Abstract
Many Pacific salmon populations are returning from sea at younger ages and smaller sizes. Hatchery culture, management practices, and environmental factors influence juvenile release size and emigration timing, which in turn affect important demographic characteristics in returning adults. We analyzed data from approximately 345,000 tagged spring Chinook Salmon juveniles exiting Cle Elum Supplementation and Research Facility (Yakima River, Washington, USA) acclimation sites over thirteen brood years (2002–2014), evaluating smolt size, emigration timing, river flow, juvenile survival, and age-at-return. We observed a relationship between size and volitional exit timing of smolts from acclimation sites, with larger fish tending to emigrate earlier than smaller fish. Early emigration was also coincident with lower river flows near acclimation sites. Later emigration timing was correlated with an increase in apparent survival of juveniles to Bonneville Dam (500–530 km downstream of acclimation sites), but also with a lower rate of survival to return from sea. In general, for juveniles successfully emigrating downstream of Bonneville Dam, age-at-return increased with decreasing juvenile fish size and later emigration timing. Our results support a growing body of evidence that hatchery practices may result in larger smolts that tend to return at younger ages. Given the biological and economic consequences of younger age-at-maturation, methods to reverse this trend should be further explored and implemented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past century, hundreds of hatchery programs have been implemented throughout the Pacific Northwest to compensate for declines in the abundance of salmon populations including Chinook Salmon (Oncorhynchus tshawytscha; Lichatowich 1999; Waples et al. 2007). Although hatchery programs vary and typically have a combination of management objectives, a consistent goal is to increase the number of returning adults to provide harvest opportunities (subsistence, ceremonial, commercial, and recreational), while meeting escapement objectives for continued health of the population. An additional goal is to mitigate for lost production and productivity as habitats are increasingly altered to serve the needs of growing human populations (Lackey 2001; Waples et al. 2009; Hartman et al. 2020). Examples of variables in hatchery operations and management that can be adjusted to optimize fish survival include broodstock selection (Hankin et al. 2009; Winsor et al. 2021), incubation temperature (Winsor et al. 2021), feed ration and concomitant growth rates (Beckman et al. 1999, 2017; Larsen et al. 2006; Spangenberg et al. 2014; Winsor et al. 2021), rearing density (Banks 1994; Beckman et al. 1999), raceway water flow (Banks 1994) and temperature (Spangenberg et al. 2014), number of juvenile fish released (Paquet et al. 2011), release size and timing (Irvine et al. 2013), acclimation (Dittman et al. 2010; Rosenberger et al. 2013), and, in some cases, migration pathways (Ward et al. 1997). All of these variables must be managed within the context of a series of complex interactions with the natural environment that may be unique to a given species, population, and river system.
In addition to regional declines in salmon productivity, evidence from recent studies in both natural and hatchery origin fish indicates that adults are returning to spawn at younger ages and smaller sizes (Lewis et al. 2015; Ohlberger et al. 2018; Oke et al. 2020). The potential anthropogenic and natural factors responsible for these changes are not fully understood, but are an active area of investigation because of their importance to salmon population demographics, sustainability, ecosystem dynamics, and fisheries (Ohlberger et al. 2020; Oke et al. 2020). In both the natural and hatchery environments, older, larger females are typically more effective breeders due to higher fecundity (Knudsen et al. 2008; Ohlberger et al. 2020; Oke et al. 2020), and in the natural environment, larger males are more effective competitors for breeding opportunities (Fleming and Gross 1994; Schroder et al. 2010, 2012). Additionally, older, larger fish increase biomass in natal streams as they contain more marine-derived nutrients that are important to maintaining primary productivity and overall ecosystem health (Bilby et al. 1998, 2003; Oke et al. 2020). Finally, larger fish are more desirable in subsistence, cultural, commercial, and recreational fisheries (Fenberg and Roy 2008; Zhou et al. 2010; Lubich et al. 2021).
Intraspecific trait variation is important to community ecology and dynamics (Bolnick et al. 2003, 2011). For example, variations in size, spatial distribution, and ability to tolerate extreme environmental conditions can allow a species to better exploit available habitats or continue to thrive under less than ideal conditions. Understanding the extent to which management protocols and environmental factors in a hatchery culture setting may influence juvenile size, emigration timing, and size and age at maturity is important for optimizing hatchery operations for both the quantity and the quality (e.g., traits such as size and age) of adults they ultimately produce. Studies in natural and hatchery origin fish have shown that larger juveniles often have a higher probability of surviving to adulthood (Zabel and Williams 2002; Tipping 2011; Brown et al. 2013; Evans et al. 2014; Faulkner et al. 2019). However, larger smolts have also been associated with younger age at maturity (Tipping 2011; Larsen et al. 2013; Harstad et al. 2014; Tattam et al. 2015). A study of artificially reared juvenile Coho Salmon (O. kisutch) in British Columbia, Canada, found a significant interaction between size and timing of releases with more age-3 male (common name: jack) production compared to older age classes observed from early release of large juveniles (> ~ 20 g in April; Bilton et al. 1982). Similarly, Tattam et al. (2015) observed a correlation between emigration timing and age-at-maturity in a wild Chinook Salmon population from the John Day River in northeast Oregon. While earlier releases in the Bilton et al. (1982) study were correlated with younger age-at-maturity, wild juveniles in the John Day River (Tattam et al. 2015) that were tagged later in the spring (later emigrants) had higher probability of maturing at age-4 rather than age-3.
The emigration timing of hatchery-released juveniles and river conditions (e.g., water temperature and flow) during emigration are among many factors affecting downstream survival, size and age at maturity, and adult return rates. Flow manipulations in impounded systems like the Columbia River that change the timing, duration, or magnitude of temperature and flow regimes may impact growth rates (Dunnigan and Terrazas 2021) and in the spring can significantly affect the emigration of salmonid smolts. Increased natural flows (e.g., spring freshets) encourage emigration, reduce travel times, and can improve survival of juvenile Chinook Salmon (Connor et al. 2003; Sommer et al. 2005; del Rosario et al. 2013; Takata et al. 2017). Warmer water temperatures, potentially indicative of drought conditions, have been associated with earlier emigration (Sykes et al. 2009). However, juvenile survival can be reduced when fish emigrate during periods of lower flows or warmer water temperatures as energetic costs increase, and these conditions may also result in longer travel times increasing exposure to predation and other mortality risks (NRC 2004; Pandit et al. 2017; Keefer et al. 2018). Previous studies have demonstrated that arrival timing to the estuary may be related to survival to adulthood (Zabel and Williams 2002) and juvenile fish that emigrate past Bonneville Dam (rkm 233; the furthest downstream dam) on the Columbia River from early to mid-May have been reported to survive at rates 4–50 times greater than those arriving in mid-June (Scheuerell et al. 2009).
Taken together, these factors illustrate the importance of using life-cycle analyses (e.g., Beckman et al. 1998, 2017; Harstad et al. 2018) to empirically evaluate and better understand the interrelationships between smolt size at release, smolt development, emigration timing, and ecosystem dynamics (e.g., freshwater, estuary, and marine conditions) and their effects on survival and adult demography. The widespread use of passive integrated transponder (PIT, Biomark, Boise, ID) tag technology (Prentice et al. 1990; Connolly et al. 2008; www.ptagis.org) and PIT-tagging programs has facilitated analyses of juvenile and adult migration and survival, habitat use, and fishery management practices (Greenberg and Giller 2000; O’Donnell et al. 2010; Thiem et al. 2013). In the Columbia River Basin, PIT tags have allowed insights into specific components of the life history of individual salmon including downstream survival and travel time through the emigration corridor as well as comparative studies of release groups and survival to adult return (McCann et al. 2022).
The Cle Elum Supplementation and Research Facility (CESRF; rkm 297) on the Yakima River, Washington, is relatively unique in its ability to facilitate the type of life-cycle investigation described above, thereby providing insights that might allow hatcheries, including the CESRF, to be adaptively managed to better meet overall program objectives. We studied detection histories from over 344,000 PIT-tagged CESRF spring Chinook Salmon spanning thirteen brood years (2002–2014; juvenile migration years 2004–2016; return/spawn years 2004–2019) and conducted post hoc analyses (stated as null hypotheses) to evaluate how present hatchery management protocols impact overall program results:
-
Ho (1): volitional exit date of juveniles from the acclimation and release sites was independent of acclimation site, smolt size, and river flow near acclimation sites,
-
Ho (2): apparent survival of juvenile emigrants at downstream detection sites was independent of acclimation site, smolt size, and volitional exit date, and
-
Ho (3): smolt-to-adult return (SAR) and age-at-return were independent of smolt size, acclimation site, and volitional exit date.
Materials and methods
Study area and CESRF facilities
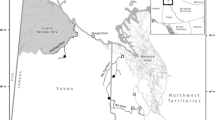
The Yakima River, located in south central Washington State, is a tributary to the Columbia River (Fig. 1). Since the establishment of the CESRF in 1997, the Yakama Nation has been collaborating with the Washington Department of Fish and Wildlife (WDFW) and other regional and national fishery management agencies to monitor and evaluate a hatchery supplemented population of spring Chinook Salmon in the upper Yakima River (the portion upstream of Roza Dam, rkm 206; Fig. 1; Fast et al. 2015). Each year fish are spawned and reared at the central hatchery facility in Cle Elum, WA (CESRF, Fig. 1; Knudsen et al. 2006; Fast et al. 2008). After approximately ten months of rearing (in October and November), fish are marked and then later transported from the CESRF (as yearling presmolts the following January or February) to three acclimation sites located at Easton (ESJ) 25 km upstream of the CESRF, Clark Flat (CFJ) 25 km downstream of the CESRF, and Jack Creek (JCJ) on the Teanaway River 27 km upstream from its confluence with the Yakima River (rkm 287; Fig. 1). Fish are reared using a combination of well and river water while at the CESRF, but once fish are moved to acclimation sites only river water is used, as the intent is to “imprint” the fish to these sites so that returning adults will home to these portions of the river system and “integrate” with the natural spawning population (Dittman et al. 2010; Fast et al. 2015). Target release for the CESRF program is 810,000 smolts at 15 fish per pound (30.2 g per fish).
Map of the study area showing the Yakima and lower Columbia River basins, Cle Elum Supplementation and Research Facility (CESRF), spring Chinook Salmon acclimation sites (Easton, Jack Creek, Clark Flat), and downstream Yakima and Columbia River dams (map courtesy of Paul Huffman, Yakama Nation Fisheries, retired)
Marking and PIT-tag detection
An annual average of 777,000 CESRF spring Chinook Salmon were released during this study period, including a subset of approximately 40,000 PIT-tagged fish (range 4.7–6.2%, mean 5.2% of each annual release). Tagged fish were distributed across all rearing raceways and acclimation sites, with site allocation based on overall experimental needs (Fast et al. 2008; Bosch 2019). PIT-tag codes allowed individual fish to be identified by brood year, acclimation site, and raceway of release. Fork lengths were taken from all fish at the time of PIT tagging, but weights were collected for only a small subsample (\(N=\mathrm{12,113}\); 3.5% of \(N\) for length-sampled fish) of individual PIT-tagged fish in a quality control exercise subsequent to tagging. PIT codes, fish lengths, and other release information were submitted annually to the PIT Tag Information System (PTAGIS; http://www.ptagis.org) database maintained by the Pacific States Marine Fisheries Commission (PSMFC). The PSMFC has also installed and maintained automatic PIT-tag detectors (with passive detection capability for both juvenile and adult fish) at several locations throughout the Columbia (e.g., Bonneville, McNary, and John Day Dams) and Yakima river systems (including the ESJ, JCJ, and CFJ acclimation sites). Thus, the PTAGIS database contains historical release and detection data for all Columbia River Basin PIT-tagged fish.
We queried the PTAGIS database in March and December 2019 to retrieve and enumerate PIT-tag detections of all spring Chinook Salmon marked at the CESRF that were released and subsequently detected volitionally exiting the acclimation sites (brood years 2002–2014; emigration years 2004–2016). For each fish detected at release, we also retrieved both downstream and upstream (return years 2004–2019) PIT-tag detection data at all monitoring locations (dams) downstream of the acclimation sites (Fig. 1). We assumed fish migrated past a given dam if a PIT tag was detected at or downstream (juvenile migrants), and at or upstream (returning fish), of that dam. Data were evaluated for interannual consistency and anomalies (described further below). Records deemed to be anomalous were excluded from statistical analyses (further explained in text following table), leaving a total of more than 344,000 unique PIT-tagged fish that were analyzed in this study (Table 1).
Statistical analyses
Volitional exit timing (day of year a fish was detected migrating out of the acclimation sites (ED)), smolt size at release (fork length (FL)), acclimation site (Ac.Site), and daily average river flow (cubic feet per second (cfs)) near each acclimation site during the emigration period (Ac.RF; https://www.usbr.gov/pn/hydromet/yakima/) were considered critical parameters for purposes of this study. Volitional exit (including fish forced from acclimation sites upon closure of the sites) occurred between March 9 and May 16 annually (68 days). Fish with exit dates outside of this range were excluded from the analyses. On average, fork length at time of PIT tagging in October–November was approximately 93.5% of fork length when fish were sampled randomly at the acclimation sites in March at the beginning of volitional release (Bosch 2019). As noted earlier, the total sample size for weights of individual PIT-tagged fish was only 3.5% of the sample size for length-sampled fish. Therefore, we used fork length at PIT tagging as a surrogate for smolt size at release in this study. Smolt sizes less than 75 or greater than 149 mm were considered outliers (< 0.15% of all fish estimated to have been released) and were excluded from the analyses. The spring Chinook Salmon program at the CESRF consists of integrated (16 raceways) and segregated (2 raceways) production (we refer readers to Mobrand et al. 2005 and Fast et al. 2015 for additional information regarding integrated and segregated hatchery production); only integrated program releases were included in this study. In brood years 2002 through 2004, one-half of the fish were subjected to a slower growth feed regime (Larsen et al. 2013) resulting in reduced size at release for these fish. The slower growth fish from these brood years were excluded from the analyses. In brood year 2006, one-half of the fish were fed an EWOS® diet; for interannual consistency, fish fed this diet were excluded from the analyses.
To evaluate hypotheses 1 and 3, we used mixed-effects linear regression (Zuur et al. 2009; Gosselin et al. 2018) to model the effects of fixed variables (e.g., ED, Ac.Site, FL, and Ac.RF) and two-way interactions between variables, with brood year (BY) as a random variable, on ED (Ho1) and SAR (Ho3). For Ho3, we assumed the underlying error distribution was binomial and used a logit link in the model (McCullagh and Nelder 1989). Ac.Site was considered a factor with 3 levels; it could have been considered a random variable, but there were only three of them and possible differences between them could be useful for management. BY was considered random since the included years were a “sample” of all possible years and we were not interested in making inference for individual years.
We first fit the largest model denoted as
where “ ~ ” denotes “as a function of,” “*” denotes interaction, (1|BY) denotes that BY was random with respect to the intercept term (i.e., the result is that predicted ED curves based on other variables would have BY = 13 “parallel” curves for the years), and the error term was assumed to be normally distributed. Inference based on the fixed variables therefore was evaluated by averaging over BYs. Note that FL and Ac.RF were standardized (i.e., subtract the overall mean and divide by the overall standard deviation) so that parameter coefficients would be on the same scale; means and predictions were back-transformed to the original scale.
Next, we fit the suite of models that were subsets of this largest model. We omitted subset models where an interaction was included but each of the pertinent main effects were not. We compared models using Akaike’s Information Criterion (AIC; Burnham and Anderson 2002). We chose the “best” model as the one with minimum AIC. If multiple models were close to the minimum, we chose the most parsimonious as “best” (e.g., FL + Ac.RF in one and only FL in another), unless they contained different types of variables (e.g., FL in one model and ED in another) in which case we model-averaged them (Burnham and Anderson 2002). These analyses were done in R (R Core Team 2021) and used the lme4 package (v1.1–26; Bates et al. 2015).
Additional information, evaluations, and analyses specific to each hypothesis were as follows.
-
Ho (1): volitional exit date of juveniles from the acclimation and release sites was independent of acclimation site, smolt size, and river flow near acclimation sites.
We used analysis of variance (ANOVA) to test whether annual trends in mean smolt size, volitional exit date, and river flow near acclimation sites varied by acclimation site and brood year.
-
Ho (2): apparent survival of juvenile emigrants at downstream detection sites was independent of acclimation site, smolt size, and volitional exit date.
To evaluate factors affecting juvenile survival from Ac.Site to Bonneville Dam (BON; rkm 234; Fig. 1), we used a mark-recapture method, the Cormack-Jolly-Seber model (CJS; Cormack 1964; Jolly 1965; Seber 1965). The CJS model allows separate estimation of the probability of apparent survival, defined as the product of the probability of true survival and study area fidelity (Lebreton et al. 1992) between time \(t\) and \(t+1\) or between dam 1 and dam 2, as well as recapture (or detection) probability (Cormack 1964; Jolly 1965; Seber 1965; Conner et al. 2020). This model has been commonly used within the Columbia River Basin to estimate survival rates for juvenile anadromous fish species (Skalski et al. 1998; Smith et al. 2002; Conner et al. 2020; Tiffan et al. 2021; McCann et al. 2022). The mark-recapture model used an encounter history for each fish, denoted “1” (released) and as “0” (not detected) or “1” (detected) at each of two detection sites: (1) BON either at the juvenile fish bypass system (PTAGIS code = BOJ) or through the corner collector (PTAGIS code = BCC) and (2) downstream of BON, via estuary PIT trawl (Ledgerwood et al. 2004) or on East Sand Island avian bird colonies (Evans et al. 2016). Thus, there were four possible detection histories: “100,” “101,” “110,” and “111,” to denote acclimation site exit detection and combined detection at and downstream of BON.
Using detection histories for each cohort, we estimated survival probability from acclimation site exit to BON for each Ac.Site (CFJ, ESJ, and JCJ) and for each BY (2002 to 2014), for a total of 39 combinations. We chose variables ED and FL as covariates to include in these models. All BY data were pooled within detection histories, and we included a series of explanatory models with and without covariates using RMark (Laake 2013; Cooch and White 2018). We tested “goodness of fit” of competing models by estimating the variance inflation factor (\(\hat{c}\)) for the largest model using the median \(\hat{c}\) test in program MARK (Cooch and White 2018). All subsequent models were corrected for overdispersion (\(\hat{c} >1.0\)). Models were again evaluated using quasi-AIC scores (QAICc), resulting in selection of the model with the minimum QAICc (Burnham and Anderson 2002). We further estimated the apparent survival probability (from acclimation site to Bonneville Dam) using the best model to illustrate how the covariates affect survival rate and detection or recapture rate.
-
Ho (3): smolt-to-adult return (SAR) and age-at-return were independent of smolt size, acclimation site, and volitional exit date.
We calculated SAR of PIT-tagged fish detected leaving each Ac.Site to adult return to Bonneville Dam for each brood year release in the study. Bonneville Dam was selected to maximize the number of fish available for this analysis. Note that we present SAR as a percentage, while modeling was based on proportions (i.e., SAR/100). Note further that these analyses and this investigation were designed only to address the null hypothesis as stated; McNary-to-McNary and Bonneville-to-Bonneville SARs for individually PIT-tagged CESRF fish detected at juvenile emigration and adult return at these dams are available in McCann et al. (2022). For age-at-return analyses, we could only include PIT-tagged fish which were detected returning upstream at Bonneville Dam (2072 total PIT detections for all brood years and acclimation sites combined). Brood year was subtracted from year of upstream passage detection to determine age. Returning fish were categorized as age-2 (minijacks or < 1-ocean year), age-3 (jacks, 1-ocean), age-4 (2-ocean), and age-5 (3-ocean). For the entire 13-year dataset, only 19 and 24 fish returned at ages 2 and 5, respectively (each age class representing only 1.2% of all returning fish). Therefore, we combined age-2 and age-3 and age-4 and age-5 fish into categories as age2&3 and age4&5 and repeated the modeling analysis detailed above substituting “age4&5” for “SAR”.
Results
Ho (1): volitional exit date of juveniles from the acclimation and release sites was independent of acclimation site, smolt size, and river flow near acclimation sites.
We observed a slight variation in mean volitional exit date by acclimation site among brood years (Fig. 2a; Online Resource Fig. S1). The mean exit dates for JCJ, ESJ, and CFJ were April 7 (day of year 97.0 ± 2.5), April 7 (97.5 ± 3.0), and April 10 (100 ± 2.6), respectively; the differences were not significant (ANOVA: F2,36 = 0.51, \(P=0.61\)). When all data were pooled, the median and mean exit dates were April 9 and April 7 (day of year 100 and 98.0 ± 1.5, mean ± SE), respectively, demonstrating a slight tendency of fish to exit earlier in the volitional emigration period than later.
Smolt size averaged over all brood years and acclimation sites was 109.0 ± 0.67 mm (mean ± SE), and differences among acclimation sites were not significant (Fig. 2b; CFJ: 109.0 ± 0.85 mm; ESJ: 108.0 ± 1.06 mm; and JCJ: 108.0 ± 1.50 mm; ANOVA: F2,36 = 0.28, P = 0.76). However, we observed a linear trend of increasing smolt size over time for all sites combined (r2 = 0.33; \(P<0.01\)) and for each acclimation site (CFJ: r2 = 0.33, \(P=0.04\); ESJ: r2 = 0.46, \(P=0.01\); JCJ: r2 = 0.31, \(P=0.05\)).
River flow averaged over all brood years and acclimation sites was 1750 ± 248 cfs (mean ± SE) during the volitional emigration period (Fig. 2c). River flow near CFJ was over four times greater (3392 ± 405 cfs) than flow near ESJ (722 ± 113 cfs) and was three times greater than flow near JCJ (1135 ± 249 cfs). There was no increasing linear trend over time for the overall river flow (r2 = 0.04, \(P=0.21\)), nor for any of the acclimation sites (CFJ: r2 = 0.24, \(P=0.09\); JCJ: r2 = 0.01, \(P=0.71\); ESJ: r2 = 0.15, \(P=0.19\)).
The relationship between exit date and smolt size ranged from − 0.37 to + 0.17. Slopes were negative and significant for most brood years (Fig. 3a; Online Resource Fig. S2a), indicating that larger smolts tended to exit the acclimation sites earlier than smaller smolts. Similarly, slopes of the relationship between volitional exit date and river flow near the acclimation sites ranged from − 0.05 to + 0.33. The majority of slopes were positive (Fig. 3b; Online Resource Fig. S2b), indicating that smolts tended to exit later with increasing river flow. Among the several models we used to evaluate how much variation in exit date was explained by smolt size and river flow near the acclimation sites, the best fit model included all variables, which explained nearly 25% of the variation (Table 2). Therefore, estimated exit date must be viewed in context with river flow and smolt size, but independent of acclimation site. We have used standardized variables in our modeling so that model coefficients are on the same scale. Therefore, we can say that the relationship between smolt size and exit date (i.e., negative slope around − 0.2 implies later exit dates for smaller fish) is opposite to that of river flow and exit date (i.e., positive slope around + 0.02 implies later exit dates at higher flows). Furthermore, the nearly 10 times larger coefficient for the smolt size relationship implies smolt size has a much larger impact than river flow on exit date.
Slope of the relationship between volitional exit date and smolt size (a) and between volitional exit date and river flow (b) for each brood year and acclimation site (see Online Resource Fig. S2). Insets illustrate slopes between the variables
At CFJ, predicted exit date increased over a range of several weeks from early April to mid-May as river flow increased across its range for both smaller (20th percentile FL = 102 mm) and larger (80th percentile FL = 116 mm) fish (Online Resource Fig. S3). At ESJ, the pattern in predicted exit date was nearly identical but with substantially lower flows than at CFJ. However, at JCJ, predicted exit date increased by only one week relative to increasing river flow across its range, regardless of smolt size.
At CFJ, predicted exit date slightly increased in the first week of April as smolt size increased and river flow was low (20th percentile, 1669 cfs), but when river flow was high (80th percentile, 4945 cfs), predicted exit date slightly decreased in the second week of April as smolt size increased (Online Resource Fig. S4). At ESJ and JCJ, predicted exit date decreased by about one week as smolt size increased, all in the first half of April regardless of river flow. One minor nuance was at ESJ where predicted exit date occurred in the first week in April at low river flow.
Ho (2): apparent survival of juvenile emigrants at downstream detection sites was independent of acclimation site, smolt size, and volitional exit date.
Average apparent survival probability from acclimation site to Bonneville Dam (with data pooled for all brood years) was \(21.85\pm 0.80\%\) (mean ± SE) and with minor variation among acclimation sites (Fig. 4). The highest average survival was for fish exiting CFJ (\(25.10\pm 1.66\%\)) with slightly lower average survival for the other two sites (ESJ: \(21.15\pm 1.30\%\); JCJ: \(20.39\pm 1.35\%\)). Average survival among brood years showed little difference; the high apparent survival for brood year 2004 fish exiting ESJ was likely an outlier as it was based on just 3 re-detections below Bonneville Dam.
Among the 69 additive and interactive models evaluated, the survival model that had an interaction between acclimation site, exit date, and smolt size had the lowest QAICc (Table 3; Online Resource Table S1). However, only exit date had an effect on detection. The model predicted that, among fish exiting earlier, smolts that were smaller in size had lower survival probability compared to larger smolts (Fig. 5). Among fish that exited later, survival probability increased at greater rates for smaller smolts relative to larger smolts, with survival probability for smaller smolts actually exceeding that of larger smolts among fish exiting latest from the CFJ site. Fish from CFJ had the highest survival probability regardless of smolt size.
Predicted apparent survival probability (acclimation site to Bonneville Dam) of small (20th percentile FL = 102 mm) and large (80th percentile FL = 116 mm) smolts as a function of exit date based on the best CJS model (Table 3). The shaded area is the standard error of the predicted mean
Ho (3): smolt-to-adult return (SAR) and age-at-return were independent of smolt size, acclimation site, and volitional exit date.
Observed SARs fluctuated between brood years (Fig. 6), beginning at approximately 0.5% for the first four BYs, then increasing threefold to over 1.5% for BY 2006, before declining to between 0.5% and 1.0% for brood years 2007 to 2014. SARs were generally similar among acclimation sites although CFJ had a higher SAR estimate in 9 of 13 years (Fig. 6).
The model including all variables was the best model (Table 4). Predicted SAR from this model showed increasing SAR for CFJ from around 0.3 to 1.7 for early-migrating fish (20th percentile ED = 77) over the range of smolt sizes, while for later emigrating smolts (80th percentile ED = 116) SAR increased from 0.3 to 0.6 (Fig. 7). This pattern was quite similar for all acclimation sites, although the predicted SAR for larger smolts exiting earlier at ESJ and JCJ was not quite as great as predicted SAR for larger smolts from the CFJ site.
Predicted smolt-to-adult return percentage (SAR) using the best model (Table 4) by acclimation site (CFJ, ESJ, and JCJ), smolt size (FL), and 20th and 80th percentile exit day of the year (ED, 77 and 116, respectively)
Conversely, the best model showed predicted SAR decreased during the emigration period for all smolt sizes (Fig. 8). Again, the pattern was fairly similar between acclimation sites. For CJF, predicted SAR decreased from around 0.75 to 0.4 over the season for smaller smolts (20th percentile FL = 102 mm) but from 1.0 to 0.4 for larger smolts (80th percentile FL = 116 mm). For ESJ, these decreases were 0.5 to 0.4 and 0.75 to 0.4 for smaller and larger smolts, respectively. For JCJ, they were 0.7 to 0.2 and 0.9 to 0.2 for smaller and larger smolts, respectively (Fig. 8).
Predicted smolt-to-adult return percentage (SAR) using the best model (Table 4) by acclimation site (CFJ, ESJ, and JCJ), exit day of year (ED), and 20th and 80th percentile smolt size (FL, 102 mm and 116 mm, respectively)
In general, predicted SAR from acclimation site smolt release to Bonneville Dam adult return increased for larger smolts and more so for those emigrating earlier. Also, predicted SAR decreased for smolts emigrating later in the season and was the lowest among larger smolts emigrating later. The relationship between SAR and smolt size was stronger than with emigration timing. Patterns were fairly similar across acclimation sites.
Based on tag detections at Bonneville Dam, the percentage of age-4 and age-5 returning adult fish varied across acclimation sites and brood years (Fig. 9). We observed no differences between acclimation sites, but there was a general trend toward younger age-at-return over time. Modeling to estimate predicted age-at-return showed the strongest support for our candidate model that included emigration timing, smolt size, and their two-way interaction (Table 5). Therefore, inference from the best model can be made pooling data across acclimation sites. For all PIT-tagged CESRF juveniles successfully emigrating below Bonneville Dam, detections for fish returning from marine waters indicated that age-at-return increased with decreasing smolt size and with later emigration from acclimation sites (Fig. 10).
Predicted age4&5 adults (2–3 years in the Pacific Ocean; as a percentage of all returning fish) using the best model (Table 5) by brood year (BY) and acclimation site (CFJ, ESJ, JCJ, and pooled for all 3 acclimation sites combined)
The predicted age4&5 percentage of all returning fish increased from approximately 60% to 90% over the course of the emigration period for smaller smolts (20th percentile FL = 102) and from 55 to 80% for larger smolts (80th percentile FL = 116) (Fig. 11a). Conversely, the predicted age4&5 percentage decreased from approximately 80% to 35% as smolt size increased earlier in the emigration period (20th percentile ED = 77) and from approximately 95% to 25% as smolt size increased with later emigration (80th percentile ED = 116) (Fig. 11b). The smolt size relationship with return age was stronger than the emigration timing relationship.
Predicted age4&5 adults (2–3 years in the Pacific Ocean; as a percentage of all returning fish) using the best model (Table 5) by a acclimation site exit day (ED), for the 20th (102 mm) and 80th (116 mm) percentile smolt sizes (FL), and b smolt size (FL), for the 20th (77) and 80th (116) percentile exit days (ED); data pooled across acclimation sites
Discussion
We observed several dependencies among emigration timing (volitional exit date), smolt size, and river flow near acclimation and release sites, in relation to juvenile survival probability, smolt-to-adult return, and age-at-return. Therefore, all of the tested null hypotheses would be rejected. Our results support and extend previously reported findings that size of juvenile salmon at emigration is an important factor affecting emigration timing (Beckman et al. 1998) and life history patterns (Larsen et al. 2004, 2013, 2019; Harstad et al. 2018), particularly age-at-maturity (Bilton et al. 1982; Tattam et al. 2015; Harstad et al. 2018). These results also support previous work indicating that seasonal growth rates in juvenile salmon (Beckman et al. 1999, 2017; Larsen et al. 2006, 2019) and demography of returning fish (e.g., size and age) should be important considerations in developing management goals for artificial production facilities (Ohlberger et al. 2020; Oke et al. 2020).
In this investigation, larger fish volitionally exited acclimation sites earlier than smaller fish. This finding is consistent with previous studies documenting that larger spring Chinook Salmon smolts tended to move downstream earlier than smaller smolts (Beckman et al. 1998; Zabel and Achord 2004). Beckman et al. (1998) reported that movement patterns were most closely related to growth rates and associated smolt development, including increased gill Na + /K + -ATPase activity, during the spring period preceding emigration (e.g., corresponding to time spent at acclimation sites in this investigation). With the exception of putative precociously maturing males (see discussion below), this investigation provides further evidence that larger smolts may tend to undergo the physiological, morphological, and behavior changes associated with smoltification earlier and emigrate earlier than smaller smolts.
River flow has been shown to influence downstream migration timing and survival of salmonid smolts (McCormick et al. 1998; Connor et al. 2003). Our analyses suggested that lower river flows near the acclimation sites may lead to earlier emigration timing. Sykes et al. (2009) found that accumulated thermal units, rather than river flow, was the parameter that best explained downstream migration timing for Chinook Salmon smolts in a river system that is flow-controlled, similar to the Yakima River. In the upper watersheds of the Yakima River system, flow and water temperature are greatly affected by winter snowpack. Emigration timing in our study was correlated with winter snowfall at Snoqualmie Pass (about 48 km west of Cle Elum, WA) with the two earliest emigration years associated with the lowest winter snowfall and the latest emigration year associated with the highest snowfall (Washington State Department of Transportation). The warmer water associated with lower flows (less snowpack) may have played a role in advancing physiological changes and inducing earlier emigration observed in this study, as even a small difference of 1–2 °C can result in a significant increase in accumulated thermal units.
We observed generally low survival (20–25%) of juveniles released from all three acclimation sites to Bonneville Dam. This compares with estimated 1999–2020 mean hatchery-origin yearling Chinook Salmon juvenile survival values of approximately 30.5% for Snake and 36.7% for Upper Columbia River fish from release to Bonneville Dam (Widener et al. 2021). All species of both natural- and hatchery-origin Yakima River juvenile salmon experience substantial survival bottlenecks due to factors such as emigration timing, flow management, and predation with earlier emigrating fish tending to have better survival from release to McNary Dam than fish emigrating from late May through July (Blodgett et al. 2022). Factors limiting salmon survival in the Yakima River Basin are being addressed through implementation of the Yakima River Basin Integrated Plan (USBOR 2011).
We observed that larger smolt size was associated with better apparent survival, based on detections of both juveniles and returning fish at Bonneville Dam for CESRF spring Chinook Salmon. However, while larger smolts tended to emigrate earlier in our study, we also found that earlier emigrating juveniles demonstrated lower survival probability to Bonneville Dam than did later emigrants, regardless of smolt size. In addition, both the largest and earliest emigrating smolts returned at younger ages (age-2 minijack and age-3 jack males) as opposed to age-4 and age-5 (males and females). The lower juvenile survival probability for earlier emigrants might be partially explained by the fact that these fish experience a longer exposure period to predation and other potential mortality risks (NRC 2004; Pandit et al. 2017; Keefer et al. 2018). Regardless of emigration timing, smaller smolts may experience greater predation risk due to slower speed in avoiding predators (Hasegawa et al. 2021), while larger smolts also experience substantial predation vulnerability due to factors such as caloric content (Werner et al. 1983), predator size (Barnes et al. 2010), or visibility (Forsman and Appelqvist 1998).
It is also possible that many of the larger, earlier emigrating juveniles in our study were precociously mature males. Larsen et al. (2004) found high rates (average ~ 40% of all males released over 10 years) of precocious male maturation in the CESRF population. Precocious maturation (at age-1 or age-2) is a known life-history strategy for male salmonids, whereby fish remain in headwater spawning areas and use their small size and agility to gain spawning opportunities (commonly referred to as “sneaking”) with anadromous females (Fleming 1996). Other studies have demonstrated that a significant proportion of the maturing minijack Chinook Salmon produced by the CESRF (Larsen et al. 2013) and other Columbia Basin hatchery programs (Zimmerman et al. 2003; Beckman and Larsen 2005; Beckman et al. 2017; Harstad et al. 2014, 2018) migrated significant distances downstream (including beyond Bonneville Dam) in the spring in a “smolt-like” manner (Larsen et al. 2010) and then were redetected shortly afterwards moving upstream in an apparent attempt to migrate back to their headwater rearing areas. Tag detection histories indicate that survival of these migrating minijacks is very low, representing a significant loss to anadromous adult (age-3, age-4, and age-5) male production (Beckman and Larsen 2005). Thus, precocial maturation among the larger or earlier emigrating smolts in this study may have contributed to the younger age-at-maturity distributions we observed for these fish.
This investigation clearly demonstrates the challenges associated with developing size-at-release targets for hatchery programs, specifically as they pertain to the survival advantages of releasing larger smolts. As observed in previous investigations (Zabel and Williams 2002; Zabel and Achord 2004; Beckman et al. 2017; Poirier and Olson 2017), we found that larger smolts produced more returning adults. However, we also found that these same larger juveniles tended to return more age-2 minijacks and age-3 jacks and fewer of the larger, more desirable age-4 and age-5 fish. In contrast, we observed that smaller juveniles generally emigrated later and had lower apparent survival both as juveniles and returning fish to Bonneville Dam, but returned a greater abundance of older age classes. Tattam et al. (2015) studied PIT-tagged wild Columbia River Chinook Salmon from 10 successive cohorts and found similar relationships between smolt length and age-at-return (larger smolts had increased probability of age-3 maturation) and between later emigration and older age-at-return. Poirier and Olson (2017) reviewed literature on subyearling and yearling hatchery release strategies for Columbia River fall Chinook Salmon and reported a similar finding: the larger yearlings generally returned at younger average ages and often returned as minijacks and jacks at a much higher frequency than did the smaller subyearling releases. Finally, in a study of artificially reared Coho Salmon, Bilton et al. (1982) found that jack (age-2) returns were maximized with earlier releases of larger smolts.
Results from this investigation and several previous studies highlight a paradox for yearling Chinook Salmon hatchery programs that endeavor to rear smolts to a size that optimizes downstream survival and smolt development for early ocean rearing and growth, while minimizing early male maturation (Larsen et al. 2006, 2013; Spangenberg et al. 2014; Beckman et al. 2017; Harstad et al. 2018). A recurring theme from all these studies, as described in Larsen et al. (2013), is that high growth rates, most notably in the first autumn–winter of rearing, create a population composed of high proportions of both large smolts and minijacks. In contrast, lower autumn feeding rates create a high proportion of relatively small smolts but a lower proportion of minijacks and jacks. Subsequent high growth rates in the spring tend to increase smolt release size and improve smolt development. This feeding regime is sometimes referred to as “the wild fish template” as it more closely mimics development patterns observed in naturally rearing fish (Beckman et al. 2000). More recently, this phenomenon was investigated at a production scale in upper Columbia River hatchery summer Chinook Salmon populations (Harstad et al. 2018). They found that colder water reduced winter growth resulting in smaller fish size at juvenile migration but that smaller smolts returned at older age-classes than did larger smolts. Further, the release group that consisted of the smallest fish and migrated the greatest distance returned as adults at the highest rate with older fish comprising more of the return than observed for other treatment groups (Harstad et al. 2018).
In the Yakima River Basin, the American River (elevation 843 m for spawning and rearing areas) has the coldest water temperatures (https://www.usbr.gov/pn/hydromet/yakima/), and spring Chinook Salmon from this subbasin demonstrate the oldest average age-at-return (Bosch 2019). For spring Chinook Salmon in the Upper Yakima River Basin that were part of this study, the JCJ (elevation 790 m) and ESJ (661 m) acclimation sites generally experienced colder water temperatures than the CFJ (499 m) site (https://www.usbr.gov/pn/hydromet/yakima/), and fish returning from these uppermost acclimation sites had slightly increased probabilities of returning at older ages. Fish from CFJ, which were larger at release and had a shorter migration distance, returned at higher rates, although with a slightly younger age distribution, a result consistent with previous findings (Tipping 2011; Larsen et al. 2013; Faulkner et al. 2019). These findings are also consistent with Spangenberg et al. (2014) and Beckman et al. (2017) who reported that the same stock of fish may respond differently to the unique environmental conditions of a different hatchery rearing environment and that these differences may in turn influence developmental physiology and life history pathways that affect smolt-to-adult return rates and the demography of returning adults.
A recent analytical model of 60 years of data from several, primarily natural origin Pacific salmon, stocks in Alaska found significant declines in size of returning adults in recent decades (Oke et al. 2020). They concluded that the decline was primarily driven by a shifting age structure toward younger ages in response to climate change and competition at sea. Thus, even in wild populations, a demographic shift toward smaller, younger fish may present significant impacts to species viability. Hatchery programs that release larger smolts that tend to return at younger ages may further amplify these demographic trends. From a management perspective, the extent to which these trends in both hatchery and wild fish may be reversed is a daunting prospect given the reliance on improved marine environmental conditions. However, there may be immediate opportunities to affect change in the freshwater environment by modifying culture and release strategies for hatchery fish.
Results from this investigation of CESRF spring Chinook Salmon may have general utility for many salmon hatchery programs throughout the Columbia Basin and broader Pacific region. While each program has unique hatchery (water temperature, density, ration, etc.) and tributary (elevation, geology, hydrology, habitat) characteristics, many regional watersheds share enough similarities that hatchery fish may experience many common factors in the freshwater migration and marine environment. Therefore, results from this study may provide useful general insights on optimal sizes and release timing for further refinement at the program level to optimize adult returns and demography. Strategies for altering size at release targets include manipulation of incubation temperature to adjust emergence and pond timing (Winsor et al. 2021), juvenile growth modulation via ration and temperature manipulation (Larsen et al. 2006; Spangenberg et al. 2014; Harstad et al. 2018; Winsor et al. 2021), photoperiod manipulation (Beckman et al. 2007; Imsland et al. 2014; Foss et al. 2020), and the use of partial reuse aquaculture systems (PRAS) for improved environmental control throughout rearing (Willard and Murauskas 2019). Furthermore, whether with protracted volitional release or a single release event, timing of juvenile releases may be calibrated to optimize adult returns based on PIT-tag histories as employed in this investigation. While declines in size and age at maturity of hatchery salmon stocks present significant ecological and socioeconomic consequences to population resilience and stock recovery, results from this investigation demonstrate that even modest adjustments to rearing and release strategies could prove effective in mitigating this trend.
Data availability
Data used in this study are available at ptagis.org, cbfish.org (see https://www.cbfish.org/Document.mvc/Viewer/P186823), and by contacting the corresponding author.
References
Banks JL (1994) Raceway density and water flow as factors affecting spring chinook (Oncorhynchus tshawytscha) during rearing and after release. Aquaculture 119:210–217
Barnes C, Maxwell D, Reuman DC, Jennings S (2010) Global patterns in predator–prey size relationships reveal size dependency of trophic transfer efficiency. Ecology 91:222–232.https://doi.org/10.1890/08-2061.1
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Beckman BR, Larsen DA (2005) Upstream migration of minijack (age-2) Chinook Salmon in the Columbia River: behavior, abundance, distribution, and origin. Trans Am Fish Soc 134:1520–1541
Beckman BR, Larsen DA, Lee-Pawlak B, Dickhoff WW (1998) Relation of fish size and growth rate to migration of spring Chinook Salmon smolts. N Am J Fish Manag 18:537–546
Beckman BR, Dickhoff WW, Zaugg WS, Sharpe C, Hirtzel S, Schrock R, Larsen DA, Middleton MA, Ewing RD, Palmisano A, Schreck CB, Mahnken CVW (1999) Growth, smoltification, and smolt-to-adult return of spring Chinook Salmon from hatcheries on the Deschutes River, Oregon. Trans Am Fish Soc 128:1125–1150
Beckman BR, Larsen DA, Sharpe C, Lee-Pawlak L, Dickhoff WW (2000) Physiological status of naturally rearing juvenile Chinook Salmon in the Yakima River: seasonal dynamics and changes associated with the parr–smolt transformation. Trans Am Fish Soc 129:727–753
Beckman BR, Gadberry BA, Parkins P, Cooper KA, Arkush KD (2007) State-dependent life history plasticity in Sacramento River winter-run Chinook Salmon (Oncorhynchus tshawytscha): interactions among photoperiod and growth modulate smolting and early male maturation. Can J Fish Aquat Sci 64:256–271
Beckman BR, Harstad DL, Spangenberg DK, Gerstenberger R, Brun CV, Larsen DA (2017) The impact of different hatchery rearing environments on smolt-to-adult survival of spring Chinook Salmon. Trans Am Fish Soc 146:539–555
Bilby RE, Fransen BR, Bisson PA, Walter JK (1998) Response of juvenile Coho Salmon (Oncorhynchus kisutch) and steelhead (Oncorhynchus mykiss) to the addition of salmon carcasses to two streams in southwestern Washington, U.S.A. Can J Fish Aquat Sci 55:1909–1918
Bilby RE, Beach EW, Fransen BR, Walter JK, Bisson PA (2003) Transfer of nutrients from spawning salmon to riparian vegetation in western Washington. Trans Am Fish Soc 132:733–745
Bilton HT, Alderdice DF, Schnute JT (1982) Influence of time and size at release of juvenile Coho Salmon (Oncorhynchus kisutch) on returns at maturity. Can J Fish Aquat Sci 39:426–447
Blodgett J, Johnston M, Bosch B (eds) (2022) Yakima/Klickitat Fisheries Project monitoring and evaluation – Yakima subbasin; Final report for the performance period May 1, 2021 through April 30, 2022, Project No. 1995–063–25, 387 electronic pages. Yakama Fish Toppenish, WA
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Natur 161:1–28
Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC, Vasseur DA (2011) Why intraspecific trait variation matters in community ecology. Trends Ecol Evol 26:183–192
Bosch B (2019) Summary of data collected by the Yakama Nation relative to Yakima River spring Chinook Salmon and the Cle Elum spring Chinook supplementation and research facility. In: Fiander B, Fast D, Bosch B (ed) Yakima/Klickitat Fisheries Project Monitoring and Evaluation – Yakima Subbasin; Final Report for the performance period May 1, 2018 through April 30, 2019, pp 110–195. https://www.cbfish.org/Document.mvc/Viewer/P166893 Accessed 3/1/2022
Brown RS, Oldenburg EW, Seaburg AG, Cook KV, Skalski JR, Eppard MB, Deters KA (2013) Survival of seaward-migrating PIT and acoustic-tagged juvenile Chinook Salmon in the Snake and Columbia Rivers: an evaluation of length-specific tagging effects. Anim Biotelem 1:8
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information–theoretic approach. Springer-Verlag, New York
Conner MM, Budy PE, Wilkison RA, Mills M, Speas D, Mackinnon PD, Mckinstry MC (2020) Estimating population abundance with a mixture of physical capture and PIT tag antenna detection data. Can J Fish Aquat Sci 77:1163–1171
Connolly PJ, Jezorek IG, Martens KD, Prentice EF (2008) Measuring the performance of two stationary interrogation systems for detecting downstream and upstream movement of PIT-tagged salmonids. N Am J Fish Manag 28:402–417
Connor WP, Burge HL, Yearsley YR, Bjornn TC (2003) Influence of flow and temperature on survival of wild subyearling fall Chinook Salmon in the Snake River. N Am J Fish Manag 23:362–375
Cooch EG, White GC (2018) Program MARK: a gentle introduction, 18th edition.http://www.phidot.org/software/mark/docs/book/ Accessed 3/1/2022
Cormack RM (1964) Estimates of survival from the sightings of marked animals. Biometrika 51:429–438. https://doi.org/10.2307/2334149
del Rosario RB, Redler YJ, Newman K, Brandes PL, Sommer T, Reece K, Vincik R (2013) Migration patterns of juvenile winter-run-sized Chinook Salmon (Oncorhynchus tshawytscha) through the Sacramento–San Joaquin delta. San Franc. Estuary Watershed Sci 11. https://doi.org/10.15447/sfews.2013v11iss1art3
Dittman AH, May D, Larsen DA, Moser ML, Johnston M, Fast D (2010) Homing and spawning site selection by supplemented hatchery and natural-origin Yakima River spring Chinook Salmon. Trans Am Fish Soc 139:1014–1028
Dunnigan JL, Terrazas MM (2021) Factors influencing rainbow trout annual growth in a large regulated river. Trans Am Fish Soc 150:89–114
Evans AF, Hostetter NJ, Collis K, Roby DD, Loge FJ (2014) Relationship between juvenile fish condition and survival to adulthood in steelhead. Trans Am Fish Soc 143:899–909
Evans AF, Payton Q, Turecek A, Cramer B, Collis K, Roby DD, Loschl PJ, Sullivan L, Skalski J, Weiland M, Dotson C (2016) Avian predation on juvenile salmonids: spatial and temporal analysis based on acoustic and passive integrated transponder tags. Trans Am Fish Soc 145:860–877. https://doi.org/10.1080/00028487.2016.1150881
Fast DE, Neeley D, Lind DT, Johnston MV, Strom CR, Bosch WJ, Knudsen CM, Schroder SL, Watson BD (2008) Survival comparison of spring Chinook Salmon reared in a production hatchery under optimum conventional and seminatural conditions. Trans Am Fish Soc 137:1507–1518
Fast DE, Bosch WJ, Johnston MV, Strom CR, Knudsen CM, Fritts AL, Temple GM, Pearsons TN, Larsen DA, Dittman AH, May D (2015) A synthesis of findings from an integrated hatchery program after three generations of spawning in the natural environment. N Am J Aquac 77:377–395
Faulkner JR, Bellerud BL, Widener DL, Zabel RW (2019) Associations among fish length, dam passage history, and survival to adulthood in two at-risk species of Pacific salmon. Trans Am Fish Soc 148:1069–1087
Fenberg PB, Roy K (2008) Ecological and evolutionary consequences of size-selective harvesting: how much do we know? Mol Ecol 17:209–220
Fleming IA (1996) Reproductive strategies of Atlantic Salmon: ecology and evolution. Rev Fish Biol Fish 6:379–416
Fleming IA, Gross MR (1994) Breeding competition in a Pacific salmon (coho: Oncorhynchus kisutch): measures of natural and sexual selection. Evolution 48:637–657
Forsman A, Appelqvist S (1998) Visual predators impose correlational selection on prey color pattern and behavior. Behav Ecol 9:409–413. https://doi.org/10.1093/beheco/9.4.409
Foss A, Siilkavuopio SI, Imsland AKD (2020) Effects of altered photoperiod regimes during winter on growth and gonadosomatic index in Arctic charr (Salvelinus alpinus) reared in freshwater. Aquac Res 51:1365–1371
Gosselin JL, Zabel RW, Anderson JJ, Faulkner JR, Baptista AM, Sandford BP (2018) Conservation planning for freshwater marine carryover effects on Chinook Salmon survival. Ecol Evol 8:319–332
Greenberg LA, Giller PS (2000) The potential of flatbed passive integrated transponder antennae for studying habitat use by stream fishes. Ecol Freshw Fish 9:74–80
Hankin DG, Fitzgibbons J, Chen Y (2009) Unnatural random mating policies select for younger age at maturity in hatchery Chinook Salmon (Oncorhynchus tshawytscha) populations. Can J Fish Aquat Sci 66:1505–1521
Harstad DL, Larsen DA, Beckman BR (2014) Variation in minijack rate among Columbia River basin Chinook Salmon hatchery populations. Trans Am Fish Soc 143:768–778
Harstad DL, Larsen DA, Miller J, Adams I, Spangenberg DK, Nance S, Rohrbach L, Murauskas JG, Beckman BR (2018) Winter-rearing temperature affects growth profiles, age of maturation, and smolt-to-adult returns for yearling summer Chinook Salmon in the Upper Columbia River Basin. N Am J Fish Manag 38:867–885
Hartman GF, Groot C, Northcote TG (2020) Science and management in sustainable salmonid fisheries: the ball is not in our court. In: Knudsen EE et al (eds) Sustainable fisheries management. CRC Press, Boca Rotan, pp 31–50
Hasegawa K, Honda K, Yoshiyama T, Suzuki K, Fukui S (2021) Small biased body size of salmon fry preyed upon by piscivorous fish in riverine and marine habitats. Can J Fish Aquat Sci 78:631–638
Imsland AK, Handeland SO, Stefansson SO (2014) Photoperiod and temperature effects on growth and maturation of pre- and post-smolt Atlantic Salmon. Aquac Int 22:1331–1345
Irvine JR, O’Neill M, Godbout L, Schnute J (2013) Effect of smolt release timing and size on the survival of hatchery-origin Coho Salmon in the Strait of Georgia. Prog Oceanogr 115:111–118
Jolly GM (1965) Explicit estimates from capture recapture data with both death and immigration-stochastic model. Biometrika 52:225–247. https://doi.org/10.2307/2333826
Keefer ML, Clabough TS, Jepson MA, Johnson EL, Peery CA, Caudill CC (2018) Thermal exposure of adult Chinook Salmon and steelhead: diverse behavioral strategies in a large and warming river system. PLoS One 13:e0204274. https://doi.org/10.1371/journal.pone.0204274
Knudsen CM, Schroder SL, Busack C, Johnston MV, Pearsons TN, Bosch WJ, Fast DE (2006) Comparison of life history traits between first-generation hatchery and wild upper Yakima River spring Chinook Salmon. Trans Am Fish Soc 135:1130–1144
Knudsen CM, Schroder SL, Busack C, Johnston MV, Pearsons TN, Strom CR (2008) Comparison of female reproductive traits and progeny of first-generation hatchery and wild upper Yakima River spring Chinook Salmon. Trans Am Fish Soc 137:1433–1445
Laake JL (2013) RMark: An R interface for analysis of capturerecapture data with MARK. AFSC Processed Rep 2013–01, 25p. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv., Seattle, WA. https://repository.library.noaa.gov/view/noaa/4372 Accessed 3/1/202
Lackey RT (2001) Defending reality. Fisheries 26(6):26–27. https://doi.org/10.1577/1548-8446-26-6
Larsen DA, Beckman BR, Cooper KA, Barrett D, Johnston M, Swanson P, Dickhoff WW (2004) Assessment of high rates of precocious male maturation in a spring Chinook Salmon supplementation hatchery program. Trans Am Fish Soc 133:98–120
Larsen DA, Beckman BR, Strom CR, Parkins PJ, Cooper KA, Fast DE, Dickhoff WW (2006) Growth modulation alters the incidence of early male maturation and physiological development of hatchery-reared spring Chinook Salmon: a comparison with wild fish. Trans Am Fish Soc 135:1017–1032
Larsen DA, Beckman BR, Cooper KA (2010) Examining the conflict between smolting and precocious male maturation in spring (stream-type) Chinook Salmon. Trans Am Fish Soc 139:564–578
Larsen DA, Harstad DL, Strom CR, Johnston MV, Knudsen CM, Fast DE, Pearsons TN, Beckman BR (2013) Early life history variation in hatchery- and natural-origin spring Chinook Salmon in the Yakima River, Washington. Trans Am Fish Soc 142:540–555
Larsen DA, Harstad DL, Fuhrman AE, Knudsen CM, Schroder SL, Bosch WJ, Galbreath PF, Fast DE, Beckman BR (2019) Maintaining a wild phenotype in a conservation hatchery program for Chinook salmon: the effect of managed breeding on early male maturation. PLoS One 14:e0216168. https://doi.org/10.1371/journal.pone.0216168
Lebreton JD, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypothesis using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Ledgerwood RD, Ferguson JW, Ryan BA, Dawley EM, Nunnallee EP (2004) A surface trawl to detect migrating juvenile salmonids tagged with passive integrated transponder tags. N Am J Fish Manag 24:440–451
Lewis B, Grant WS, Brenner RE, Hamazaki T (2015) Changes in size and age of Chinook Salmon Oncorhynchus tshawytscha returning to Alaska. PLoS One 10:e0132872. https://doi.org/10.1371/journal.pone.0130184
Lichatowich JA (1999) Salmon without rivers: a history of the Pacific salmon crisis. Island Press, Washington D.C.
Lubich C, Campos C, Freitas C, Siqueira-Souza F (2021) Effects of fishing on the population of Speckled Pavon Cichla temensis in the Middle Negro River (Amazonas State, Brazil): a decrease in the size of the trophy fish? Trans Am Fish Soc 150:667–678
McCann J, Chockley B, Cooper E, Scheer G, Haeseker S, Lessard R, Copeland T, Ebel J, Storch A, Rawding D (2022) Comparative survival study of PIT-tagged spring/summer/fall Chinook, summer steelhead, and Sockeye. https://www.fpc.org/documents/CSS/2021_CSS_Annual_Report.pdf Accessed 3/1/2022
McCormick SD, Hansen LP, Quinn TP, Saunders RL (1998) Movement, migration, and smolting of Atlantic Salmon (Salmo salar). Can J Fish Aquat Sci 55(Supplement 1):71–92
McCullagh P, Nelder J (1989) Generalized linear models, 2nd edn. CRC Press, Boca Rotan
Mobrand L, Barr J, Blankenship HL, Campton DE, Evelyn TTP, Flagg T, Mahnken C, Seeb LW, Seidel PR, Smoker WW (2005) Hatchery reform in Washington State: principles and emerging issues. Fisheries 30(6):11–23
National Research Council (NRC) (2004) Managing the Columbia River: instream flows, water withdrawals, and salmon survival. The National Academies Press, Washington, D.C.
O’Donnell MJ, Horton GE, Letcher BH (2010) Use of portable antennas to estimate abundance of PIT-tagged fish in small streams: factors affecting detection probability. N Am J Fish Manag 30:323–336
Ohlberger J, Ward EJ, Schindler DE, Lewis B (2018) Demographic changes in Chinook Salmon across the Northeast Pacific Ocean. Fish Fish 19:533–546
Ohlberger J, Schindler DE, Brown RJ, Harding JMS, Adkison MD, Munro AR, Horstmann L, Spaeder J (2020) The reproductive value of large females: consequences of shifts in demographic structure for population reproductive potential in Chinook Salmon. Can J Fish Aquat Sci 77:1292–1301
Oke KB, Cunningham CJ, Westley PAH, Baskett ML, Carlson SM, Clark J, Hendry AP, Karatayev VA, Kendall NW, Kibele J, Kindsvater HK, Kobayashi KM, Lewis B, Munch S, Reynolds JD, Vick GK, Palkovacs EP (2020) Recent declines in salmon body size impact ecosystems and fisheries. Nat Commun 11:1–13
Pandit SN, Maitland BM, Pandit LK, Poesch MS, Enders EC (2017) Climate change risks, extinction debt, and conservation implications for a threatened freshwater fish: Carmine Shiner (Notropis percobromus). Sci Total Environ 598:1–11
Paquet PJ, Flagg T, Appleby A, Barr J, Blankenship L, Campton D, Delarm M, Evelyn T, Fast D, Gislason J, Kline P, Maynard D, Mobrand L, Nandor G, Seidel P, Smith S (2011) Hatcheries: conservation, and sustainable fisheries- achieving multiple goals: results of the Hatchery Scientific Review Group’s Columbia River Basin Review. Fisheries 36:547–561
Poirier J, Olson D (2017) Literature review of survival, age at return, straying, life history diversity and ecological effects of subyearling and yearling hatchery release strategies for fall Chinook Salmon. U.S. Fish and Wildlife Service, Columbia River Fish and Wildlife Conservation Office, Vancouver, WA. https://www.fws.gov/columbiariver/publications/Fall%20Chinook%20Literature%20Review%2020%20April%202017.pdf Accessed 3/1/2022
Prentice EF, Flagg TA, McCutcheon CS, Brastow DF (1990) PIT-tag monitoring systems for hydroelectric dams and fish hatcheries. In: Parker NC, Giorgi AE, Heidinger RC, Jester DBJ, Prince ED, Winans GA (ed) Fish-marking techniques, Am Fish Soc Symp 7, Bethesda, Maryland, pp 323–334
R Core Team (2021) R: A language and environment for statistical computing. R Found Stat Comput, Vienna, Austria
Rosenberger SJ, Connor WP, Peery CA, Milks DJ, Schuck ML, Hesse JA, Smith SG (2013) Acclimation enhances postrelease performance of hatchery fall Chinook Salmon subyearlings while reducing the potential for interaction with natural fish. N Am J Fish Manag 33:519–528
Scheuerell M, Zabel R, Sandford B (2009) Relating juvenile migration timing and survival to adulthood in two species of threatened Pacific salmon (Oncorhynchus spp.). J Appl Ecol 46:983–990
Schroder SL, Knudsen CM, Pearsons TN, Kassler TW, Young SF, Beall EP, Fast DE (2010) Behavior and breeding success of wild and first generation hatchery male spring Chinook Salmon spawning in an artificial stream. Trans Am Fish Soc 139:989–1003
Schroder SL, Knudsen CM, Pearsons TN, Kassler TW, Beall EP, Young SF, Fast DE (2012) Breeding success of four male life history types of spring Chinook Salmon spawning in an artificial stream. Environ Biol Fish 94:231–248
Seber GAF (1965) A note on the multiple recapture census. Biometrika 52:249–259. https://doi.org/10.2307/2333827
Skalski JR, Smith SG, Iwamoto RN, Williams JG, Hoffmann A (1998) Use of passive integrated transponder tags to estimate survival of migrant juvenile salmonids in the Snake and Columbia Rivers. Can J Fish Aquat Sci 55:1484–1493
Smith SG, Muir WD, Williams JG, Skalski JR (2002) Factors associated with travel time and survival of migrant yearling Chinook Salmon and steelhead in the Lower Snake River. N Am J Fish Manag 22:385–405
Sommer T, Harrell W, Nobriga M (2005) Habitat use and stranding risk of juvenile Chinook Salmon on a seasonal floodplain. N Am J Fish Manag 25:1493–1504
Spangenberg D, Larsen DA, Gerstenberger R, Brun C, Beckman BR (2014) The effects of variation in rearing conditions on growth, smolt development, and minijack rate in yearling Chinook Salmon: a hatchery scale experiment. Trans Am Fish Soc 143:1220–1230
Sykes GE, Johnson CJ, Shrimpton JM (2009) Temperature and flow effects on migration timing of Chinook Salmon smolts. Trans Am Fish Soc 138:1252–1265
Takata L, Sommer TR, Conrad JL, Schreier BM (2017) Rearing and migration of juvenile Chinook Salmon (Oncorhynchus tshawytscha) in a large river floodplain. Environ Biol Fish 100:1105–1120
Tattam IA, Ruzycki JR, McCormick JL, Carmichael RW (2015) Length and condition of wild Chinook Salmon smolts influence age at maturity. Trans Am Fish Soc 144:1237–1248
Thiem JD, Binder TR, Dumont P, Hatin D, Hatry C, Katopodis C, Stamplecoskie KM, Cooke SJ (2013) Multispecies fish passage behaviour in a vertical slot fishway on the Richelieu River, Quebec, Canada. River Res Appl 29:582–592
Tiffan KF, Rhodes TN, Bickford BK, Lebeda DD, Connor WP, Mullins FL (2021) Performance of subyearling fall Chinook Salmon tagged with 8-, 9-, and 12-mm passive integrated transponder tags in the Snake River. N Am J Fish Manag 41:176–186
Tipping JM (2011) Effect of juvenile length on Chinook Salmon survivals at four hatcheries in Washington state. N Am J Aquac 73:164–167
USBOR (U.S. Bureau of Reclamation 2011) Yakima River basin study, volume 1. Proposed integrated water resource management plan. USBOR, Yakima, WA
Waples RS, Beechie T, Pess GR (2009) Evolutionary history, habitat disturbance regimes, and anthropogenic changes: what do these mean for resilience of Pacific salmon populations? Ecol Soc 14(1):3
Waples RS, Ford MJ, Schmitt D (2007) Empirical results of salmon supplementation in the Northeast Pacific: a preliminary assessment. In: Bert TM (ed) Ecological and genetic implications of aquaculture activities, Springer, Dordrecht, The Netherlands, pp 383–403
Ward DL, Boyce RR, Young FR, Olney FE (1997) A review and assessment of transportation studies for juvenile Chinook Salmon in the Snake River. N Am J Fish Manag 17:652–662
Werner EE, Mittelbach GG, Hall DJ, Gilliam JF (1983) Experimental tests of optimal habitat use in fish: the role of relative habitat profitability. Ecology 64:1525–1539. https://doi.org/10.2307/1937507
Widener DL, Faulkner JR, Smith SG, Marsh TM, Zabel RW (2021) Survival Estimates for the passage of spring migrating juvenile salmonids through Snake and Columbia River dams and reservoirs, 2020. NOAA NW Fish Sci Ctr Seattle, WA. https://doi.org/10.25923/g1b4-gc05
Willard C, Murauskas J (2019) Chinook reared in partial RAS outshine its raceway-grown counterparts. https://www.hatcheryinternational.com/chinook-raised-in-partial-ras-outshine-its-raceway-grown-counterparts/ Accessed 3/1/2022
Winsor S, Blumenshine S, Adelizi P, Bigelow M (2021) Precocious maturation in spring-run Chinook Salmon is affected by incubation temperature, feeding regime, and parentage. Trans Am Fish Soc 150:578–592
Zabel RW, Achord S (2004) Relating size of juveniles to survival within and among populations of Chinook Salmon. Ecology 85:795–806
Zabel RW, Williams JG (2002) Selective mortality in Chinook Salmon: what is the role of human disturbance? Ecol Appl 12:173–183
Zhou S, Smith ADM, Punt AE, Richardson AJ, Gibbs M, Fulton EA, Pascoe S, Bulman C, Bayliss P, Sainsbury K (2010) Ecosystem-based fisheries management requires a change to the selective fishing philosophy. Proc Natl Acad Sci USA 107:9485–9489
Zimmerman CE, Stonecypher RWJ, Hayes MC (2003) Migration of precocious male hatchery Chinook Salmon in the Umatilla River, Oregon. N Am J Fish Manag 23:1006–1014
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
Monitoring and evaluation efforts for the Cle Elum Supplementation and Research Facility are the result of a cooperative effort by many individuals from a variety of agencies including the Yakama Nation Fisheries Program, the Washington Department of Fish and Wildlife, the Columbia River Inter-Tribal Fish Commission, Pacific States Marine Fisheries Commission, and NOAA Fisheries. We would especially like to thank Yakama Nation for their ongoing support of this project, Dr. David Fast (senior research scientist, retired), Charles Strom and all of the fish culture staff at the CESRF complex, all of the technicians who have marked and tagged fish through the years, and David Lind and Daylen Isaac who provided considerable data retrieval and analysis contributions in support of this work. Finally, we would like to thank two anonymous reviewers for their very constructive comments and recommendations that helped improve this publication.
Funding
This work is funded by the Bonneville Power Administration (BPA) through the Northwest Power and Conservation Council’s Fish and Wildlife Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Ethical approvals were not applicable to the present study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bosch, W.J., Pandit, S.N., Sandford, B.P. et al. Effects of volitional emigration timing and smolt size on survival and age-at-return in a Pacific salmon hatchery population. Environ Biol Fish 106, 1037–1059 (2023). https://doi.org/10.1007/s10641-023-01395-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01395-0