distance (i.e., speed, km/day) and total seasonal distance were determined when individual migrants were outside of Hamilton Harbour and the Niagara River. These two areas have dense arrays of receivers with overlapping detection ranges, such that even if a fish had not moved subsequent detections could occur on another receiver and give a false movement distance. Any detections in these areas were given a single location to determine distances and movement speeds of individuals in Lake Ontario proper. To get speed, distances between receivers from chronological detections were summed across each day, and then divided by the number of days between detections to get daily distance. For example, if the receiver distance between two detections was 10 km and the detections occurred 2 days apart, the daily distance would be 5 km. To determine migration speeds, instances of zero movements were removed for the analyses. Total distances in each season were summed for each individual per year. Both daily distances and total distances moved for migrants were separately compared across seasons using a GLMM with individuals and year as random effects with the ‘lmer’ function in lme4 package (Bates et al. 2015), to account for individual variation and the change in the receiver array over time. A type II analyses of deviance (which uses a Wald chi-square test statistic) was used to determine the significance of season as a predictor variable. If season was significant, a Tukey post hoc test was conducted to determine differences among seasons. For all statistical analyses, assumptions of normality and homoscedasticity were visually assessed using qqplots and fitted versus residual plots, respectively; ACF plots verified the assumption of independence via temporal autocorrelation, and significance was determined at α = 0.05.
Results
Movement groups
Three distinct movement groups were observed based on the duration of time spent in Hamilton Harbour: residents, migrants, and sporadic migrants (Supplementary Figs. 1A-L and 2A-L). Three of twelve individuals (25%) were considered residents, in which they almost fully resided in Hamilton Harbour, specifically at or near the location of tagging. Seven individuals (58%) left the harbour annually or would make large spatial movements on an annual basis and were considered migrants. Lastly, two individuals (17%) were considered sporadic migrants as they were resident in Hamilton Harbour for over a year but in another year would emigrate, with no particular pattern. Overall, 75% (n = 9 of 12) of tagged gar made long-distance excursions out of the harbour at some point during the 4 year tracking period. The mean total length and mass appeared to be slightly smaller in sporadic migrants (mean: 716 mm and 825 g, n = 2) than in residents (mean: 792 mm and 1220 g, n = 3) or migrants (mean: 844 mm and 1396 g, n = 7).
Depth use varied significantly by both movement group (χ22 = 27.139, p < 0.001), season (χ23 = 307.613, p < 0.001), and their interaction (χ26 = 111.069, p < 0.001, R2 = 0.62). Residents remained shallow (~ 0.5 m) year-round; migrants were shallow in the spring and summer (~ 1 m), and were deeper in the fall (~ 3.5 m) and winter (~ 6.5 m)'; and sporadic migrants were shallow (~ 0.5 m) from spring to fall and used deeper waters (~ 3 m) in the winter only (Fig. 3).
Seasonal depth use of different movement groups of Longnose Gar. Different letters indicate significant differences across groups and seasons. Lower and upper box boundaries are the 25th and 75th percentiles, respectively; line inside box is the median; lower and upper error lines are the lower box boundary − 1.5 * inter-quartile range (IQR) and upper box boundary + 1.5*IQR, respectively. Coloured squares indicate mean values
Harbour residency
Seasonally, resident fish were almost entirely in the Ottawa St. Slip of Hamilton Harbour at consistent shallow depths year-round (Fig. 4). Similarly, sporadic migrants had highest residency year-round in the Ottawa St. Slip; however, there was a slight increase in residency in the east end in the summer and fall and in the west end in the fall and winter when using deeper depths (Fig. 4). Conversely, for migrants, residency in the spring and summer was greatest outside of the harbour, followed by the Ottawa St. Slip when at shallower depths (Fig. 4). In the fall, residency was still highest outside of the harbour but increased in the harbour relative to spring or summer. Finally, in the winter, migrant residency was roughly split between the west end of the harbour and outside of the harbour (near the Niagara River) when at their deepest depths (Fig. 4).
Seasonal mean residency index (RI) at each receiver location in Hamilton Harbour based on detections of acoustically tagged Longnose Gar (N = 12) of different movement groups. Size increases with increased RI. Colours were added to help distinguish the high RI values where brighter/warmer colours indicate higher RI. The single point to the east of Hamilton Harbour represents all points outside of the harbour
Detections by migrants in the Ottawa St. Slip generally occurred in the spring and/or summer (Supplementary Figs. 2A-L). When migrants returned to Hamilton Harbour, they were all detected at some point in the Ottawa St. Slip, but for variable durations.
Lake Ontario home range and movement patterns
Changes occurred seasonally for both core (50% KUD) and general (95% KUD) home ranges of migrant Longnose Gar. Seasonally, core and general home ranges of migrant Longnose Gar were smallest in winter, occurring in the western end of Hamilton Harbour and near the Niagara River (Fig. 5). In spring, there was a slight expansion of home ranges compared to winter, with further expansion in summer, which had the largest seasonal home ranges. These expansions occurred in southwestern Lake Ontario and also along the south central part of Lake Ontario near Rochester, NY (Fig. 5). In fall, home ranges started contracting and were more focused along the southern shoreline between Hamilton Harbour and the Niagara River, as well as near Toronto.
When individual detection pathways were assessed across years, migrant Longnose Gar exhibited different migration and movement patterns (Fig. 6). Four of seven (57%) migrants showed annual site fidelity to Hamilton Harbour, three returning every winter and spring (Tag IDs 15202, 15211, 15212, 15213) with one migrant returning in the spring and summer (15209; Fig. 6 — top panel; Supplementary Figs. 1A-L; Supplementary Fig. 2A-L). Some migrants did not return to Hamilton Harbour and appeared to disperse eastward over the years (15210; Fig. 6 — middle panel) or remain in the Niagara River region for over a year (15203). One migrant dispersed to Toronto in the first year of tagging and was never detected afterwards (15214), while one sporadic migrant went to Toronto and returned and remained in Hamilton Harbour for two years (15217; Fig. 6 — bottom panel). In total, four of 12 (33%) tagged Longnose Gar were detected at Toronto, specifically three of seven (43%) migrants and one of two (50%) sporadic migrants. Longnose Gar detected in Toronto visited a number of areas including the inner harbour quays, Toronto Islands, and outer harbour embayments, but generally only staying for several days before leaving again. Most tagged Longnose Gar (7 of 12; 58%) were detected in the Niagara River region with six of them spending time in the river proper (six of seven migrants and one of two sporadic migrants). Most migrant and sporadic migrants maximum displacement distances were ~ 60 km — roughly the distance to Niagara River (Table 1). Only three migrant Longnose Gar were detected in 2019 when the array expanded beyond the Niagara River, and two were detected as far as Rochester, NY (15209 and 15210 with maximum displacement distances of 156 and 184 km, respectively).
Example of long distance, annual movement paths (as the crow flies at receiver sites) by Julian date of migrant (ID 15209 and 15210) and sporadic migrant (ID 15217) Longnose Gar tagged in Hamilton Harbour. Some long distance migrants showed annual site fidelity, returning each year to Hamilton Harbour (15209), while others may move long distances each year without site fidelity (15210), or vary annually between short- or long-distance movements (15217). Note, as the receiver array (grey dots) expanded over the years, Longnose Gar could be detected further east in Lake Ontario
When moving long-distance in Lake Ontario (excluding days with zero movement), outside of Hamilton Harbour or the Niagara River region (areas with close groups of receivers), migrant Longnose Gar varied seasonally in their movement speed (χ23 = 11.029, p = 0.012, R2 = 0.04). Longnose Gar moved slower in winter (2.6 ± 3.9 km/day) compared to summer (16.7 ± 4.2 km/day), while movement speed did not vary for spring (13.0 ± 4.6 km/day) and fall (15.2 ± 4.5 km/day) from any other season (Fig. 7A). When both offshore and nearshore receivers were deployed in Lake Ontario (i.e., after 2017), Longnose Gar tended to be detected more on nearshore receivers (~ 20 m depth contour) than on offshore, pelagic receivers; Figs. 1 and 6; Supplementary Figs. 1A-L). The maximum movement speed detected among Longnose Gar was 74.0 km/day (Tag ID 15209 in Sept 2019 moving from Niagara River east towards Rochester, NY), although across seasons on average they moved 13.5 ± 6.3 km/day. Total distances moved in Lake Ontario by migrant Longnose Gar also varied seasonally (χ23 = 19.213, p < 0.001, R2 = 0.23). Longnose Gar generally travelled further in summer (162.7 ± 32.3 km) compared to spring (27.7 ± 32.3 km) and winter (12.0 ± 32.3 km), but not fall (104.7 ± 32.3 km; Fig. 7B).
Model predicted seasonal differences in A daily movement speeds (km/day) and B total distances moved by migrant Longnose Gar in Lake Ontario. Different letters indicate significant differences across seasons. Lower and upper box boundaries are the 25th and 75th percentiles, respectively; line inside box is the median, lower, and upper error lines are the lower box boundary − 1.5* inter-quartile range (IQR) and upper box boundary + 1.5*IQR, respectively. Red points indicate the mean
Discussion
This study represents the first use of acoustic telemetry to document the spatial ecology of Longnose Gar in a large lake. Three distinct movement patterns were observed over the study period. Longnose Gar in Hamilton Harbour were primarily migrants, making large spatial movements, with a smaller proportion of Longnose Gar either being residents of where they were tagged or sporadic migrants that exhibited the characteristics of residents and migrants across different years. Although previous studies in different aquatic systems with Longnose Gar did observe large-scale movements (roughly 50–70 km; Johnson and Noltie 1996; McGrath et al. 2012), the larger extent (max 184 km displacement) and annual repeatability with seasonal patterns in horizontal and vertical spatial use has not been documented. Similar movement patterns were found with juvenile Alligator Gar (Atractosteus spatula) in the Mississippi floodplains, with fish having either strong fidelity to their tagging/release location (70% of radio-tagged juveniles) or exhibiting larger movement patterns (30% of radio-tagged juveniles; Solomon et al. 2013). The Longnose Gar movement patterns in this study support Clark's (1968) contingent hypothesis, where different contingents, or groups of a population exhibit different migratory patterns based on factors such as genetics, environment, and behaviour (Secor 1999). Although it is still unclear what drives migrant Longnose Gar to move beyond Hamilton Harbour, we found no differences in length or mass of the fish between movement groups; it is unclear if age or sex (which were not recorded) of the fish would influence Longnose Gar movement patterns. Due to the size of the acoustic transmitter, only adult fish were tagged so it is possible juveniles may exhibit different patterns of site fidelity and movement. Regardless, the different movement patterns observed in the present study explain the past characterization of Longnose Gar as permanent residents in Great Lakes coastal wetlands (Jude and Pappas 1992) since, even if the migratory component of the population was missed, resident or sporadic migrants could still be captured during routine biological monitoring activities.
In Hamilton Harbour, the Ottawa St. Slip has a warmwater outfall that provides a unique, although unnatural habitat for fish. Both resident and sporadic migrant Longnose Gar could be found in the Ottawa St. Slip year-round; however, returning migrants would also be detected in the slip, at least for a short period in spring and early summer. Winter water temperatures in Hamilton Harbour range from 2 to 4 °C, whereas the Ottawa St. Slip temperatures hover around 16 °C (Smith 2006; Gertzen et al. 2016). In general, slip temperatures are roughly 12–14° C warmer in the winter and 3–10° C warmer in the summer compared to Hamilton Harbour (Smith, P.D unpublished data; Smith 2005, 2006). During a 3-year (2004–2006) observational study in the Ottawa St. Slip, Longnose Gar were present year-round with the highest counts in the winter and spring (Smith 2006). Counts of over 100 individuals were not unusual in the winter, with multiple age classes present (size ranges of 300–900 mm; Smith 2006). The Ottawa St. Slip likely provides all necessary aspects for some Longnose Gar to complete their lifecycle and sufficient forage base for a portion of the Longnose Gar population; hence, residents can have a smaller home range over multiple years.
Direct evidence of Longnose Gar spawning in the Ottawa St. Slip has not been observed, but anecdotally eggs attached to rocks have been observed (J. Midwood, pers. comm.) coincident with the Longnose Gar spawning window (19 °C; May–July; Holm et al. 2022). Another potential spawning area is Cootes Paradise Marsh, which lies at the western end of Hamilton Harbour but is separated from the harbour by a fishway designed to exclude Common Carp (Cyprinus carpio; Royal Botanical Gardens 1998). Only ten Longnose Gar were caught at the fishway trying to enter Cootes Paradise Marsh from 1996 to 2021 (Bendo et al. 2021), and none of the tagged fish were found in the marsh. There are several potential explanations for these observations that warrant further study including the spacing of bars in the fishway barrier limiting Longnose Gar passage (although juveniles could get through the barrier), a distinct sub-population of Longnose Gar that uses Cootes Paradise Marsh, or our limited sample size did not include the proportion of Longnose Gar that frequent the marsh. Regardless of where else Longnose Gar may be spawning in the harbour, the conditions in the Ottawa St. Slip are clearly an attractant for gar and its role as a source or sink for gar populations should be explored further.
The spatial ecology of fishes during winter is an understudied time of year (Cunjak 1996; Marsden et al. 2021) but we were able to observe movement and depth use of Longnose Gar during this season. Resident Longnose Gar were found predominantly in the Ottawa St. Slip during the winter months, whereas migrants used either the western portion of Hamilton Harbour, the south shore of Lake Ontario, or the Niagara River. All tagged Longnose Gar were found to have smaller core home ranges in the winter compared to other seasons. Movement speeds and distances traversed by migrant Longnose Gar in Lake Ontario were also slowest and smallest, respectively, during the winter. While some fishes actively forage during the winter (e.g., Northern Pike; Pierce et al. 2013), little is known about Longnose Gar dietary and metabolic requirements in the winter. In Chesapeake Bay tributaries, three Longnose Gar collected in the winter had empty stomachs (McGrath et al. 2013), and in South Carolina estuaries, 76% of Longnose Gar stomachs in winter were empty compared to only 33% in summer (Henzler 2011). Evidence of reduced feeding in some systems, the reductions in home range and movements observed in the present study, and observations of more quiescent winter strategies in warmwater fishes in general (Shuter et al. 2012) likely support overall reductions in winter locomotor activity for Longnose Gar.
Depth use was also found to vary by season. In the artificially warm waters of the Ottawa St. Slip during winter, the resident Longnose Gar stayed within 1 m of the surface and depth did not vary year-round. Outside of the slip and under more natural conditions, migrant Longnose Gar moved out of shallow wetlands and nearshore habitat into deeper waters (~ 6.5 m) to overwinter. Many different fish species have been found to use deeper depths in this season (Larocque et al. 2020) and such winter shifts could be a response to finding comparatively warmer oxygen rich waters while also avoiding ice dynamics at the surface and in littoral areas. For gar, the transition back to shallow waters occurred in mid- to late-April, and as water temperatures warmed, gar likely began to seek foraging and spawning habitats. Having unique summer and winter habitat adds additional complexities for species management and confirming such seasonal shifts in depth use can help better define seasonally important habitat.
The majority of acoustically tagged Longnose Gar made long excursions into Lake Ontario, generally towards the Niagara River and occasionally towards Toronto, while some fish showed annual site fidelity to Hamilton Harbour. Long-distance excursions paired with 57% (4 of 7) of migrants repeatedly returning to Hamilton Harbour every year indicate fidelity to Hamilton Harbour, and specifically the Ottawa St. Slip. However, a few fish showed site fidelity to the Niagara River, or large-scale eastward dispersal over several years towards Rochester. The maximum displacement of two fish being greater than 150 km, and the average movement of migrants being ~ 160 km in the summer season alone, is the greatest movement of Longnose Gar documented to date. Spawning site fidelity has been observed in a variety of fishes (Miller et al. 2001; Zemeckis et al. 2014; Binder et al. 2015; Hayden et al. 2018), and is considered an evolutionary adaptation to maximize reproductive success (Leggett 1977). Longnose Gar could be homing back towards Hamilton Harbour for the winter in preparation for spawning in the Ottawa St. Slip in the late spring/early summer, while other gar, during summer dispersal, may have found suitable spawning locations to head towards in subsequent years (e.g., Niagara River area or near Rochester). The more productive waters of Hamilton Harbour (and nearshore areas in general) relative to Lake Ontario may also provide important foraging opportunities to improve spawning success. It is unknown exactly why Longnose Gar migrated such long distances in Lake Ontario, or what method of navigation helps orient return trips. Fish that explored the north shore towards Toronto did not remain in that area (although one individual was not detected returning), and there appears to be greater affinity towards the south shore of Lake Ontario. While the Toronto area supports a diverse fish community (Midwood et al. 2022), cold water upwellings from Lake Ontario into the system can negatively influence warmwater fishes (Murphy et al. 2011; Hlevca et al. 2015) and few Longnose Gar are typically captured there (OMNRF 2020). In contrast, the Niagara River and Rochester areas are at natural river mouths that can be productive environments and bioenergetically favourable (warmer temperatures, increased prey availability) for fishes (Larson et al. 2013), and may make the > 50 km excursion to these areas energetically beneficial for Longnose Gar.
While we cannot confirm the drivers of Longnose Gar migration, resource limitations and density dependence in the Ottawa St. Slip may force some individuals out into the harbour (Taylor et al. 2013). The harbour itself has impaired water quality with high nutrient levels causing hypolimnetic anoxia during the summer as waters warm (Polak and Haffner 1978; Gertzen et al. 2016), which may reduce the availability of habitat for gar. Furthermore, habitat quality and food availability in nearshore areas can also be reduced due to upwellings of this anoxic water (Flood et al. 2021) and Longnose Gar may leave Hamilton Harbour as a result. However, since Longnose Gar are facultative air breathers (Scott and Crossman 1998), such anoxia-related issues may not influence gar directly, but rather may affect the availability of prey resources, which are confined to surface waters during the summer months (Midwood et al. 2019). Adult Walleye (Sander vitreus) in eastern Lake Ontario have shown large summer migrations out of the Bay of Quinte that were related to water temperatures and food availability (Bowlby and Hoyle 2011). Other acoustically tagged species have also been found to leave Hamilton Harbour in the summer and return in the fall/winter (e.g., Walleye, Freshwater Drum (Aplodinotus grunniens), and White Sucker (Catostomus commersonii); Brooks et al. (2019); Larocque, S., unpublished data). Whether all of these species, Longnose Gar included, are leaving and returning to the harbour simultaneously and whether this timing is related to water quality, environmental variables, or food availability it is an important area of future research. Documenting such drivers would improve our understanding of the dynamics of the Hamilton Harbour fish community including seasonal and interannual variation in productivity and would also identify potential limitations related to the quality of fish habitat, which may help with the development of habitat restoration plans for the AOC.
Understanding the spatial ecology of Longnose Gar and other fishes can assist with fisheries management and conservation. Longnose Gar had variable movement patterns but showed extensive long-distance migrations with a high degree of site fidelity to the tagging location in Hamilton Harbour. These movement patterns indicate that monitoring of Longnose Gar in Hamilton Harbour may have reduced catches and lower population estimates if sampling outside the main spawning site and time window. Understanding the residency of fish species to an area can help determine if it is an appropriate species for monitoring local trends (e.g., contaminants, fish productivity). With other species also leaving Hamilton Harbour during the summer, it suggests that there may be shared drivers of emigration and determining these factors may help direct Hamilton Harbour ecosystem restoration projects. These movements of gar into the open lake highlight the connection and energy transfer in fish communities between the lake and protected embayments. From a conservation standpoint, studying the spatial ecology of rare or threatened species may be difficult logistically and consequently aspects of their ecology may remain unknown. However, information extrapolated by studying a similar congeneric can be used to assist with imperiled species restoration and conservation (Cooke 2008). Documenting Longnose Gar movement patterns can thus potentially be used to inform the conservation of Spotted Gar (Lepisosteus oculatus), an endangered species in Canadian waters (COSEWIC 2015). Past studies of this species at risk have documented limited movements (Glass et al. 2012); however, tracking was spatially limited and would have missed larger-scale movements. If Spotted Gar have similar movement patterns as Longnose Gar, results from the present study can be used to help set the spatial scale for population recovery plans in Canadian waters. In the present work, acoustic telemetry provided a useful tool to study Longnose Gar and revealed unexpected and previously undocumented large-scale spatial movements that will influence management of Longnose Gar and congenerics.
Data availability
Data are available on reasonable request.
Code availability
Data was analysed using the R version 4.0.2. The R code is available on request.
References
Allen AM, Singh NJ (2016) Linking movement ecology with wildlife management and conservation. Front Ecol Evol 3:1–13. https://doi.org/10.3389/fevo.2015.00155
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using Lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bendo N, Rebalka A, Bowman JE (2021) Wetland restoration season summary 2021. Royal Botanical Gardens Report No. 2022–05, Hamilton, Ontario
Binder TR, Riley SC, Holbrook CM et al (2015) Spawning site fidelity of wild and hatchery lake trout (Salvelinus namaycush) in northern Lake Huron. Can J Fish Aquat Sci 73:18–34. https://doi.org/10.1139/cjfas-2015-0175
Boston CM, Randall RG, Hoyle JA et al (2016) The fish community of Hamilton Harbour, Lake Ontario: status, stressors, and remediation over 25 years. Aquat Ecosyst Heal Manag 19:206–218. https://doi.org/10.1080/14634988.2015.1106290
Bowlby JN, Hoyle JA (2011) Distribution and movement of Bay of Quinte walleye in relation to temperature, prey availability and Dreissenid colonization. Aquat Ecosyst Heal Manag 14:56–65. https://doi.org/10.1080/14634988.2011.548298
Brooks JL, Boston C, Doka S, Gorsky D, Gustavson K, Hondorp D, Isermann D, Midwood JD, Pratt TC, Rous AM, Withers JL, Krueger CC, Cooke SJ (2017) Use of fish telemetry in rehabilitation planning management and monitoring in areas of concern in the Laurentian Great Lakes. Environ Manag 60(6):1139–1154. https://doi.org/10.1007/s00267-017-0937-x
Brooks JL, Midwood JD, Gutowsky LFG et al (2019) Spatial ecology of reintroduced walleye (Sander vitreus) in Hamilton Harbour of Lake Ontario. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2018.11.011
Brownscombe JW, Adams AJ, Young N et al (2019) Bridging the knowledge-action gap: a case of research rapidly impacting recreational fisheries policy. Mar Policy 104:210–215. https://doi.org/10.1016/j.marpol.2019.02.021
Brownscombe JW, Shipley ON, Griffin LP, Morley D, Acosta A, Adams AJ, Boucek R, Danylchuk AJ, Cooke SJ, Power M (2022) Applications of telemetry to fish habitat science and management. Can J Fish Aquat Sci 79(8):1347–1359. https://doi.org/10.1139/cjfas-2021-0101
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Modell 197:516–519
Clark J (1968) Seasonal movements of striped bass contingents of Long Island Sound and the New York Bight. Trans Am Fish Soc 97:320–343
COA (1992) Remedial action plan for Hamilton Harbour stage 1 second edition: environtmental conditions and problem definition. Prep for Ont. Min of Env and Env. Canada. Ottawa, Canada, Canada Ontario Agreement
Cooke SJ (2008) Biotelemetry and biologging in endangered species research and animal conservation: relevance to regional, national, and IUCN Red List threat assessments. Endanger Species Res 4:165–185. https://doi.org/10.3354/esr00063
Cooke SJ, Martins EG, Struthers DP et al (2016) A moving target—incorporating knowledge of the spatial ecology of fish into the assessment and management of freshwater fish populations. Environ Monit Assess 188:239. https://doi.org/10.1007/s10661-016-5228-0
Cooke SJ, Bergman JN, Twardek WM et al (2022) The movement ecology of fishes. J Fish Biol 101:756–779. https://doi.org/10.1111/jfb.15153
COSEWIC (2015) COSEWIC assessment and status report on the Spotted Gar Lepisosteus oculatus in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa, Canada
Cunjak RA (1996) Winter habitat of selected stream fishes and potential impacts from land-use activity. Can J Fish Aquat Sci 53:267–282. https://doi.org/10.1139/cjfas-53-s1-267
Daly R, Filmalter JD, Daly CAK et al (2019) Acoustic telemetry reveals multi-seasonal spatiotemporal dynamics of a giant trevally Caranx ignobilis aggregation. Mar Ecol Prog Ser 621:185–197. https://doi.org/10.3354/meps12975
Dudgeon CL, Pollock KH, Braccini JM et al (2015) Integrating acoustic telemetry into mark–recapture models to improve the precision of apparent survival and abundance estimates. Oecologia 178:761–772. https://doi.org/10.1007/s00442-015-3280-z
Espinoza M, Lédée EJI, Smoothey AF et al (2021) Intra-specific variation in movement and habitat connectivity of a mobile predator revealed by acoustic telemetry and network analyses. Mar Biol 168:1–15. https://doi.org/10.1007/s00227-021-03886-z
Flood B, Wells M, Midwood JD et al (2021) Intense variability of dissolved oxygen and temperature in the internal swash zone of Hamilton Harbour, Lake Ontario. Inl Waters 11:162–179. https://doi.org/10.1080/20442041.2020.1843930
Gatch AJ, Furgal SL, Gorsky D et al (2022) Evaluation of post-stocking dispersal and mortality of juvenile lake trout Salvelinus namaycush in Lake Ontario using acoustic telemetry. J Great Lakes Res 48:572–580. https://doi.org/10.1016/j.jglr.2022.01.014
Gertzen EL, Doka SE, Tang RW et al (2016) Long-term dissolved oxygen and temperature monitoring in Hamilton Harbour, Lake Ontario (2006–2013). Can Manuscr Rep Fish Aquat Sci 3092:x+29
Glass WR, Corkum LD, Mandrak NE (2012) Spring and summer distribution and habitat use by adult threatened Spotted Gar in Rondeau Bay, Ontario, using Radiotelemetry. Trans Am Fish Soc 141:1026–1035. https://doi.org/10.1080/00028487.2012.675904
Hayden TA, Holbrook CM, Fielder DG et al (2014) Acoustic telemetry reveals large-scale migration patterns of walleye in Lake Huron. PLoS ONE 9:1–19. https://doi.org/10.1371/journal.pone.0114833
Hayden TA, Binder TR, Holbrook CM et al (2018) Spawning site fidelity and apparent annual survival of walleye (Sander vitreus) differ between a Lake Huron and Lake Erie tributary. Ecol Freshw Fish 27:339–349. https://doi.org/10.1111/eff.12350
Henzler JM (2011) Aspects of reproduction and diet in a coastal South Carolina population of Longnose Gar, Lepisosteus osseus. MSc Thesis, School of the Environment, University of South Carolina
Hlevca B, Cooke SJ, Midwood JD et al (2015) Characterisation of water temperature variability within a harbour connected to a large lake. J Great Lakes Res 41:1010–1023. https://doi.org/10.1016/j.jglr.2015.07.013
Holbrook C, Hayden T, Binder T, Pye J (2020) GLATOS: a package for the Great Lakes acoustic telemetry observation system. package version 0.1.3. Retrieved from. https://gitlab.oceantrack.org/GreatLakes/glatos [Accessed Dec 2022]
Holm E, Mandrak NE, Burridge ME (2022) A field guide to freshwater fishes of Ontario, 2nd edn. Royal Ontario Museum, Toronto
Johnson B, Noltie D (1996) Migratory dynamics of stream-spawning longnose gar (Lepisosteus osseus). Ecol Freshw Fish 5:97–107
Jude DJ, Pappas J (1992) Fish utilization of Great Lakes coastal wetlands. J Gt Lakes Res 18:651–672. https://doi.org/10.1016/S0380-1330(92)71328-8
Klinard NV, Matley JK (2020) Living until proven dead: addressing mortality in acoustic telemetry research. Rev Fish Biol Fish 30:485–499. https://doi.org/10.1007/s11160-020-09613-z
Larocque SM, Lake C, Johnson TB, Fisk AT (2022) Patterns in spatial use of land-locked Atlantic salmon (Salmo salar) in a large lake. J Great Lakes Res 48:381–391. https://doi.org/10.1016/j.jglr.2021.12.002
Larocque SM, Boston CM, Midwood JD (2020) Seasonal daily depth use patterns of acoustically tagged freshwater fishes informs nearshore fish community sampling protocols. Can Tech Rep Fish Aquat Sci 3409:viii + 38
Larson JH, Trebitz AS, Steinman AD et al (2013) Great Lakes rivermouth ecosystems: scientific synthesis and management implications. J Great Lakes Res 39:513–524. https://doi.org/10.1016/j.jglr.2013.06.002
Leggett WC (1977) The ecology of fish migrations. Annu Rev Ecol Syst 8:285–308
Lenth RV (2023) emmeans: estimated marginal means, aka least-squares means. package version 1.8.8. Retrieved from. https://CRAN.R-project.org/package=emmeans
Marsden JE, Blanchfield PJ, Brooks JL et al (2021) Using untapped telemetry data to explore the winter biology of freshwater fish. Rev Fish Biol Fish 31:115–134. https://doi.org/10.1007/s11160-021-09634-2
Matley JK, Klinard NV, Barbosa Martins AP et al (2022) Global trends in aquatic animal tracking with acoustic telemetry. Trends Ecol Evol 37:79–94. https://doi.org/10.1016/j.tree.2021.09.001
McGrath PE (2010) The life history of Longnose Gar, Lepisosteus osseus, an apex predator in the tidal waters of Virginia. PhD Thesis, Faculty of the School of Marine Science, The College of William and Mary. https://scholarworks.wm.edu/etd/1539616772/. Accessed November 2023
McGrath PE, Hilton EJ, Musick JA (2012) Seasonal distributions and movements of Longnose Gar (Lepisosteus osseus) within the York River System, Virginia. Southeast Nat 11:375–386
McGrath PE, Hilton EJ, Musick JA (2013) Temporal and spatial effects on the diet of an estuarine piscivore, Longnose Gar (Lepisosteus osseus). Estuaries Coasts 36:1292–1303. https://doi.org/10.1007/s12237-013-9637-9
Midwood JD, Reddick DT, Brooks JL et al (2018) Intracoelomic Implantation of Transmitters in Longnose Gar. Trans Am Fish Soc 147:704–710. https://doi.org/10.1002/tafs.10044
Midwood JD, Leisti KE, Milne SW, Doka SE (2019) Assessing seasonal changes in pelagic fish density and biomass using hydroacoustics in Hamilton Harbour, Lake Ontario in 2016. Can Tech Rep Fish Aquat Sci 3299:x + 63. https://publications.gc.ca/collections/collection_2019/mpo-dfo/Fs97-6-3299-eng.pdf
Midwood JD, Blair SG, Boston CM et al (2022) First assessment of the fish populations beneficial use impairment in the Toronto and region area of concern. Can Tech Rep Fish Aquat Sci 3503:283. https://publications.gc.ca/collections/collection_2022/mpo-dfo/Fs97-6-3503-eng.pdf
Miller LM, Kallemeyn L, Senanan W (2001) Spawning-site and natal-site fidelity by Northern Pike in a large lake: mark–recapture and genetic evidence. Trans Am Fish Soc 130:307–316. https://doi.org/10.1577/1548-8659(2001)130%3c0307:ssansf%3e2.0.co;2
Murphy SC, Collins NC, Doka SE (2011) Thermal habitat characteristics for warmwater fishes in coastal embayments of Lake Ontario. J Great Lakes Res 37:111–123. https://doi.org/10.1016/j.jglr.2010.12.005
OMNRF (2020) Lake Ontario fish communities and fisheries: 2019 annual report of the Lake Ontario management unit. Picton, Ontario
Pierce RB, Carlson AJ, Carlson BM et al (2013) Depths and thermal habitat used by large versus small northern pike in three Minnesota Lakes. Trans Am Fish Soc 142:1629–1639. https://doi.org/10.1080/00028487.2013.822422
Pincock DG (2012) False detections: what they are and how to remove them from detection data. Amirix Doc DOC-004691 Version 03, Vemco Division, Amirix Systems Inc., Halifax, Nova Scotia
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-Plus. Springer, New York
Polak J, Haffner GD (1978) Oxygen depletion of Hamilton Harbour. Water Res 12:205–215. https://doi.org/10.1016/0043-1354(78)90088-X
R Core Team (2020) R: a language and environment for statistical computing
Royal Botanical Gardens (1998) The Cootes Paradise fishway: carp control techniques at Royal Botanical Gardens. Hamilton, Ontario, Canada
Scott WB, Crossman EJ (1998) Freshwater fishes of Canada. Galt House Publications Ltd, Oakville, Ontario
Secor D (1999) Specifying divergent migrations in the concept of stock: the contingent hypothesis. Fish Res 43:13–34. https://doi.org/10.1016/S0165-7836(99)00064-8
Shuter BJ, Finstad AG, Helland IP et al (2012) The role of winter phenology in shaping the ecology of freshwater fish and their sensitivities to climate change. Aquat Sci 74:637–657. https://doi.org/10.1007/s00027-012-0274-3
Smith PD (2005) Longnose Gar (Lepisosteus osseus) in Hamilton Harbour: part 1 — spotted in the Ottawa Street Slip. Wood Duck J Hamilt Nat Club 58
Smith PD (2006) Longnose Gar (Lepisosteus osseus) in Hamilton Harbour: Part 2 - What’s Happening Under the Slip. Wood Duck J Hamilt Nat Club 59
Solomon LE, Phelps QE, Herzog DP et al (2013) Juvenile alligator gar movement patterns in a disconnected floodplain habitat in southeast Missouri. Am Midl Nat 169:336–344. https://doi.org/10.1674/0003-0031-169.2.336
Taylor MD, Fairfax AV, Suthers IM (2013) The race for space: using acoustic telemetry to understand density-dependent emigration and habitat selection in a released predatory fish. Rev Fish Sci 21:276–285. https://doi.org/10.1080/10641262.2013.796813
Zemeckis DR, Hoffman WS, Dean MJ et al (2014) Spawning site fidelity by Atlantic cod (Gadus morhua) in the Gulf of Maine: implications for population structure and rebuilding. ICES J Mar Sci 71:1356–1365. https://doi.org/10.1093/icesjms/fsu117
Acknowledgements
We thank Susan Doka, Christine Boston, and Jill Brooks for assistance with planning and designing the Hamilton Harbour array. Jill Brooks, Erin Budgell, Dallas Linley, Rex Tang, Jesse Gardner Costa, Emily Marshall, Andrew Fernley, Filipe Aguiar, and Alex Price assisted with receiver servicing and/or acoustic tagging. We also thank the various collaborators and respective field crews for servicing the array in Lake Ontario (Carleton University, Fisheries and Oceans Canada, New York State Department of Environmental Conservation, OMNRF, Queens University, University of Windsor, US Geological Survey, US Fish and Wildlife). We would like to thank Craig McGinlay from ArcelorMittal for facilitating access to the Ottawa St. Slip. Animal care approval for this study was provided by the Carleton University Animal Care Committee under permit 102935.
Funding
Open Access funding provided by Fisheries & Oceans Canada. The Hamilton Harbour acoustic telemetry study was funded by the Great Lakes Action Plan, Great Lakes Sustainability Fund, and Great Lakes Protection Initiative all administered by Environment and Climate Change Canada, as well as the Canada Research Chairs Program (Cooke) and Fisheries and Oceans Canada. This paper is contribution 114 of the Great Lakes Acoustic Telemetry Observation System (GLATOS), which was partially funded by the Great Lakes Fishery Commission (Grant ID #2013_BIN_44024) by way of Great Lakes Restoration Initiative appropriations (Grant ID #GL-00E23010).
Author information
Authors and Affiliations
Contributions
Jon Midwood, David Reddick, Steven Cooke, and Paul Smith contributed to study conception and design. Material preparation, data collection, and analysis were performed by David Reddick, Sarah Larocque, Jon Midwood, and Melanie Croft-White. The first draft was written by Melanie Croft-White and Sarah Larocque, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This scientific research meets all Canadian ethics and animal care regulations. Animal care approval for this study was provided by the Carleton University Animal Care Committee under permit 102935.
Ethical statement
All authors of this scientific research followed the ethical rules stated in the submission guidelines, according to COPE. Data was analyzed with honesty and integrity. All data and text are the authors’ own original work.
Consent to participate
All authors have consented to participate in this paper and approve of the submission to Environmental Biology of Fishes.
Consent for publication
All authors have provided consent for this manuscript to be published in Environmental Biology of Fishes.
Conflict of interest
Dr. Steven J. Cooke is on the Editorial Board of this journal, but he was not involved in the peer review of this article, and he had no access to information regarding its peer review. The authors have no other conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Croft-White, M.V., Larocque, S.M., Reddick, D.T. et al. Diversity of movement patterns of Longnose Gar tracked in coastal waters of western Lake Ontario. Environ Biol Fish (2023). https://doi.org/10.1007/s10641-023-01491-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10641-023-01491-1