Abstract
The European eel (Anguilla anguilla) has undergone an unprecedented population decline since the 1980s, with current recruitment levels fluctuating from 3 to 15% of historical levels for the last 20 years. Monitoring of glass eels and elvers as 0 + recruitment is an essential step in helping to understand the trend in recruitment and to better quantify the current recruitment time series. Two locations within the Shannon estuary on the west coast of Ireland were monitored for glass eel recruitment from January to April in 2017 and 2018. This study used a generalised linear mixed model to examine a range of environmental variables impacting on glass eel abundance in transitional waters. Results found that water temperature and moon phase were the most important variables. Tidal height and cloud cover also influenced the abundance of glass eels but to a lesser extent. This study found that focussing survey efforts on nights around the full moon when water temperatures exceed 5℃ will allow a catch which is representative of the population in an estuary. Glass eel monitoring needs a long-term sampling plan in order to account for annual fluctuations apparent in glass eel recruitment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recruitment of the European eel (Anguilla anguilla L.) has experienced an unprecedented decline since the late 1970s and early 1980s. Recent estimates have put the decline at 90% with recruitment levels fluctuating from 3 to 15% of historical levels for the last 20 years (Dekker 2003, 2019; Dekker and Casselman 2014). The International Union for Conservation of Nature placed the European eel on its critically endangered list in 2008; this listing was reviewed in 2020 without changing the status (Freyhof and Kottelat 2008; Pike et al. 2020). The decline in eel numbers is due to the interaction between human activities, oceanic fluctuations and possibly climate change (Feunteun 2002; Dekker 2003; Knights 2003; van Ginneken and Maes 2005). The current low level of the eel stock, in conjunction with the legacy of reduced recruitment for the past 40 years, could hinder the recovery of this species (Dekker 2003). In 2007, the EU established a management framework (EU Regulation No. 1100/2007), the objective of which is to protect the eel and promote recovery and the sustainable use of the EU eel stock. Each European Union member state developed an Eel Management Plan with the aim of ensuring at least 40% of historical levels of silver eels escape continental waters.
The European eel (Anguilla anguilla L.) is a catadromous species, whose range extends over Europe and northern Africa (Tesch and Thorpe 2003). European eels leave continental waters from September to December as silver eels crossing the Atlantic Ocean to their spawning grounds in the Sargasso Sea. The resulting eel larvae, called leptocephalus, make their way to Europe on oceanic currents (Kettle and Haines 2006; Friedland et al. 2007; Bonhommeau et al. 2008a, 2009, 2010). Before migrating into inshore waters, they undergo metamorphosis into the glass eel stage.
The signal to migrate inshore is signalled by numerous environmental cues such as freshwater lure (Tosi and Sola 1993; Sullivan et al. 2006; Crivelli et al. 2008), salinity gradients (Tosi et al. 1990; Edeline et al. 2005), water temperature (Jessop 2003; Briand et al. 2005; Edeline et al. 2006; Laffaille et al. 2007), tidal cycles (Laffaille et al. 2007; Cresci et al. 2017), lunar phases and light intensity (Jellyman and Lambert 2003; Bardonnet et al. 2005; Jellyman et al. 2009). Using Selective Tidal Stream Transport, the eels enter estuaries and the lower tidal reaches of rivers between December and April (Harrison et al. 2014; Trancart et al. 2014). Once in contact with estuarine water, they start to pigment, with pigmentation increasing with time and water temperature (Briand et al. 2005). As eels reach the upper estuary, their behaviour changes from Selective Tidal Stream Transport to counter current active migration in order to move upstream against the flow of water (Briand et al. 2005). Glass eels develop into yellow eels and remain in freshwater for 5–49 years before maturing and undergoing their final transformation as silver eels and migrating to sea to complete their life cycle (Poole and Reynolds 1996; Aoyama and Miller 2003; van Ginneken and Maes 2005).
As part of the assessment on the status of the European Eel stock, the Joint EIFAAC/ICES/GFCM Working group on Eel uses the trend in eel recruitment (Amilhat et al. 2019). This recruitment trend comprises of catch data submitted annually by countries and is based on glass eel commercial fisheries data, research surveys and eel traps located at barriers. However, since the introduction of the EU eel regulation, commercial glass eel fisheries have experienced management measures such as quotas and reduced fishing season. This has resulted in a reduction in the commercial glass eel time series available for this important analysis. As a result, a number of authors have recommended that countries increase the number of recruitment surveys to improve the data available for modelling the annual recruitment of the European eel (Knights 2003; ICES 2008; Bornarel et al. 2018).
Many fishery independent recruitment series focus on the actively migrating eel in the upper estuary due to the ease of capturing eels at this life stage. Eels are migrating upstream against the flow of the river and are concentrated along the banks making them easy to intercept or are captured in eel ladders located at barriers (Moriarty 1978; White and Knights 1997; Piper et al. 2012; Harrison et al. 2014; Pecorelli et al. 2019; Tamario et al. 2019). However, at this point, the recruitment reflects the quantity of eels moving into freshwater and does not take into account the amount of eels settling in the estuarine environment. Growth rates are higher for eels captured in transitional waters compared with freshwater caught eels and if conditions in the estuary are suitable, eels can remain in estuarine and coastal waters until outward migration at the silver eel stage (Tsukamoto and Arai 2001; Marohn et al. 2013); therefore, it is important to capture this cohort of the population.
Commercial glass eel fishing has been prohibited in Ireland under the 1959 Fisheries Act. However, there was a glass eel and elver stocking programme operated by the Electricity Supply Board (ESB Ireland) from 1959 to 2007. The aim of the programme was to source eels from the surrounding river estuaries for stocking above a hydropower dam on the River Shannon to support the commercial eel fisheries located upstream (Reynolds et al. 1994 and O'Connor 2003). One of the locations of these stocking programmes was used in this current study.
The first objective of this study was to examine the most influential environmental variables on glass eel abundance in the literature and develop a standardised sampling method to target the optimum glass eel migration period. This study then examined the resulting catch of glass eels in the upper Shannon estuary to refine the list of environmental variables that influence this catch.
Materials and methods
Study site
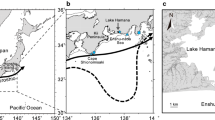
At 240 km in length, the River Shannon is the longest river in Ireland. The river rises in County Cavan in the north of the country and meets the Atlantic Ocean in the southwest (Fig. 1). The Shannon estuary is the largest in Ireland with a southwest orientation which is similar to the orientation of the Severn estuary, a well-known location for glass eel (White and Knights 1997; Walmsley et al. 2018). The estuary is macro tidal, with the tidal range in the inner estuary at Limerick being the largest on the Irish coast at 5.4 m (Healy and Hickey 2002). The Shannon estuary has vast expanses of intertidal mudflats often fringed with saltmarsh vegetation; these exposed mudflats have been listed as a Special Area of Conservation and a Special Protected Area.
In this study, glass eel surveys were conducted in the upper Shannon estuary on the Owengarney River at Bunratty Bridge and at Latoon Bridge located on the Rine River (Fig. 1). These locations were chosen based on the advice and knowledge of Mr. H. Power following his involvement with the ESB stocking programme carried out up to 2007. The original sampling design was to fish 2 years at Latoon Bridge; however, due to essential construction works being carried out by the local council on Latoon Bridge in late 2017 and early 2018, a second location was required.
Glass eel collection
Glass eels were collected using standard conical-shaped plankton nets with a 1-l collection bottle attached. The net had a diameter of 0.5 m, and a length of 2 m with a mesh size of 800 µm. A flowmeter was suspended in the middle of the opening of each net to calculate the volume of water filtered. To sample the incoming tide, three plankton nets were set from the bridge in Latoon (two from the middle arch and one alternating between the right and left arch). Bunratty Bridge is a narrower single span bridge and two plankton nets were set in the middle of the channel concentrated in the fastest flow. As glass eels are proportionally dispersed throughout the water column on a rising tide, the nets were set so that they fished within the first metre of the water column (Witting et al. 1999; Jellyman and Lambert 2003; Laffaille et al. 2007).
Surveys were conducted on a rising tide over two consecutive nights during each new and full moon phase from January to April. The survey dates were selected for nights when the tidal range exceeded 4.7 (Reynolds et al. 1994) and when high tide occurred before sunrise (McCleave and Kleckner 1982; Wippelhauser and McCleave 1987). This resulted in a total of 4 survey nights per calendar month (Table 1). Many studies show that glass eels use the rising tide to migrate upstream so the plankton nets were set 2.5 h before high water, with the nets fishing until the tide turned to ebb (Fukuda et al. 2016). Each net fished for 30 min before the catch was removed and the net reset; each net had a total of 3 hauls per night. This design was chosen to ensure the glass eels were not in the net for excessive amount of time. Depth measurements were taken at the start of each net haul. Time at which the nets started actively fishing and time the nets were removed at end of the survey were recorded. The net was washed down and shaken to ensure all glass eels sampled were collected in the sample bottle. Sample bottles were then removed and contents passed through a sieve to collect any glass eels and by-catch. The total number and weight of glass eels was recorded per net haul.
At each sampling session, a sample of glass eels were euthanized in anaesthetic and taken back to the laboratory, where length (cm) and weight (g) were recorded. Pigment stage was determined using a binocular dissecting microscope and the classification system based on the index used in Briand et al. 2005 (adapted from Elie 1982; Strubberg 1913; Lecomte-Finiger 1984). This classification with seven stages was chosen as it reviews preceding classifications, in particular Strubberg (1913) that considers six stages (Supplementary Information Fig. 1). Otoliths were extracted from 50 individuals from Bunratty Bridge. The otoliths were examined for age using the radii measurements. The radii of each otolith is measured and plotted against glass eel length. To verify this method, a subsample of otoliths were aged (n = 15) via a modification of the burn and crack method (Christensen 1964; Moriarty 1983). This same subsample of 15 individuals was also examined for the presence of a zero band (transition ring).
Environmental variables
Nine environmental variables were recorded at each survey, namely water temperature, depth, water conductivity, salinity, freshwater discharge, atmospheric pressure, moon phase, percentage illumination and cloud cover. A water temperature data logger was deployed at each site from October to May each year. At each net haul, onsite water temperature and salinity readings were recorded using a handheld multi probe (YSI in 2017, EXTECH ExStik®II Conductivity/TDS/Salinity Meter in 2018). Moon phase is a qualitative variable with two levels: new moon and full moon. Percentage illumination was downloaded for the period of the survey (https://www.moongiant.com/). Mean cloud cover, cumulative rainfall 5 days before survey, wind speed, wind direction and air temperature were taken from the meteorological recordings from Met Eireann at Shannon Airport (https://data.gov.ie/dataset/shannon-airport-daily-weather-station-data). Sea surface water temperature was taken from the Marine Institute weather buoys M4 and M6 (https://data.gov.ie/dataset/weather-buoy-network). Tidal heights were taken from local tide tables (Shannon Foynes Port Company). Sunrise timings were recorded for Ennis County Clare, Ireland (https://www.timeanddate.com). For each net, the number of flow metre revolutions was converted to the volume of water filtered (m3). These environmental variables are used in the model selection to assess glass eel recruitment.
Data Analysis
The data from the 3 individual net hauls are pooled into 1 net value and this was examined with the environmental data. Data exploration was carried out using the guidelines from Zuur et al. (2009) and Zuur et al. (2010). This involved checking for outliers and collinearity along with examining the relationship between the response and the predictor variables was examined. The data was assessed for spatial and temporal dependency and for zero inflation. As a result of this exploratory analysis, we removed samples for the month of May as it was only sampled in 1 year and is at the tail end of the glass eel season. Three samples with a fishing time of less than 90 min were removed from the analysis to standardise the fishing effort. These samples appeared as outliers as they were so different to the other values, and as the flow was so slow the concern for the accuracy of the flow metres and the impact this difference could have on the models. As a result of this exercise, the sample size was reduced from 61 to 55 samples.
A generalised linear mixed model (GLMM) was used to analyse the count of glass eels and environmental variables in the two rivers using R (R Core Team 2018). To take account of repeated measurements from the same net location and month, these variables were added to the random structure of the model (Millar and Anderson 2004; Wagner et al. 2006). Year could not be used as a random effect as there were only 2 years sampled. To account for difference in fishing effort, the volume of water filtered was used as an offset in the model. Due to overdispersion in the data, a negative binomial model was used.
Due to the small sample size (n = 55) and the dependency structure, model creation was limited to 3 additional environmental parameters to ensure there were enough samples per covariate. The variables site and year are the same as Latoon Bridge was surveyed in 2017 and Bunratty Bridge was surveyed in 2018 so the variable site was used in the analysis. Following the exploratory analysis, the list of environmental variables available were site, tidal height, atmospheric pressure, salinity, water temperature, cloud cover, cumulative rainfall, wind degrees, percentage illumination, moon phase and time difference between high tide and sunrise. The following packages were used in the analysis: glmmTMB, ggplot2, dplry (Wickham 2016; Brooks et al. 2017; Wickham et al. 2018). Model selection was based on the lowest Akaike information criterion. The minimum value of AIC indicates the best compromise between the fit of the model and the number of parameters. Model validation was carried out by assessing the model residuals for normality, homogeneity, violations in the assumption of independence and overdispersion.
Results
Description of glass eel catch
A total of 15 nights were surveyed at Latoon Bridge between January and May 2017, and 9 nights at Bunratty Bridge between February and April 2018. Surveys were not possible at Bunratty in January 2018 due to adverse weather conditions. Overall, there were 61 pooled net hauls (Latoon Bridge n = 43, Bunratty Bridge n = 18) over the entire survey period, resulting in a capture of 1081 glass eels with a total weight of 398.7 g (see Supplementary Fig. 2 and 3). Length, weight and pigment stage were recorded for 467 glass eels (Latoon Bridge n = 242, Bunratty Bridge n = 225). The length of glass eels caught at Latoon Bridge ranged from 6.0 to 8.0 cm (mean 7.0 cm) while those caught at Bunratty Bridge ranged from 5.8 to 8.0 cm (mean 6.9 cm; Fig. 2). The by-catch in the plankton nets consisted of Stickleback and Gammarus sp. and also included pipefish, flounder, sprat, shrimp and molluscs. The by-catch diversity increased with temperature in both locations, but the Bunratty Bridge site was less diverse overall in terms of by-catch than Latoon Bridge.
Glass eel pigmentation
The proportion of glass eels in the different pigmentation stages changes throughout the sampling season. At Latoon Bridge in January and February, the catch was dominated by glass eels in the early pigmentation stages of VA, VB and VIA0 (86% of the catch for those months; Fig. 3), reducing to just 3.3% by April, by which time VIA2, VIA3 and VIA4 made up 85% of that month’s catch. In March and April, the later-stage pigmented eels were clearly dominant, but the early stages (VA, VB and VIA0) were still present in low numbers (23% of the catch for March/April). By late April, the early-stage pigmentation eels (VA, VB and VIA0) were absent, and the glass eels present were representative of the VIA2, VIA3, VIA4 and VIB stages. Elver level pigmentation (VIB) did not occur until late April and early May sampling. In 2017, at Latoon Bridge, the average water temperature ranged from 7.1 to 15.2° (Fig. 3). At Bunratty Bridge in 2018, the same trend was observed but with a slightly delayed pigmentation. The VA, VB and VIA0 pigment stages were dominant in the February samples (86% of catch; Fig. 4) dropping to 26% by April, when VIA2, VIA3 and VIA4 made up 74% of the catch. The last pigmentation stage VIB (elver stage) was not detected during the fishing season at Bunratty Bridge. Average temperature at this site ranged from 5.2 to 10.1 °C (Fig. 4).
Glass eel otolith results
A total of 50 eels from Bunratty Bridge (10 per sampling occasion) were assessed for radii length, with a subsample aged using the burn and crack technique. All glass eels sampled were 0 + years. The deposition of a transition ring (zero band) was assessed for 15 randomly selected individuals from Bunratty Bridge with only four showing a zero band. These eels were from pigment stage VIA0 to VIA4. No glass eel in the VA or VB pigment stages had deposited a transition ring in this study.
Generalized linear mixed modelling
Fourteen models were created to examine the effect of environmental variables on glass eel catch (Table 2). There were 2 models with the lowest Akaike information criterion (AIC) and within 2 units of each other. Model 14 with the variables site, water temperature and moon phase, and model 12 with site, water temperature and percentage illumination. Both models are accounting for the same variables but with different variable type; percentage illumination is a continuous variable and moon phase is a categorical variable. The model with the lowest AIC value was model 14 with model outputs presented in Table 3.
The model validation process showed no pattern in the plots of residuals against the fitted values and with all the parameters in the model and those excluded from the model. A simulation study using the model to predict new data did not highlight any issues with the model fit (Zuur and Ieno 2016; Harrison et al. 2018). The number of zeros simulated multiple times were assessed and compared favourably with the observed raw data. A likelihood ratio test was carried out to test the importance of the two categorical variables (site and moon phase) in the final model; both categorical variables are required as when they are left out individually the AIC increases.
The model interpretation indicates that the count of glass eels increased with water temperature (Fig. 5) and there were higher counts of glass eels on nights around the full moon compared with the new moon (Fig. 6). The effect of site had a large effect on glass eels with more eels recorded at Bunratty Bridge under the different environmental conditions compared with Latoon Bridge; however, the effects of site and year are confounded due to the sampling design. The third and fourth lowest models contained the variables water temperature and cloud cover (model 6) and water temperature and tidal height (model 1, Table 1) suggesting that these variables are also important for glass eel recruitment.
Discussion
The glass eel catch at both locations showed the continuous recruitment of early-stage pigmented glass eels into both sites during the survey periods. However, the amount of glass eels recorded in each pigment stage shifted from early stages to late stages as the season progressed. This study found that the zero band deposition began at the VIA0 pigmentation stage at the earliest, which is in line with previous research (Lecomte-Finiger 1992). However, out of the 8 individual glass eels representing pigmentation stages from VIA1 to VIA4, 5 had not deposited a transition ring at this point. It is unclear whether this result was linked to the colder temperature experienced at Bunratty Bridge in 2018. Umezawa and Tsukamoto (1991) found that otolith increment deposition in Japanese elvers was disrupted by low water temperatures as well as food deprivation.
This study examined a range of environmental parameters that can influence glass eel recruitment and found that water temperature and moon phase were the most important variables in glass eel migrations in estuaries, confirming findings of previous surveys using different methods (Jellyman and Lambert 2003; Bardonnet et al. 2005; Sullivan et al. 2009; Podda et al. 2020). This study targeted nights on the new and full moon phases as Tzeng (1985) reported that fishing would often be stopped during nights either side of these moon phases. Tzeng (1985) found catches were higher for the nights around the full moon compared with the new moon. Other studies have found the opposite, thought to be an effect of water clarity and a predator avoidance strategy (Aranburu et al. 2015; Fukuda et al. 2016). Podda et al. (2020) only targeted new moon nights which could be a reflection of the difference in recruitment and environmental conditions between the Atlantic Ocean and the Mediterranean Sea.
This study found a larger difference between full moon and new moon in Bunratty Bridge compared with Latoon Bridge; however, the confounding of site and year has affected the influence of moon phase on glass eel migrations. The Latoon site was used as a source of glass eels for restocking into the Shannon Catchment by the ESB for many years. The poor glass eel abundance recorded compared with Bunratty site is unexpected unless it is reflected in poor recruitment for that particular year. This is not reflected in the ICES advice for eel which estimated a similar recruitment trend for 2017 and 2018 (ICES 2021); however, this estimated trend is at the European scale and does not take into account fine-scale spatial variation between sampling locations. Jellyman and Lambert (2003) created two models to account for the variables affecting glass eel counts over 2 different years; however, we kept both locations together using a mixed effects modelling technique to capture the difference in site/year. The data shows that glass eel monitoring needs a long-term sampling plan in order to account for annual fluctuations in glass eel recruitment.
Laffaille et al. (2007) found sea level and water temperature were important in their models of recruitment below a dam but moon phase had no impact on the data. This current study took place in a natural setting without the influence of the hydropower station which is located further upstream on the River Shannon main channel. Tidal height and cloud cover were also important variables but did not have the lowest AIC value in the model selection. However, there is collinearity between moon phase and tidal height. In this study, tidal height is taken into account within moon phase, as all sampling took place on high tides associated with the new and full moon with tidal heights ranging from 6.4 to 7.3 m. Cloud cover would represent darker nights and is often used as a predator avoidance strategy.
Arribas et al. (2012) also found a difference in abundance at survey locations during rising tides at night and during the day. They found that in the lower estuary, the glass eels were present during rising tides during the day but further up the estuary that the abundance decreased, and nocturnal rising tides had higher abundance. The location of our study sites in the upper estuary would confirm the absence of eels in the rising tide during daylight as proposed by Arribas et al. (2012). This study observed that abundance of glass eels in the nets dropped immediately when the rising tide and sunrise coincided as indicated in our samples from May. Further research at various locations along the estuary gradient will determine if glass eels are present during the day as turbidity could also play a part in the distribution of glass eels during daylight hours and will be required to refine a standardised glass eel monitoring methodology (Jellyman and Lambert 2003; Bardonnet et al. 2005; Bru et al. 2009; Prouzet et al. 2009).
The dependence on environmental cues for glass eel migrations is a cause of concern as the link between migration and water temperature may be interrupted under different climate change scenarios in the ocean and in continental waters (Bonhommeau et al. 2008b; Drouineau et al. 2018). August and Hicks (2008) highlighted the risk of climate change for eels in New Zealand (A. australis and A. dieffenbachia) with a reduction in recruitment when water temperatures exceeded 22 °C. Warmer monthly water temperature could move the migration period of glass eels from estuarine into freshwater to earlier in the year resulting in a mismatch in the phenology for this species. This could interrupt the timing of suitable prey items for elvers in freshwater systems and the inherent knock on effects on the ecosystem functioning in general; further research is required into the effects of climate change on the recruitment process.
Fortnightly sampling of glass eels from January to May for 2 years was labour intensive, and expensive in terms of staff costs. However, this method is cost-efficient compared with boat surveys which require a minimum team of 4–6 staff coupled with fuel costs. While this bi-weekly survey method is not suitable for a long-term monitoring programme, it did highlight when to target sampling efforts. Focusing efforts on nights around the high tide when water temperature exceeds 5 °C could result in a representative catch of glass eels during the migration run. Maintaining this data set by fishing 4–5 nights per year as opposed to 12 to 15 nights and repeating annually could result in an index time series suitable for recording long-term changes to the eel recruitment in the area.
This study used a generalised linear mixed model (GLMM) to examine a range of environmental variables impacting on glass eel recruitment in transitional waters. Results found that water temperature and moon phase were the most important variables in glass eel migrations in the Shannon estuary. Tidal height and cloud cover were also important variables influencing glass eel abundance. These variables are drivers in glass eel recruitment and need to be taken into account when setting up a monitoring programme. This study used a standardised research method coupled with targeted environmental conditions to create a monitoring programme for glass eels.
Availability of data and material
The data will be made available on request to Inland Fisheries Ireland.
Code availability
The data will be made available on request to Inland Fisheries Ireland.
References
Amilhat E, Basic T, Beaulaton L, Belpaire C, Bernotas P, Briand C, …, Dekker W (2019) Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL)
Aoyama J, Miller MJ (2003) The silver eel. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Tokyo: Springer Japan pp 107–117. https://doi.org/10.1007/978-4-431-65907-5_8
Aranburu A, Díaz E, Briand C (2015) Glass eel recruitment and exploitation in a South European estuary (Oria, Bay of Biscay). ICES J Mar Sci 73(1):111–121. https://doi.org/10.1093/icesjms/fsv116
Arribas C, Fernández-Delgado C, Oliva-Paterna FJ, Drake P (2012) Oceanic and local environmental conditions as forcing mechanisms of the glass eel recruitment to the southernmost European estuary. Estuar Coast Shelf Sci 107:46–57. https://doi.org/10.1016/j.ecss.2012.04.024
August SM, Hicks BJ (2008) Water temperature and upstream migration of glass eels in New Zealand: implications of climate change. Environ Biol Fishes 81(2):195–205
Bardonnet A, Bolliet V, Belon V (2005) Recruitment abundance estimation: role of glass eel (Anguilla anguilla L.) response to light. J Exp Mar Biol Ecol 321(2):181–190. https://doi.org/10.1016/j.jembe.2005.02.004
Bonhommeau S, Chassot E, Rivot E (2008a) Fluctuations in European eel (Anguilla anguilla) recruitment resulting from environmental changes in the Sargasso Sea. Fish Oceanogr 17(1):32–44. https://doi.org/10.1111/j.1365-2419.2007.00453.x
Bonhommeau S, Chassot E, Planque B, Rivot E, Knap AH, Le Pape O (2008b) Impact of climate on eel populations of the Northern Hemisphere. Mar Ecol Prog Ser 373:71–80. https://doi.org/10.3354/meps07696
Bonhommeau S, Castonguay M, Rivot E, Sabatié R, Le Pape O (2010) The duration of migration of Atlantic Anguilla larvae. Fish Fish 11(3):289–306. https://doi.org/10.1111/j.1467-2979.2010.00362.x
Bonhommeau S, Blanke B, Tréguier AM, Grima N, Rivot E, Vermard Y, …, Le Pape O (2009) How fast can the European eel (Anguilla anguilla) larvae cross the Atlantic Ocean? Fish Oceanogr 18(6):371–385.https://doi.org/10.1111/j.1365-2419.2009.00517.x
Bornarel V, Lambert P, Briand C, Antunes C, Belpaire C, Ciccotti E, …, Secor D (2018) Modelling the recruitment of European eel (Anguilla anguilla) throughout its European range. ICES J Mar Sci 75(2):541–552.https://doi.org/10.1093/icesjms/fsx180
Briand C, Fatin D, Ciccotti E, Lambert P (2005) A stage-structured model to predict the effect of temperature and salinity on glass eel Anguilla anguilla pigmentation development. J Fish Biol 67(4):993–1009. https://doi.org/10.1111/j.0022-1112.2005.00798.x
Brooks ME, Kristensen K, van Benthem J, Magnusson A, Berg CW, Nielsen A, …, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9(2):378–400
Bru N, Prouzet P, Lejeune M (2009) Daily and seasonal estimates of the recruitment and biomass of glass eels runs (Anguilla anguilla) and exploitation rates in the Adour open estuary (Southwestern France). Aquat Living Resour 22(4):509–523. https://doi.org/10.1051/alr/2009050
Christensen JM (1964) Burning of otoliths, a technique for age determination of soles and other fish. ICES J Mar Sci 29(1):73–81. https://doi.org/10.1093/icesjms/29.1.73
Cresci A, Paris CB, Durif CMF, Shema S, Bjelland RM, Skiftesvik AB, Browman HI (2017) Glass eels (Anguilla anguilla) have a magnetic compass linked to the tidal cycle. Sci Adv 3(6):e1602007. https://doi.org/10.1126/sciadv.1602007
Crivelli AJ, Auphan N, Chauvelon P, Sandoz A, Menella JY, Poizat G (2008) Glass eel recruitment, Anguilla anguilla (L.), in a Mediterranean lagoon assessed by a glass eel trap: factors explaining the catches. In: Dufour S, Prévost E, Rochard E, Williot P (eds) Fish and diadromy in Europe (ecology, management, conservation): Proceedings of the symposium held 29 March – 1 April 2005, Bordeaux, France. Dordrecht: Springer Netherlands, pp 79–86. https://doi.org/10.1007/978-1-4020-8548-2_6
Dekker W (2019) The history of commercial fisheries for European eel commenced only a century ago. Fish Manage Ecol 26(1):6–19. https://doi.org/10.1111/fme.12302
Dekker W, Casselman JM (2014) The 2003 Québec declaration of concern about eel declines—11 years later: are eels climbing back up the slippery slope? Fisheries 39(12):613–614. https://doi.org/10.1080/03632415.2014.979342
Dekker W (2003) Status of the European eel stock and fisheries. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Tokyo: Springer Japan. https://doi.org/10.1007/978-4-431-65907-5_17 pp 237–254
Drouineau H, Durif C, Castonguay M, Mateo M, Rochard E, Verreault G, …, Lambert P (2018) Freshwater eels: a symbol of the effects of global change. Fish Fish 19(5):903–930.https://doi.org/10.1111/faf.12300
Edeline E, Lambert P, Rigaud C, Elie P (2006) Effects of body condition and water temperature on Anguilla anguilla glass eel migratory behavior. J Exp Mar Biol Ecol 331(2):217–225. https://doi.org/10.1016/j.jembe.2005.10.011
Edeline E, Dufour S, Elie P (2005) Role of glass eel salinity preference in the control of habitat selection and growth plasticity in Anguilla anguilla. Mar Ecol Prog Ser 304:191–199. Retrieved from https://www.int-res.com/articles/meps2005/304/m304p191.pdf
Elie P (1982) Definition of the limits of the pigmented stages of the civelle Anguilla anguilla L. Vie Milieu 32:149–197
Feunteun E (2002) Management and restoration of European eel population (Anguilla anguilla): an impossible bargain. Ecol Eng 18(5):575–591. https://doi.org/10.1016/S0925-8574(02)00021-6
Freyhof J, Kottelat M (2008) Anguilla anguilla. The IUCN red list of threatened species 2008: e.T60344A12354180. Downloaded on 02 December 2020
Friedland KD, Miller MJ, Knights B (2007) Oceanic changes in the Sargasso Sea and declines in recruitment of the European eel. ICES J Mar Sci 64(3):519–530. https://doi.org/10.1093/icesjms/fsm022
Fukuda N, Aoyama J, Yokouchi K, Tsukamoto K (2016) Periodicities of inshore migration and selective tidal stream transport of glass eels, Anguilla japonica, in Hamana Lake, Japan. Environ Biol Fish 99(2):309–323. https://doi.org/10.1007/s10641-016-0475-z
Harrison AJ, Walker AM, Pinder AC, Briand C, Aprahamian MW (2014) A review of glass eel migratory behaviour, sampling techniques and abundance estimates in estuaries: implications for assessing recruitment, local production and exploitation. Rev Fish Biol Fisheries 24(4):967–983. https://doi.org/10.1007/s11160-014-9356-8
Harrison XA, Donaldson L, Correa-Cano ME, Evans J, Fisher DN, Goodwin CED, …, Gray A (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794. https://doi.org/10.7717/peerj.4794
Healy MG, Hickey KR (2002) Historic land reclamation in the intertidal wetlands of the Shannon estuary, western Ireland. J Coast Res 36(1):365–374. Retrieved from https://doi.org/10.2112/1551-5036-36.sp1.365
ICES (2008) Report of the 2008 session of the Joint EIFAAC/ICES Working Group on Eels
ICES (2021) European eel (Anguilla anguilla) throughout its natural range. In Report of the ICES Advisory Committee, 2021. ICES Advice 2021, ele.2737. nea, https://doi.org/10.17895/ices.advice.7752
Jellyman DJ, Lambert PW (2003) Factors affecting recruitment of glass eels into the Grey River, New Zealand. J Fish Biol 63(5):1067–1079. https://doi.org/10.1046/j.1095-8649.2003.00220.x
Jellyman DJ, Booker DJ, Watene E (2009) Recruitment of Anguilla spp. glass eels in the Waikato River, New Zealand. Evidence of declining migrations?. J Fish Biol 74(9):2014–2033
Jessop BM (2003) Annual variability in the effects of water temperature, discharge, and tidal stage on the migration of American eel elvers from estuary to river. Am Fish Soc Symp 33:3–16
Kettle AJ, Haines K (2006) How does the European eel (Anguilla anguilla) retain its population structure during its larval migration across the North Atlantic Ocean? Can J Fish Aquat Sci 63(1):90–106. https://doi.org/10.1139/f05-198
Knights B (2003) A review of the possible impacts of long-term oceanic and climate changes and fishing mortality on recruitment of anguillid eels of the Northern Hemisphere. Sci Total Environ 310(1):237–244. https://doi.org/10.1016/S0048-9697(02)00644-7
Laffaille P, Caraguel JM, Legault A (2007) Temporal patterns in the upstream migration of European glass eels (Anguilla anguilla) at the Couesnon estuarine dam. Estuar Coast Shelf Sci 73(1):81–90. https://doi.org/10.1016/j.ecss.2006.12.011
Lecomte-Finiger R (1992) Growth history and age at recruitment of European glass eels (Anguilla anguilla) as revealed by otolith microstructure. Mar Biol 114(2):205–210. https://doi.org/10.1007/BF00349520
Lecomte-Finiger R (1984) Contribution à la connaissance de l’écobiologie de l’anguille Anguilla anguilla L., 1758 des milieux lagunaires méditerranéens du Golfe du Lion: Narbonnais et Roussillon. Cybium (Paris) 8(2):102–103
Marohn L, Jakob E, Hanel R (2013) Implications of facultative catadromy in Anguilla anguilla. Does individual migratory behaviour influence eel spawner quality? J Sea Res 77:100–106. https://doi.org/10.1016/j.seares.2012.10.006
McCleave JD, Kleckner RC (1982) Selective tidal stream transport in the estuarine migration of glass eels of the American eel (Anguilla rostrata). ICES J Mar Sci 40(3):262–271. https://doi.org/10.1093/icesjms/40.3.262
Millar RB, Anderson MJ (2004) Remedies for pseudoreplication. Fish Res 70(2):397–407. https://doi.org/10.1016/j.fishres.2004.08.016
Moriarty C (1978) Eels: a natural and unnatural history. Vancouver, David and Charles
Moriarty C (1983) Age determination and growth rate of eels, Anguilla anguilla (L). J Fish Biol 23(3):257–264. https://doi.org/10.1111/j.1095-8649.1983.tb02903.x
O’Connor W (2003) Biology and management of European eel (Anguilla anguilla, L) in the Shannon Estuary, Ireland (PhD). National University of Ireland, Galway
Pecorelli JP, Macphie KH, Hebditch C, Clifton-Dey DR, Thornhill I, Debney AJ (2019) Using citizen science to improve the conservation of the European Eel (Anguilla anguilla) in the Thames River Basin District. Freshw Sci 38(2):281–291
Pike C, Crook V, Gollock M (2020) Anguilla anguilla. The IUCN Red List of Threatened Species 2020: e.T60344A152845178 https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T60344A152845178.en. Downloaded on 02 December 2020
Piper AT, Wright RM, Kemp PS (2012) The influence of attraction flow on upstream passage of European eel (Anguilla anguilla) at intertidal barriers. Ecol Eng 44:329–336
Podda C, Palmas F, Frau G, Chessa G, Culurgioni J, Diciotti R, …, Sabatini A (2020) Environmental influences on the recruitment dynamics of juvenile European eels, Anguilla anguilla, in a small estuary of the Tyrrhenian Sea, Sardinia, Italy. Aquat Conserv Mar Freshwat Ecosyst 30(8):1638–1648. https://doi.org/10.1002/aqc.3362
Poole WR, Reynolds JD (1996) Age and growth of yellow eel, Anguilla anguilla (L.), determined by 2 different methods. Ecol Freshw Fish 5(2):86–95
Prouzet P, Odunlami M, Duquesne E, Boussouar A (2009) Analysis and visualization of the glass eel behavior (Anguilla anguilla) in the Adour estuary and estimate of its upstream migration speed. Aquat Living Resour 22(4):525–534. https://doi.org/10.1051/alr/2009041
R Core Team (2018) R [Computer software]. Retrieved from URL https://www.R-project.org/
Reynolds JD, Donnelly R, Molloy S, Walsh T (1994) Glass eel, elver and juvenile eel programme: a report presented to Electricity Supply Board
Strubberg A (1913) The metamorphosis of elvers as influenced by outward conditions. Meddr Kommn Havunders Ser Fisk 4:1–11
Sullivan MC, Wuenschel MJ, Able KW (2009) Inter and intra-estuary variability in ingress, condition and settlement of the American eel Anguilla rostrata: implications for estimating and understanding recruitment. J Fish Biol 74(9):1949–1969. https://doi.org/10.1111/j.1095-8649.2009.02252.x
Sullivan MC, Able KW, Hare JA, Walsh HJ (2006) Anguilla rostrata glass eel ingress into two, U.S. east coast estuaries: patterns, processes and implications for adult abundance. J Fish Biol 69(4):1081–1101. https://doi.org/10.1111/j.1095-8649.2006.01182.x
Tamario C, Calles O, Watz J, Nilsson PA, Degerman E (2019) Coastal river connectivity and the distribution of ascending juvenile European eel (Anguilla anguilla L.): Implications for conservation strategies regarding fish-passage solutions. Aquat Conserv Mar Freshwat Ecosyst 29:612–622
Tesch FW, Thorpe JE (2003) The eel: Wiley. https://doi.org/10.1002/9780470995389
Tosi L, Spampanato A, Sola C, Tongiorgi P (1990) Relation of water odour, salinity and temperature to ascent of glass-eels, Anguilla anguilla (L.): a laboratory study. J Fish Biol 36(3):327–340. https://doi.org/10.1111/j.1095-8649.1990.tb05613.x
Tosi L, Sola C (1993) Role of geosmin, a typical inland water odour, in guiding glass eel Anguilla anguilla (L.) migration. Ethology 95:177–185
Trancart T, Lambert P, Daverat F, Rochard E (2014) From selective tidal transport to counter-current swimming during watershed colonisation: an impossible step for young-of-the-year catadromous fish? Knowl Managt Aquatic Ecosyst. https://doi.org/10.1051/kmae/2013086
Tsukamoto K, Arai T (2001) Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Mar Ecol Prog Ser 220:265–276
Tzeng WN (1985) Immigration timing and activity rhythms of the eel, Anguilla japonica, elvers in the estuary of northern Taiwan, with emphasis on environmental influences. Bull Jpn Soc Fish Oceanogr 47(48):11–28
Umezawa A, Tsukamoto K (1991) Factors influencing otolith increment formation in Japanese eel, Anguilla japonica T. and S., elvers. J Fish Biol 39(2):211–223. https://doi.org/10.1111/j.1095-8649.1991.tb04357.x
Van Ginneken VJT, Maes GE (2005) The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: a literature review. Rev Fish Biol Fisheries 15(4):367–398. https://doi.org/10.1007/s11160-006-0005-8
Wagner T, Hayes DB, Bremigan MT (2006) Accounting for multilevel data structures in fisheries data using mixed models. Fisheries 31(4):180–187. https://doi.org/10.1577/1548-8446(2006)31[180:AFMDSI]2.0.CO;2
Walmsley S, Bremner J, Walker A, Barry J, Maxwell D (2018) Challenges to quantifying glass eel abundance from large and dynamic estuaries. ICES J Mar Sci 75(2):727–737
White EM, Knights B (1997) Environmental factors affecting migration of the European eel in the Rivers Severn and Avon, England. J Fish Biol 50(5):1104–1116. https://doi.org/10.1111/j.1095-8649.1997.tb01634.x
Wickham H, Romain F, Henry L, Muller K (2018) dplyr: a grammar of data manipulation: R package version 0.8.0.1. https://CRAN.R-project.org/package=dplyr
Wickham H (2016) ggplot2:: elegant graphics for data analysis: Springer-Verlag New York. Retrieved from http://ggplot2.org
Wippelhauser GS, McCleave JD (1987) Precision of behavior of migrating juvenile American eels (Anguilla rostrata) utilizing selective tidal stream transport. ICES J Mar Sci 44(1):80–89. https://doi.org/10.1093/icesjms/44.1.80
Witting DA, Able KW, Fahay MP (1999) Larval fishes of a Middle Atlantic Bight estuary: assemblage structure and temporal stability. Can J Fish Aquat Sci 56(2):222–230. https://doi.org/10.1139/f98-175
Zuur AF, Ieno EN (2016) A protocol for conducting and presenting results of regression-type analyses. Methods Ecol Evol 7(6):636–645. https://doi.org/10.1111/2041-210X.12577
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York, London
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
The authors would like to express their thanks to Mr. Herbie Power for his assistance, local knowledge, and expertise which was a vital part of the study. Local Inland Fisheries Ireland staff in Limerick are acknowledged for their cooperation and use of laboratory facilities. The Department of Communications, Climate Action and Environment (DCCAE) provided funding for the Scientific Eel Fishery under which this study was completed. The authors would like to thank the 2 anonymous reviewers whose comments helped improve and clarify this manuscript.
Funding
This study was funded by the Department of Communications, Climate Action and Environment (DCCAE), under the Scientific Eel Fishery programme 2016–2020.
Author information
Authors and Affiliations
Contributions
Ciara O’Leary: funding acquisition; conceptualization; methodology; investigation; formal analysis; writing—original draft; writing—review and editing; visualisation; project administration; final approval of MS.
Sarah Healy: conceptualization; methodology; investigation data curation; writing—review and editing; final approval of MS.
Robert Cruikshanks: investigation; writing—review and editing; final approval of MS.
Karen Kelly: investigation; writing—review and editing; final approval of MS.
Patrick Gargan: funding acquisition; writing—review and editing; supervision; final approval of MS.
Corresponding author
Ethics declarations
Ethics approval
There are no conflicts with animal ethics; the authors involved in the investigation contain individual authorisations under the Health Products Regulatory Authority and in line with the Animal Welfare legislation in Ireland.
Consent to participate
N/A
Consent to publication
All authors consent to the publication of this study in the journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

10641_2022_1340_Fig8_ESM.png
Supplementary Information Fig. 1 Pigmentation development scoring and descriptions of pigment stages [Briand et al. 2005 (adapted from Elie 1982; Strubberg 1913; Lecomte-Finiger 1984)]. (PNG 60 kb)

10641_2022_1340_Fig9_ESM.png
Supplementary Information Fig. 2 Density of glass eel catches at Latoon Bridge 2017. Net 1 is located on left hand bank looking downstream, net 2 and 3 are in the centre of the channel and net 4 is right hand bank. (PNG 8 kb)

10641_2022_1340_Fig10_ESM.png
Supplementary Information Fig. 3 Density of glass eel catches at Bunratty Bridge 2018. Net 1 is located in centre of channel on right looking downstream and net 2 is centre on the left. (PNG 6 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Leary, C., Healy, S., Cruikshanks, R. et al. Assessment of the environmental drivers of European glass eel (Anguilla anguilla) recruitment in transitional waters. Environ Biol Fish 105, 1203–1217 (2022). https://doi.org/10.1007/s10641-022-01340-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01340-7