Abstract
Maintaining natural thermal regimes in montane stream networks is critical for many species, but as climate warms, thermal regimes will undoubtedly change. Mitigating impacts of changing thermal regimes on freshwater biodiversity requires knowledge of which elements of the thermal regime are limiting factors for aquatic biota. We used full-year stream temperature records sampled across a broad latitudinal gradient to describe the diversity of the thermal landscapes that bull trout (Salvelinus confluentus) occupy and identify potential divergences from thermal regimes where this species has been studied previously. Populations of bull trout occupied stenothermic, cold thermal niches in streams that exhibited low to moderate thermal sensitivity throughout the species’ range. However, winter thermal regimes in the central and northernmost streams were colder and more stable than in the southernmost streams, reflecting differences in sensitivity to air temperature variation and contributions of perennial groundwater to baseflow. In the southernmost streams, bull trout distributions appeared to be regulated by warm summer temperatures, whereas in northern streams, unsuitably cold temperatures may be more limiting. Our results also suggest that local differences in the extent of complete freezing during winter among northern streams may further limit the distributions of suitable habitats. Contrasts in limiting factors at bull trout range extents would suggest differential responses to climate warming wherein northern populations extend their range while southern populations contract, and an overall change in species status that is less dire than previously anticipated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate warming is a pervasive global stressor that continues to have wide-ranging impacts on a variety of organisms and ecosystems (Walther et al. 2002; Parmesan 2006; Sunday et al. 2012). Given that temperature is an important dimension of an organism’s ecological niche, the geographic distributions of taxa across the globe are determined, at least in part, by the range of temperatures species can occupy—i.e., realized thermal niche (Angilletta 2009; Sexton et al. 2009; Peterson 2011). Therefore, temperatures that species experience at their north–south distributional limits provide a high-level constraint often associated with physiological limitations (Pörtner and Farrell 2008; Somero 2010; Bates and Morley 2020). When organisms experience temperatures outside their thermal niche, they must disperse to track thermally suitable habitats, adjust behaviorally or phenotypically (acclimate), adapt (over the course of generations), or risk local extirpation (Parmesan 2006; Sunday et al. 2012; Sunday et al. 2014). As climate warms, populations of species occupying warm-edge niche boundaries in the Northern Hemisphere are likely to experience range contractions as individuals are forced to move to higher elevations or poleward to track suitable thermal habitats (Parmesan 2006; Deutsch et al. 2008; Sunday et al. 2014). Less studied, however, are the range extensions and associated mechanisms that may occur in populations of the same species that occupy cold-edge niche boundaries near a northern range terminus (Chamaillé-Jammes et al., 2006; Clarke and Zani 2012; Campana et al. 2020). The discrepancy in the effects of climate warming at range extents could lead to outcomes wherein no net loss occurs for a species or the loss that occurs is smaller than otherwise estimated by studies focusing on range subsets where losses are most likely to occur.
The above considerations are particularly relevant for lotic ectotherms that are strongly controlled by temperatures and inhabit linear networks where dispersal opportunities are limited (Fagan 2002; Somero 2010; Sunday et al. 2014). Streams and rivers have a demonstrated sensitivity to air temperature increases and have been warming in association with regional climate trends (Isaak et al. 2017a; Michel et al. 2020; Su et al. 2021) that are often most pronounced at higher latitudes (Prowse et al. 2006a, b; Heino, 2020). This has precipitated numerous regional climate risk assessments for societally important cold-water fishes such as salmon, trout, and char (Isaak et al. 2015; Lynch et al. 2016; Wenger et al. 2011), but most assessments focus on southern subsets of species’ ranges in mid-latitude areas where the preponderance of researchers also live. The vulnerability of these populations has been confirmed by observations showing range contractions and phenological adjustments in populations that are attempting to avoid unsuitably warm conditions (Lynch et al. 2016; LeMoine et al. 2020). The importance of temperature in driving these changes highlights the critical nature of maintaining or restoring natural thermal regimes for preserving aquatic biota (Olden and Naiman 2009). Doing so, however, requires a better understanding and description of thermal regimes, especially from high latitude areas where temperature records have traditionally been lacking. The advent of inexpensive, miniature temperature sensors and robust data collection protocols over the last decade (Stamp et al. 2014) have reduced this constraint, and broad comparisons with a focus on the ecologically relevant aspects of stream thermal regimes are becoming possible.
Bull trout (Salvelinus confluentus) is a char species that has experienced significant population declines throughout much of its range in western North America. Populations in the USA and Canada have protected status under the Endangered Species Act and Species at Risk Act, respectively (USFWS 1999; COSEWIC 2012), and the species is classified as being highly sensitive to climate change (Rieman et al. 2007). Bull trout typically occupies montane watersheds and is patchily distributed in headwater streams that are most conducive to adult spawning and subsequent survival of natal and juvenile life stages (Rieman and McIntyre 1995; Isaak et al. 2017b; Mochnacz et al. 2021). The occurrence of bull trout populations has been strongly linked with the distribution of the coldest water temperatures within landscapes (Benjamin et al. 2016; Isaak et al. 2017b; Kovach et al. 2017). However, this view is derived almost entirely from research conducted at the southern extent of the species’ range where the primary focus has been on limitations associated with maximum summer stream temperatures (Isaak et al. 2015; Kovach et al. 2017). Maximum temperatures, however, represent only one component of annual thermal regimes, and it is well documented that there is substantial spatial–temporal heterogeneity in thermal regimes across stream networks (Steel et al. 2016; Isaak et al. 2020). One element that has not been examined for bull trout is whether streams near the northern geographic range extent may be cold-limiting. For example, studies have shown two ways in which cold stream temperatures act to limit fish survival. First, some streams do not provide enough thermal units throughout the growing season for juveniles to attain sufficient size and lipid stores to survive throughout the winter (Finstad et al. 2004b; Coleman and Fausch 2007a; Berg et al. 2009). Secondly, some streams may freeze completely during intense winters or experience high variability in habitat availability during this period and therefore provide little viable year-round habitat to support populations (Cunjak 1988; Cunjak et al. 1998). Here, we explore these issues using full-year temperature data records compiled from three montane areas distributed across the range-wide distribution of bull trout in North America. We use these datasets to describe the annual thermal regimes that these populations experience and gain a broader understanding of which elements might act as factors limiting distributions (Fig. 1). Our objectives were to (1) compare annual temperature records and juvenile distribution data from representative streams across the bull trout range and (2) compare biologically relevant aspects of thermal regimes across the range.
Methods
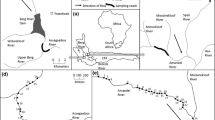
Study areas
The study area encompasses three montane stream networks situated in the southern (45° N, 124° W), central (55° N, 124° W), and northern (61° N, 124° W) regions of the bull trout range, spanning approximately 2000 km (Fig. 1). The three areas have topographically complex terrain but experience different climatic conditions. The southern area (Idaho, USA) has cold, wet winters, and dry, hot summers, whereas the central (Alberta, Canada) and northern (Northwest Territories, Canada) areas experience colder and longer winters, and shorter, hot, dry summers (Holland and Coen 1983; Halliwell and Catto 2003; Isaak et al. 2018; see differences in air temperatures, Table 1). The dominant vegetation in all three areas is mixed coniferous forests in higher elevation areas and shrubs, grasses, and willows in lower elevation areas, but the overall density of vegetation is far less in the northern area than the two others. The geology in the south consists of resistant granites and volcanics (Isaak et al. 2018), whereas the central and northern locations are composed of limestone, dolomite, shale mantled by till, and sandy fluvioglacial drift (Holland and Coen 1983; Halliwell and Catto 2003). Unpaved roads/trails are present in all three areas but are least extensive in the north. All three stream networks have self-sustaining, healthy bull trout populations in multiple streams (M. Taylor unpublished data; Isaak et al. 2015; Mochnacz et al. 2021). The southern area has the most diverse fish assemblage with 12 species (Isaak et al. 2017a), followed by the central and northern areas which each have four species (Schindler 2000; Babaluk et al. 2015).
Stream temperature datasets
Hourly temperature data were collected from 15 sites across bull trout streams of similar size and gradient in each of the three areas using Tidbit temperature sensors (Onset Computer Corporation, Pocasset, Massachusetts, USA; Table 1). These sensors have measurement accuracies of 0.21 °C and resolutions of 0.02 °C. Most site records had temperature recordings on at least 60% of the days (but average completeness of most records was ≥ 80%) during a 3-year period. Records from northern and southern areas covered the period of 1 August 2013 to 31 August 2016, whereas the central area data ran from 1 July 2016 to 26 July 2019. Although it would have been desirable to have monitoring sites that ran concurrently over a longer time span to inform thermal regime research (Jones and Schmidt 2018), 2 or 3 years of data have been shown sufficient for representing many key aspects of thermal regimes (Isaak et al. 2020). In areas where site monitoring records were spatially dense at the stream scale, only records from streams that were ≥ 2.5 km apart (with one exception in the northern area; Fig. S1) were selected to achieve spatial balance and minimize the probability of spatial dependency that often occurs in temperature records that are close to one another (Isaak et al. 2010).
Because some study streams freeze during winter, we only used records with monthly mean daily water temperatures that were warmer than − 1.2 °C. This threshold was based on both experimental and field data, which show that supercooling temperatures in rivers range between 0.07 and − 1.0 °C (Devik 1949; Nafziger et al. 2013) and, because the accuracy of our temperature sensors was ± 0.21 °C, we assumed that any values < − 1.2 °C were not from flowing waters.
In some instances, records were missing daily stream temperature data because loggers were lost due to high flows or removed from sites earlier than anticipated for logistical reasons. Missing daily stream temperature values were imputed using the missMDA package (Josse and Husson 2016) in R (R Development Core Team 2018). This technique uses correlations among sites in time-series records to accurately estimate missing values by first applying standard principal components analysis to the incomplete dataset where missing values have been replaced with column means. Data are then reconstructed from the principal components and the initial analysis step repeated but with missing values replaced using estimates from the reconstructed data (Josse and Husson 2016). Similar to what others have shown (Isaak et al. 2018; Johnson et al. 2021), we found that the quality of imputed data based on temporal covariation from nearby stream sites was good, and all of the correlations between daily observed records and predictions from the imputation were high (r ≥ 0.99). After imputation, all stream site records consisted of 1127 mean daily temperature records across 3 years. To provide measures of the climatic variability that streams experienced, mean daily air temperature data were downloaded from local monitoring stations within 100 km of sites in each area (Idaho: Cooperative Observer Network, https://www.ncdc.noaa.gov/data-access, last access, 01 July 2020; Canada: Environment Canada, https://climate.weather.gc.ca/historical_data/search_historic_data_e.html, last access, 01 July 2020) (Environment Canada 2020; NOAA 2020). Mohseni et al. (1998) has shown that air temperature stations up to 250 km from stream monitoring sites can be useful in this regard.
Bull trout thermal metrics
Metrics were calculated to describe the thermal regimes of streams from each region based on magnitude, variability, and timing (Table 2). Although dozens of metrics are available, many are strongly correlated and redundant, so we focused on a smaller subset that was most relevant to key elements of bull trout biology (Fig. S2) (Chu et al. 2010; Arismendi et al. 2013; Isaak et al. 2018). For example, August mean temperature and winter mean temperature were used as magnitude metrics. The former has been used to define the thermal niche that bull trout occupy in southern latitudes (Dunham et al. 2003; Isaak et al. 2015, 2017b), but August mean temperature has not been reported from mid- and high-latitude streams across the range. Juveniles and adults in the southern and central regions of the range prefer to occupy streams with mean summer stream temperatures < 11 °C and, although adults can survive in warmer water (> 16 °C; Isaak et al. 2015; Parkinson et al. 2016), growth is typically poor at these temperatures (Selong et al. 2001). Accounts of winter stream thermal regimes that bull trout experience are rare, but the winter season is considered by many as a survival bottleneck for freshwater salmonids as it influences development and growth of eggs, timing of hatching and juvenile emergence (i.e., free swimming fish), and survival of adults and juveniles (Cunjak 1988; Shuter et al. 2012). During winter, early-life stages of salmonids often experience high mortality due to exhaustion of energy reserves (Finstad et al. 2004b).
Temperature is also an important determinant of the phenology of bull trout life history events, such as hatching and emergence timing, as well as growth rates that may reflect either local phenotypic adjustments, or adaptations to unique thermal regimes that act to maximize individual fitness (Sparks et al. 2017; Austin et al. 2019; Campbell et al. 2019). Moreover, the timing of phenological events varies across the species’ range because both climate and the length of development and growing seasons differ across latitude—i.e., winter begins earlier and is longer in the north than it is in the south (Reist et al. 2006b; Shuter et al. 2012). Once eggs are deposited in the gravel, the rate and magnitude of thermal units that accumulate throughout the incubation and initial growth period determine when hatching occurs and juveniles emerge—i.e., where the yolk sac is absorbed and juveniles can swim on their own (Neuheimer and Taggart 2007; Fuiman and Werner 2009). Accumulated thermal units (ATU) are often used to quantify how temperature influences developmental rates, and is quantified by calculating cumulative thermal units over time, where 1 °C for 24 h = 1 thermal unit (Neuheimer and Taggart 2007). Laboratory and field studies on bull trout show that individuals require approximately 800 ATU from egg deposition to 50% juvenile emergence (Gould 1987; Bowerman et al. 2014), and Bebak et al. (2000) show that survival of juvenile Arctic char (Salvelinus alpinus) is highest when fish experience an additional 800 ATU after emergence. Together, these data suggest that chars require 1600 ATU from egg deposition to the onset of winter. Based on this assumption, we used 1600 ATU as a guideline for estimating the minimum number of thermal units that bull trout require to survive the winter.
ATU are also important for understanding differences in fish growth across latitudinal gradients and during important life stages, such as the summer-early fall (Coleman and Fausch 2007b; Neuheimer and Taggart 2007; Neuheimer and MacKenzie 2014). The latter can be particularly important for successful recruitment and survival of salmonids in cold-edge boundary habitats (Coleman and Fausch 2007a, 2007b; Berg et al. 2009). ATU were analyzed across annual, incubation, and growing periods (Table 1), as all are biologically relevant periods for growth during early-life stages. The incubation period began at the mid-point of the spawning season and extended until 50% emergence, defined as the stage where juveniles swim freely and can feed on their own. Calculations for analyses during this period started on different dates (NT: 01 September; AB: 15 September; ID: 01 October) to reflect latitudinal differences in the median spawning date across these areas. These dates were selected based on known spawning dates published in the literature and unpublished data for central and northern sites (N. Mochnacz and M. Taylor, unpublished data; Baxter and McPhail 1999; Guzevich and Thurow 2017; Austin et al. 2019). Because emergence dates were not known for these populations, we used a reciprocal hatch/emergence timing model developed by Sparks et al. (2019) for Oncorhynchus spp. in Alaska, and refined by Austin et al. (2019), to estimate emergence date. It is known as the Effective Value model and expressed as:
where E is an effective value (range of 0–1) describing the relative daily contribution to development; \({log}_{e} a=5.59\) and b = 0.126 are model coefficients, based on thermal relationships and emergence timing for bull trout (Austin et al. 2019); and T is the daily mean water temperature on each day of incubation. Because fish eggs accumulate E over the course of the incubation period, the model predicts 50% emergence when the sum of E = 1. The initial model developed by Beacham and Murray (1990) requires an estimate of mean water temperature during incubation but, when this is unknown, the Sparks et al. (2019) model allows one to predict hatch timing by using daily mean water temperature and each day’s respective contribution towards development. The growing period was an estimate of emergence date for each respective area through to the onset of winter, defined as the period when stream temperatures were ≤ 1.5 °C for 7 consecutive days (1 November in the south and 15 October in the central and northern areas). We did not choose these timing windows to predict exactly when timing of key events happens, but rather to compare differences in the estimated hatch dates and magnitude of thermal units accumulated across developmental periods and among regions.
Finally, we calculated thermal sensitivity, which is a measure quantifying how streams respond to air temperature variation and an important metric for understanding the thermal stability of streams (Snyder et al. 2015; Bolduc and Lamoureux 2018). Streams with low to moderate thermal sensitivity (0.10–0.45) are defined as thermally resilient because they have more stable stream temperatures throughout the year due to minimal effects imposed by changes in air temperature. Conversely, streams with thermal sensitivities greater than 0.55 are defined as thermally reactive, where the thermal response to air temperature is more intense (Kelleher et al. 2012; Mayer 2012; Piccolroaz et al. 2016). Additionally, perennial groundwater is an underlying mechanism driving low thermal sensitivity in streams, and areas associated with perennial groundwater constitutes high-quality spawning and rearing habitat for bull trout (Baxter and McPhail 1999; Baxter and Hauer 2000). To identify variations in water temperature relative to changes in air temperature, we calculated the thermal sensitivity of all sites across years in each area. Thermal sensitivity was expressed as the slope of the linear regression relationship between weekly water temperature (Tw) and weekly air temperature (Tw) records across a given year. Weekly time steps were used because they typically provide more precise thermal sensitivity relationships than daily time steps, and full-year records were used to capture the broadest range of temporal variability in this relationship (Kelleher et al. 2012). Negative air temperatures were not included in this calculation because linear relationships below this threshold poorly predict stream temperatures and less accurately represent the influence of groundwater buffering on thermal sensitivity (Morrill et al. 2005; Kelleher et al. 2012; Mayer 2012).
Data analyses
We used a combination of generalized linear (binomial) and linear mixed models to test for differences in thermal regime metrics among regions because these models can handle unbalanced data and account for differences associated with random effects among datasets (Zuur et al. 2009). Year was set as a fixed effect in all models to account for potential differences between years because data were not collected across the same periods in all regions. Site was specified as a random intercept to account for repeated measures across years for models examining winter stream temperature, thermal sensitivity, emergence date, and accumulated thermal units. Variation in winter mean temperature, thermal sensitivity index, estimated egg hatch date, and ATU were examined using linear mixed models with location (i.e., region—as factor), elevation, their interaction (location × elevation), and year (factor) included as fixed effects. Seasonal differences in ATU were compared among regions across incubation, growing, and annual periods. We modeled mean August stream temperature using a combination of linear and generalized linear models (see details below). Model selection was performed using backwards stepwise regression with marginal F tests. No data transformations were performed for linear mixed models but, for the generalized linear model, continuous variables were standardized to a mean of 0 and a standard deviation of 1. The assumptions of models were tested following the methods of Zuur and Leno (2016). Tukey pairwise post hoc multiple comparisons tests were used for among-region comparisons when location was found to be an influential fixed effect. For linear mixed models, marginal (R2m) and conditional (R2c) coefficients of determinations were used to quantify the proportion of variance explained by fixed factors and fixed and random factors, respectively. All analyses and figures were completed in R (R Development Core Team 2018) and significance was assessed at the 0.05 level. Analyses were performed using the following R packages: Tukey tests with lsmeans (Lenth 2016), LMM with nlme (Pinheiro et al. 2016), and R2m and R2c with MuMIn (Barton 2016).
Because juvenile bull trout distributional data were available across all three areas (ID, n = 180; AB, n = 185; NT, n = 415) and was collected using similar methods—i.e., two spatial/temporal replicates across sites allocated using a stratified random design (Isaak et al. 2017b; Mochnacz et al. 2021)—we combined these data with mean August stream temperatures to portray the available and occupied thermal niche in each respective area. To provide a consistent means of comparison across the fish survey sites which lacked co-located temperature sensors, spatially explicit temperature models were built for streams in each of the three study areas that predicted mean August temperatures (Isaak et al. 2017a; Mochnacz 2021). By modeling August stream temperatures across these three areas, we were able to precisely predict temperatures (≤ 1.0 RMSPE) across a broader spatial scale than we could have using the limited number of full-year temperature records available in each area (n = 15). These data were analyzed as a two-step process. First, full-year temperature records (n = 15) were used to test for regional differences in mean August stream temperature (dependent variable), at sites occupied by bull trout, using a linear model with location (as factor), elevation, and mean August air temperature included as fixed effects, and site as a random effect. Second, the distributional datasets were input into a generalized linear model (GLM) to define the realized summer thermal niche that juvenile bull trout occupy in each respective region (south, central, north) as well as a global model to represent a range-wide thermal niche. For each GLM, juvenile presence-absence data (dependent variable) was regressed against mean August stream temperature. This model was fit with both linear and quadratic terms for mean August stream temperature because both relationships have been shown to explain variation in occupancy of stream-dwelling salmonids elsewhere (Isaak et al. 2017a). Thermal response curves were plotted as the probability of occupancy versus mean August stream temperatures across the range of values from the dataset. Modeled thermal response curves for each region were plotted together to visualize the degree of similarity in the realized summer thermal niche among populations. Because prevalence differed across each region and resulted in differences in peak probability of thermal response curves (i.e., height of response curves), probabilities were rescaled to the maximum value observed (0.80) for visual comparison. In the northern area, most streams freeze completely during the winter; therefore, full-year temperature records are sparse. Consequently, small sample sizes of other metrics associated with full-year records (e.g., mean winter stream temperature, ATU) precluded integration with distributional data and modeling, as described above in step 2.
As latitude increases, winters become colder and longer, which translates into more extensive freezing of freshwater rivers in montane systems (Prowse et al. 2006a, b; Crites et al. 2020). However, at higher latitudes, perennial groundwater discharge in some streams attenuates the magnitude of freezing, creating areas that are ice free or do not completely freeze to the bottom (Utting et al. 2013; Crites et al. 2020). This phenomenon is reflected in the thermal sensitivity of streams, whereby as perennial groundwater contributions increase, thermal sensitivity declines (Kelleher et al. 2012; Bolduc and Lamoureux 2018; Hare et al. 2021). Given that perennial groundwater is the primary mechanism preventing shallow (< 1.5 m) streams from freezing at higher latitudes (Utting et al. 2013), it follows that streams which remain unfrozen in the winter should have lower thermal sensitivity. To investigate whether this relationship was present in our dataset, we randomly selected 80% of the available full-year temperature records from each region (ID, n = 314; AB, n = 84; NT, n = 68), and calculated thermal sensitivity. Sites were classified as frozen or unfrozen, based on mean daily winter temperatures being below or above the flowing water threshold of − 1.21 °C. Pairwise t-tests were used to determine if thermal sensitivity of frozen versus unfrozen sites differed within regions.
Results
A comparison of daily mean air temperatures to daily mean water temperatures showed that stream temperatures from all three regions exhibited a dampened response to air temperature fluctuations during the summer months (Fig. 2). Results of our linear mixed model support these trends and showed that the mean (± SD) thermal sensitivity was low to moderate for all three areas (ID: 0.43 ± 0.06; AB: 0.35 ± 0.09; NT: 0.35 ± 0.12) but differed between southern and central-northern regions (location: F2,42 = 4.03, p = 0.02; Tukey test: ID-AB, Z = 0.08, p < 0.0001; Tukey test: ID-NT, Z = 0.09, p < 0.0001; Tukey test: AB-NT, Z = 0.005, p = 0.955; Fig. 3(A)) and varied across years, but the year effect size was relatively small (year: F6,129 = 8.03, p < 0.0001, coefficient range 2014–2019 = − 0.004 to − 0.10, SE = 0.01–0.02). Elevation was removed from the model because it was not significant (F2,39 = 1.10, p = 0.34). Location accounted for 20% of the variation in thermal sensitivity, whereas the random effect of site accounted for 65%.
Comparison of thermal sensitivity (A); thermal sensitivity across sites in the south (ID), central (AB), and north (NT) using full-year temperature records from streams that do not freeze during the winter (B; n = 15); mean August stream temperature (C); and mean winter stream temperatures (D). The thermal sensitivity of unfrozen and frozen sites is shown (B), based on a random selection of 80% of records from each area. For each boxplot, the thick horizontal line represents the median and values within the box represent the interquartile range. Whiskers below and above the boxes represent the 10th and 90th percentiles and observations falling outside these percentiles are shown as points. Lower case letters denote significant differences in each metric across locations
Thermal sensitivity was lower in unfrozen sites than in frozen sites in the north (t-test, t66 = − 5.4, p = 0.003), but did not differ in the central or south (central: t-test, t82 = 0.29, p = 0.77; south: t-test, t312 = − 0.15, p = 0.88; Fig. 3(B)). The proportion of sites that froze in each location was lowest in the south at < 1% (frozen = 1, unfrozen = 313), followed by the central at 13% (frozen = 11, unfrozen = 73), and highest in the north at 47% (frozen = 32, unfrozen = 36).
The breadth of the available mean August stream temperature niche (hereafter referred to as summer stream temperature) differed across locations and was widest in the north (1.2–10.6 °C) and narrower in both the central (3.5–11.6 °C) and south (7.9–14.1 °C). Overall, streams in the south were warmest (Fig. 3(C)) and, as expected, the summer thermal niche that bull trout occupied transitioned from warmest to coldest following a south to north latitudinal gradient (Fig. 3(C)). Consequently, summer temperatures in streams occupied by bull trout (mean ± SE) differed among regions, with the southern areas being the warmest (9.3 °C ± 0.11), followed by central (6.7 °C ± 0.18) and northern (4.6 °C ± 0.10) areas (location: F2,41 = 42.6, p < 0.001; Tukey test: ID_AB, t = − 5.46, p < 0.0001; Tukey test: ID-NT, Z = − 6.46, p < 0.0001; Tukey test: AB-NT, Z = 2.30, p < 0.06). Elevation did not have a significant effect on summer stream temperature, but year did, although the effect size was relatively small (elevation: F1,41 = 1.69, p = 0.2002; year: F5,115 = 7.2, p = 0.0001, coefficient range 2014–2018 = − 0.68–0.15, SE = 0.14–0.23). Year and location accounted for 72% of the variation in summer stream temperature and the random effect of site accounted for 22%.
Mean winter stream temperature (mean ± SE) differed among regions where temperatures in the south (0.71 °C ± 0.10) were warmer than both the central (0.20 °C ± 0.14) and north (0.07 °C ± 0.19) (location: F2,41 = 16.8, p < 0.0001; Fig. 3(D)), although the magnitude of differences was relatively small (Tukey test: ID_AB, Z = 0.52, p < 0.001; Tukey test: NT-ID, Z = − 0.64, p < 0.001). There was no difference in mean winter stream temperature between the central and northern sites (Tukey test: Z = − 0.12, p = 0.24). Elevation did not influence mean winter stream temperatures and there was a significant year effect (elevation: F1,41 = 2.17, p = 0.41; year: F6,90 = 3.64, p = 0.0008, coefficient range 2014–2019 = 0.08–0.31, SE = 0.07–013). The fixed effects in the model accounted for 32% of the variation in mean winter stream temperatures and the random effect of site accounted for 55%.
Thermal response curves indicated that the realized thermal niches of these populations overlapped, but the northern population occupied a colder and narrower thermal niche than both the central and southern populations (Fig. 4A). The curves for the central and southern populations showed that occurrence probabilities peaked at 8.3 °C and 6.2 °C, respectively, and both exhibited cold- and warm-edge transition boundaries. The thermal curve for the southern population did not show a cold-edge boundary because sufficiently cold streams were not available to populations in this portion of the range, but this curve did display a warm-edge transition boundary at temperatures ≥ 11 °C (Fig. 4B).
Comparison of mean August stream temperature observed across occupied sites (A), and modeled thermal response curves (B) in south (ID), central (AB), north (NT), and for all sites across the three areas (Global). The black arrow shows where peak occurrence occurs (8.4 °C) on the global response curve. Variation in juvenile occupancy was best described by a quadratic relationship with mean August stream temperature in northern and central areas and a negative linear relationship in the southern area (ID). The hatched blue line represents the theoretical curve extending beyond data used to build this model. Untransformed regression equations for each respective curve are shown. Models were built using standardized values for temperature terms and values were back transformed for the probability plot. Aug_Temp, is the mean August stream temperature and Aug_Temp2, is mean August stream temperature raised to the second power for use in the quadratic model
Estimated number of days to emergence (mean ± SD) differed among regions with days to emergence in the south (157 ± 34.9) being fewer than the north (270 ± 7.0) and central (283 ± 5.9) areas (location: F2,42 = 41.8, p = 0.0003; Table 3). Interestingly, the length of time to emergence was longest in the central region followed closely by the northern region (Tukey test: NT-AB, Z = − 0.19, p = 0.01; NT-ID, Z = 53.6, p = 0.13; ID-AB, Z = − 73.0, p = 0.0002). However, this was not an unexpected result because the winter thermal regime was coldest in streams that did not freeze in the central location, and the Sparks et al. (2019) model uses daily thermal degree units to predict hatch dates (Fig. 5).
Annual time series of accumulated thermal units (°C∙day) for all stream temperature records from Idaho (ID, blue), Alberta (AB, yellow), and the Northwest Territories (NT, gray). The range of thermal units for all sites are shown as vertical lines and dark lines are means. Data for ID and NT are from the years indicated on the plots. Alberta data are from 2016 to 2017, 2017 to 2018, and 2018 to 2019, respectively, and were superimposed on these plots for a visual comparison. Mean emergence dates for each region are shown on the top plot and periods coinciding with incubation and growing seasons are indicated on the middle plot. Data spanning the entire growing season were not available in 2015–2016 and 2018–2019 for each respective area
ATU differed among regions across all developmental periods, but differences were smallest during the incubation period (Table 3). In the south, ATU during the annual and growing periods were more than double the central region and 1.8 to 2.1 times greater than the northern region. Conversely, differences in mean ATU between central and northern locations were much smaller (Table 3). Time series plots of ATU are illustrated in Fig. 5 for southern, central, and northern areas. These data show that in all years, ATU from all sites show an immediate increase during the initial part of the incubation period shortly after spawning, followed by a plateau during the winter months, an increase during the early spring, and then a more pronounced increase throughout the growing period. During the growing period, the northern and central areas showed a slower rate of increase in ATU than the southern location (Fig. 5).
Results of the linear mixed models for the incubation, growing, and annual development periods support the patterns shown in Fig. 5, as ATU differed between southern and central-northern locations during all periods (incubation: location—F2,41 = 24.5, p < 0.001; growing: location—F2,41 = 120.0, p < 0.0001; annual: location—F2,41 = 120.0, p < 0.0001), but intercepts did not differ between the central and northern locations (incubation: Tukey—T41 = − 0.12, p = 0.97; growing: Tukey, NT-AB—T41 = 53, p = 0.44; annual: Tukey, NT-AB—T41 = 99.8, p = 0.12). The effect of year was significant for all periods and elevation was significant only during the growing and annual periods (incubation: elevation—F1,41 = 1.8, p = 0.19; year—F4,86 = 15.1, p < 0.0001; growing: elevation—F1,41 = 6.89, p = 0.01; year—F3,72 = 45.3, p < 0.0001; annual: elevation—F1,41 = 7.21, p = 0.01; year—F3,72 = 46.0, p < 0.0001). Although year was a significant effect in the models, it had a relatively small effect on ATU across all three periods with model coefficients (± SE) ranging from − 0.003(0.004) to − 0.006(0.004). The interaction between location and elevation was not significant and was removed from all models. The random effect of repeated measures across sites accounted for 37%, 10%, and 11% of the variation in ATU during the incubation, growing, and annual periods, respectively, while the fixed effects accounted for 50%, 82%, and 83%, respectively.
Discussion
We show that bull trout occupy a relatively cold thermal niche, relative to other cold-water fishes, across a broad latitudinal gradient (mean annual stream temperatures across the three study areas ranged from 1 to 5 °C). Further results of a range-wide species thermal response curve model predicted site occupancy peaked at 8.4 °C mean August stream temperatures. In the southern portion of the species’ range, however, bull trout appeared to be regulated by warmer temperatures and occurrence probabilities declined to < 0.1 once summer temperatures exceeded 13 °C (Isaak et al. 2017b; Selong et al. 2001). Temperatures that warm were rarely observed in the central and northern study streams where bull trout appeared instead to be regulated by unsuitably cold temperatures and a lack of thermally suitable habitat in many areas throughout the year. This finding further reinforces that some populations in the southern distributional range extent occupy streams that are at the species’ warm-edge range boundary where site extirpation is more likely as streams continue to warm (Eby et al. 2014; LeMoine et al. 2020). Regardless of the mechanism controlling population persistence (i.e., warm- vs. cold-limiting habitat), all the streams we examined exhibited high thermal stability, which suggests this is a key abiotic property of core habitat across the species’ range (Luce et al. 2014; Isaak et al. 2016). However, in northern areas, this thermal property acts to stabilize stream temperatures to prevent freezing during the winter, whereas in the south, high thermal stability mediates air temperature warming effects in the summer because streams rarely freeze in the winter (Isaak et al. 2016).
These results highlight an important dichotomy in the prevailing thermal mechanisms governing the distributions of bull trout populations at southern-northern range limits and may have important implications for the species as climate warms. In the south, populations inhabiting streams that warm beyond acceptable upper thermal limits will need to disperse upstream to colder, higher elevation streams, or adjust or adapt to occupy warmer thermal regimes. In the Bitteroot basin in Montana, both Eby et al. (2014) and LeMoine et al. (2020) report range contraction of bull trout through abandonment of previously occupied sites that have warmed, suggesting either dispersal or extirpation as possible mechanisms. In most instances, fish will probably move from warmer downstream habitat upstream to colder habitats at higher elevations; however, in some systems, these habitats may be at their carrying capacity or occupied by competitors (e.g., brook trout Salvelinus fontinalis; Paul and Post, 2001). In the north, our results suggest that the distribution of populations is currently limited by streams being too cold in both the summer and winter (i.e., streams freeze to the bottom). However, as streams warm in these very cold systems, there is potential for expansion of thermally suitable habitat whereby streams that are currently too cold to support populations warm enough to provide new colonization opportunities. In addition, productivity may increase as the severity (e.g., warmer, less ice cover) and length of winters decrease (Prowse et al. 2006a, b), resulting in greater growth potential and better recruitment via higher survival throughout the winter. Campana et al. (2020) show that as climate warms in the north, lake trout (Salvelinus namaycush) populations will realize gains in productivity and experience potential range expansion by colonization of new habitats. Species geographic ranges are an artifact of physiological thermal safety margins, which are typically smaller at upper thermal limits (Sunday et al. 2014; Sandblom et al. 2016). Given this and our findings, we would expect that, as streams warm, bull trout will continue to experience range contraction in the south and potential expansion in the north. However, the overall net effect on the species’ distribution will probably be driven by the proportion of cold-limiting areas versus warm-limiting areas found across the geographic range.
Potential for local adaptation
To our knowledge, the northern bull trout populations from this study occupy the coldest and narrowest summer thermal niche on record for the species, and represents a downward shift from thermal niches reported elsewhere across the range (Benjamin et al. 2016; Isaak et al. 2017b). However, this is not particularly surprising given this is the most northerly situated area for which thermal regimes have been described, and it is near the species’ northernmost range boundary (Mochnacz et al. 2013). The summer stream temperature of 6.2 °C, where occurrence peaked, aligns well with the thermal optimum (≤ 10 °C) for fishes in the Arctic thermal guild (Reist et al. 2006a). The colder streams that the northern populations occupy is partially a reflection of a colder summer thermal niche that is available compared to others we examined. This implies that northern populations may be forced to occupy a colder summer thermal niche compared to those in central and southern areas. However, it is interesting that despite northern populations having access to relatively warm streams (7.0–9.0 °C), occurrence peaks at a much lower temperature of 6.2 °C, which is 2 °C below the peak temperature of the global response curve. This suggests that northern populations have either adapted to occupy a colder thermal niche than those found further south, possess the phenotypic plasticity to acclimate to a colder thermal regime, or occupancy of this cold thermal niche is driven by other factors (e.g., predation by older bull trout, competition with other species, access to resources). Local adaptation to different environmental conditions is common in salmonids (Eliason et al. 2011; Narum et al. 2013) and arises more often as geographic distance between population increases (Fraser et al. 2011). However, chars are also renowned for exhibiting exceptional phenotypic plasticity; therefore, it would not be surprising if acclimation was the prevailing mechanism (Klemetsen 2010; Chavarie et al. 2016).
Thermal regime contrasts
Thermal regimes across all three areas exhibit similar insensitivity to seasonal fluctuations in air temperature and highlights a common element of thermal stability in core habitat that these populations require. Similar stability in thermal regimes has been reported for bull trout in other regions (Isaak et al. 2016) but, to our knowledge, this is the first account of disparate populations experiencing similar thermal stability across a wide geographic area. Occupying habitat with stable thermal regimes is important for persistence of cold-water salmonids because streams with these properties are projected to mediate effects of climate warming (Lisi et al. 2015; Snyder et al. 2015; Mauger et al. 2017) and could promote positive growth during winter (French et al. 2017). The average thermal sensitivity in the southern area was slightly higher than that in the central and northern areas, but it was still well below the 0.55 threshold differentiating insensitive versus sensitive streams (Kelleher et al. 2012; Piccolroaz et al. 2016). Differences in thermal sensitivity could be related to the characteristics of these streams (i.e., elevation, sun angle, riparian canopy) and the prominent mechanism driving thermal sensitivity. Very stable water temperatures throughout the summer in the central and northern areas produced flatter air–water temperature hysteresis cycles (i.e., a cyclical lag between input and output) and much lower thermal sensitivities that are consistent with systems where perennial groundwater is the prevalent underlying mechanism (Kelleher et al. 2012). Conversely, the streams in the south exhibit slightly broader and higher air–water temperature hysteresis cycles that are reflective of systems where thermal sensitivity is driven more by a combination of precipitation, snow melt, riparian shading, and, to a lesser extent, groundwater (Kelleher et al. 2012; Lisi et al. 2015). Although it is not possible to define the exact mechanism(s) responsible for the patterns we report, a first-order understanding of the most probable mechanism(s) will be useful for predicting how climate warming may affect these systems differently. For example, Lisi et al. (2015) show that, as climate warms, the heterogeneity pattern of thermal sensitivity in high elevation snow-melt stream systems may be lost, resulting in more uniform warming across all streams.
Our analysis also demonstrates that in the north, only streams which do not freeze completely during the winter have low thermal sensitivities, suggesting groundwater as the primary mechanism maintaining warm enough environments for fish to survive throughout the winter. It follows that the distribution of suitable spawning and rearing habitat in this area appears to be driven by a balance between streams being warm, and presumably productive enough in the summer, but do not freeze completely during the winter due to perennial groundwater input. Similarly, the distribution of Dolly Varden (Salvelinus malma) spawning and overwintering habitat is also associated with prevalence of groundwater (Dunmall et al. 2016), and suggests the pattern we report reflects an important mechanism governing suitability of riverine char habitat north of the 60th parallel. Further, Crites et al. (2020) mapped the distribution of perennial groundwater sources in the western Arctic, and these align well with the broader distributions of riverine chars in this region, further reinforcing the importance of groundwater prevalence for sustaining riverine char populations at higher latitudes (Mochnacz et al. 2013).
The finding that central and northern populations experience colder thermal regimes than the southern population also represents a divergence in patterns reported by others (Benjamin et al. 2016), and highlights a potential recruitment bottleneck associated with growth potential and emergence phenology. Our results show how populations exposed to colder thermal regimes will emerge later and have access to a shorter open water season, thereby reducing growth potential before the onset of winter. Variability in emergence timing across streams with different thermal regimes was also reported by Austin et al. (2019) for bull trout in Washington State and Sparks et al. (2019) for sockeye salmon (Oncorhynchus nerka) in Alaska. However, our results are contrary to those of Campbell et al. (2019), who reported high synchrony in hatch dates for coho salmon (O. kisutch) across streams with vastly different thermal regimes. This discrepancy could be related to an indirect interaction (i.e., not statistical) between latitude and winter thermal regime that are present in our results but not considered by Campbell et al. (2019), a missing co-variate, or interspecific differences in life history strategies that represent local adaptations to unique thermal regimes (Shuter et al. 2012). Determining hatch and emergence dates using otoliths would refine our understanding of hatch phenology for populations from these areas and validate emergence model estimates (Fuiman and Werner 2009; Campbell et al. 2019). In addition, the fact that interannual variation (i.e., year effect) had a relatively small influence on most metrics we examined suggests some level of stability in the thermal regimes we report and may also reflect a broader temporal signature in these systems. It is worth noting, for models of thermal sensitivity and winter stream temperatures, the random effects explained more variation than fixed effects, and may suggest that these two metrics vary more within regions (i.e., site effects) rather than among them (i.e., distributional effects). However, high site-level variability could also be present because few fixed effects were included in our models and important covariate controls associated with subsurface hydrology, and the amount of groundwater inputs, are not well represented by things estimable from digital elevation models, such as stream elevation.
The divergence in growth potential through exposure to different annual thermal regimes presents some interesting hypotheses that warrant consideration. Results suggest that limits on habitat carrying capacity currently force northern populations to occupy streams near cold-edge thermal boundaries. So how do these fish manage to survive in such cold and presumably unproductive environments? Northern populations may possess a compensatory mechanism to grow more efficiently in colder environments during shorter growing seasons (i.e., countergradient variation) (Conover and Schultz 1995). All of the streams in the northern area, where we describe full-year thermal regimes, have abundant, self-sustaining bull trout populations (Mochnacz et al. 2021), yet several of these streams accumulate very few thermal units throughout the annual growing season (i.e., ≤ 800). However, we would not expect populations to occupy these streams if they could not survive under these growing conditions; therefore, they appear to have adjusted (i.e., genetically or via phenotypic plasticity/acclimation) to be successful in these colder thermal regimes. Such adaptations to cold winter thermal regimes have been observed in other salmonids (Finstad et al. 2004a). Furthermore, the acclimation time to readjust efficiency of physiological energy pathways is reduced as a species’ fundamental thermal niche gets closer to ambient temperature (Pörtner and Farrell 2008). In this context, it is worthwhile noting that, in the northern area, the difference between the mean annual stream temperature (2.4 °C) and optimal temperature where summer occurrence probability peaks (6.2 °C) is approximately 3.8 °C. Others report similar compensatory growth in Arctic char (Chavarie et al. 2010; Sinnatamby et al. 2015), and we think this observation warrants further investigation in bull trout.
Conclusion
Examination of full-year thermal regimes across a broad latitudinal gradient reinforces what others report from the southern range extent, which is that bull trout populations occupy a cold, narrow thermal niche relative to other cold-water salmonids (Benjamin et al. 2016; Isaak et al. 2017b). However, we show how divergent elements of annual thermal regimes appear to limit populations at southern and northern range extents (i.e., warm- versus cold-limiting streams). Consequently, as air temperatures continue to rise, managers will need to consider how these two areas differ if they wish to manage thermal regimes effectively to ensure long-term survival of core populations (Isaak et al. 2016). Even if managers carefully monitor and manage thermal regimes (e.g., mitigate flow disruption and maintain riparian zones), it seems inevitable that streams will continue to warm (Isaak et al. 2017a), which makes it important to understand the adaptive capacity that populations possess to adjust to potential changes in stream temperature. Given that local adaptations to unique thermal regimes occur in other salmonids (Eliason et al. 2011; Sparks et al. 2019), we view this as an important avenue of future research for conserving bull trout populations. A key element in this process is broadening understanding of thermal regimes that bull trout populations experience across a wide latitudinal gradient by further expanding stream temperature monitoring networks in both central and northern Canada. Doing so will determine if the patterns we report are a local area effect or are reflective of a broader classification of cold-water thermal regimes in montane areas across the geographic range.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, New York
Arismendi I, Johnson SL, Dunham JB, Haggerty R (2013) Descriptors of natural thermal regimes in streams and their responsiveness to change in the Pacific Northwest of North America. Freshwat Biol 58(5):880–894
Austin CS, Essington TE, Quinn TP (2019) Spawning and emergence phenology of bull trout Salvelinus confluentus under differing thermal regimes. J Fish Biol 94(1):191–195
Babaluk J, Sawatzky C, Watkinson DA, Tate D, Mochnacz N, Reist J (2015) Distributions of fish species within the South Nahanni River watershed, Northwest Territories. Can Manuscr Rep Fish Aquat Sci 3064: vii + 91 pp
Barton K (2016) MuMIn: Multi-Model Inference. R package version 1.43.17. https://CRAN.Rproject.org/package=MuMIn
Bates AE, Morley SA (2020) Interpreting empirical estimates of experimentally derived physiological and biological thermal limits in ectotherms. Can J Zool 98:237–244
Baxter CV, Hauer FR (2000) Geomorphology, hyporheic exchange, and selection of spawning habitat by bull trout (Salvelinus confluentus). Can J Fish Aquat Sci 57(7):1470–1481
Baxter JS, McPhail JD (1999) The influence of redd site selection, groundwater upwelling, and over-winter incubation temperature on survival of bull trout (Salvelinus confluentus) from egg to alevin. Can J Zool 77(8):1233–1239
Beacham TD, Murray CB (1990) Temperature, egg size, and development of embryos and alevins of five species of Pacific salmon: a comparative analysis. Trans Am Fish Soc 119(6):927–945
Bebak J, Hankins J, Summerfelt S (2000) Effect of water temperature on survival of eyed eggs and alevins of Arctic char. N Am J Aquacult 62(2):139–143
Benjamin JR, Heltzel JM, Dunham JB, Heck M, Banish N (2016) Thermal regimes, nonnative trout, and their influences on native bull trout in the Upper Klamath River Basin. Oregon Trans Am Fish Soc 145(6):1318–1330
Berg O et al (2009) Pre-winter lipid stores in young-of-year Atlantic salmon along a north–south gradient. J Fish Biol 74(7):1383–1393
Bolduc C, Lamoureux SF (2018) Multiyear variations in high Arctic river temperatures in response to climate variability. Arctic Sci 4(4):605–623
Bowerman T, Neilson BT, Budy P (2014) Effects of fine sediment, hyporheic flow, and spawning site characteristics on survival and development of bull trout embryos. Can J Fish Aquat Sci 71(7):1059–1071
Campana SE et al (2020) Arctic freshwater fish productivity and colonization increase with climate warming. Nat Clim Change 10(5):428–433
Campbell EY, Dunham JB, Reeves GH, Wondzell SM (2019) Phenology of hatching, emergence, and end-of-season body size in young-of-year coho salmon in thermally contrasting streams draining the Copper River Delta Alaska. Can J Fish Aquat Sci 76(2):185–191
Chamaillé-Jammes S, Massot M, Aragon P, Clobert J (2006) Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara. Global Change Biol 12(2):392–402
Chavarie L, Dempson JB, Schwarz CJ, Reist JD, Power G, Power M (2010) Latitudinal variation in growth among Arctic charr in eastern North America: evidence for countergradient variation? Hydrobiologia 650(1):161–177
Chavarie L et al (2016) Multiple generalist morphs of Lake Trout: avoiding constraints on the evolution of intraspecific divergence? Ecol Evol 6(21):7727–7741
Chu C, Jones NE, Allin L (2010) Linking the thermal regimes of streams in the Great Lakes Basin, Ontario, to landscape and climate variables. River Res Appl 26(3):221–241
Clarke DN, Zani PA (2012) Effects of night-time warming on temperate ectotherm reproduction: potential fitness benefits of climate change for side-blotched lizards. J Exp Biol 215(7):1117–1127
Coleman MA, Fausch KD (2007a) Cold summer temperature limits recruitment of age-0 cutthroat trout in high-elevation Colorado streams. Trans Am Fish Soc 136(5):1231–1244
Coleman MA, Fausch KD (2007b) Cold summer temperature regimes cause a recruitment bottleneck in age-0 Colorado River cutthroat trout reared in laboratory streams. Trans Am Fish Soc 136(3):639–654
Conover DO, Schultz ET (1995) Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol Evol 10(6):248–252
COSEWIC (2012) COSEWIC assessment and status report on the Bull Trout Salvelinus confluentus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. iv + 103 pp
Crites H, Kokelj SV, Lacelle D (2020) Icings and groundwater conditions in permafrost catchments of northwestern Canada. Sci Report 10(1):1–11
Cunjak R, Prowse T, Parrish D (1998) Atlantic salmon (Salmo salar) in winter:" the season of parr discontent"? Can J Fish Aquat Sci 55(S1):161–180
Cunjak RA (1988) Physiological consequences of overwintering in streams: the cost of acclimitization? Can J Fish Aquat Sci 45(3):443–452
Deutsch CA et al (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci U S A 105(18):6668–6672
Devik O (1949) Freezing water and supercooling: anchor ice and frazil ice. J Glaciol 1(6):307–309
Dunham J, Bruce R, Gwynne C (2003) Influences of temperature and environmental variables on the distribution of bull trout within streams at the southern margin of its range. N Am J Fish Manage 23(3):894–904
Dunmall KM, Mochnacz NJ, Zimmerman CE, Lean C, Reist JD (2016) Using thermal limits to assess establishment of fish dispersing to high-latitude and high-elevation watersheds. Can J Fish Aquat Sci 73(999):1–9
Eby LA, Helmy O, Holsinger LM, Young MK (2014) Evidence of climate-induced range contractions in bull trout Salvelinus confluentus in a Rocky Mountain watershed, USA. PLoS ONE 9(6):e98812
Eliason EJ et al (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332(109):109–112. https://doi.org/10.1126/science.1199158
Environment Canada. (2020) Past weather and climate data. https://climate.weather.gc.ca/historical_data/search_historic_data_e.html. Accessed 01 July 2020
Fagan WF (2002) Connectivity, fragmentation, and extinction risk in dendritic metapopulations. Ecology 83(12):3243–3249. https://doi.org/10.2307/3072074
Finstad AG, Naesje TF, Forseth T (2004a) Seasonal variation in the thermal performance of juvenile Atlantic salmon (Salmo salar). Freshwat Biol 49(11):1459–1467. https://doi.org/10.1111/j.1365-2427.2004.01279.x
Finstad AG, Ugedal O, Forseth T, Næsje TF (2004b) Energy-related juvenile winter mortality in a northern population of Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 61(12):2358–2368
Fraser DJ, Weir LK, Bernatchez L, Hansen MM, Taylor EB (2011) Extent and scale of local adaptation in salmonid fishes: review and meta-analysis. Heredity 106(3):404–420
French WE, Vondracek B, Ferrington LC, Finlay JC, Dieterman DJ (2017) Brown trout (Salmo trutta) growth and condition along a winter thermal gradient in temperate streams. Can J Fish Aquat Sci 74(1):56–64. https://doi.org/10.1139/cjfas-2016-0005
Fuiman LA, Werner RG (2009) Fishery science: the unique contributions of early life stages. Blackwell Science, Victoria
Gould WR (1987) Features in the early development of bull trout (Salvelinus confluentus). Northwest Sci 23(4):264–268
Guzevich JW, Thurow RF (2017) Fine-scale characteristics of fluvial bull trout redds and adjacent sites in Rapid River, Idaho, 1993–2007. Northwest Sci 91(2):198–213
Halliwell DR, Catto S (2003) How and why is aquatic quality changing at Nahanni National Park Reserve, NWT, Canada? Environ Monit Assess 88:243–281
Hare DK, Helton AM, Johnson ZC, Lane JW, Briggs MA (2021) Continental-scale analysis of shallow and deep groundwater contributions to streams. Nat Commun 12:1450
Heino J et al (2020) Abruptly and irreversibly changing Arctic freshwaters urgently require standardized monitoring. J Appl Ecol 57:1192–1198
Holland W, Coen GM (1983) Ecological (biophysical) land classification of Banff and Jasper National Parks. Alberta Institute of Pedology, Calgary
Isaak DJ, Luce CH, Chandler GL, Horan DL, Wollrab SP (2018) Principal components of thermal regimes in mountain river networks. Hydrol Earth Syst Sci 22(12):6225–6240
Isaak DJ et al (2020) Thermal regimes of perennial rivers and streams in the western United States. J Amer Water Res Assoc 56(5):842–867
Isaak DJ et al (2010) Effects of climate change and wildfire on stream temperatures and salmonid thermal habitat in a mountain river network. Ecol Appl 20(5):1350–1371. https://doi.org/10.1890/09-0822.1
Isaak DJ et al (2017a) The NorWeST summer stream temperature model and scenarios for the western US: A crowd-sourced database and new geospatial tools foster a user community and predict broad climate warming of rivers and streams. Water Resour Res 53(11):9181–9205
Isaak DJ, Wenger SJ, Young MK (2017b) Big biology meets microclimatology: defining thermal niches of ectotherms at landscape scales for conservation planning. Ecol Appl 27(3):977–990
Isaak DJ et al (2016) Slow climate velocities of mountain streams portend their role as refugia for cold-water biodiversity. Proc Nat Acad Sci 113(16):4374–4379
Isaak DJ, Young MK, Nagel DE, Horan DL, Groce MC (2015) The cold-water climate shield: delineating refugia for preserving salmonid fishes through the 21st century. Glob Change Biol 21:2540–2553. https://doi.org/10.1111/gcb.12879
Johnson ZC, Johnson BG, Briggs MA, Snyder CD, Hitt NP, Devine WD (2021) Heed the data gap: guidelines for using incomplete datasets in annual stream temperature analyses. Ecol Indic 122:107229
Jones N, Schmidt B (2018) Thermal regime metrics and quantifying their uncertainty for North American streams. River Res Appl 34(4):382–393
Josse J, Husson F (2016) missMDA: A package for handling missing values in multivariate data analysis. 2016 70(1):31 doi:https://doi.org/10.18637/jss.v070.i01
Kelleher C, Wagener T, Gooseff M, McGlynn B, McGuire K, Marshall L (2012) Investigating controls on the thermal sensitivity of Pennsylvania streams. Hydrol Process 26(5):771–785
Klemetsen A (2010) The charr problem revisited: exceptional phenotypic plasticity promotes ecological speciation in postglacial lakes. Freshw Rev 3:49–74
Kovach RP, Al-Chokhachy R, Whited DC, Schmetterling DA, Dux AM, Muhlfeld CC (2017) Climate, invasive species and land use drive population dynamics of a cold-water specialist. J Appl Ecol 54(2):638–647
LeMoine MT, Eby LA, Clancy CG, Nyce LG, Jakober M, Isaak DJ (2020) Landscape resistance mediates native fish species distribution shifts and vulnerability to climate change in riverscapes. Glob Change Biol 26(10):5492–5508
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69(1):1–33. https://doi.org/10.18637/jss.v069.i01
Lisi PJ, Schindler DE, Cline TJ, Scheuerell MD, Walsh PB (2015) Watershed geomorpholosgy and snowmelt control stream thermal sensitivity to air temperature. Geophys Res Lett 42(9):3380–3388
Luce C, Staab B, Kramer M, Wenger S, Isaak D, McConnell C (2014) Sensitivity of summer stream temperatures to climate variability in the Pacific Northwest. Water Resourc Res 50(4):3428–3443
Lynch AJ et al (2016) Climate change effects on North American inland fish populations and assemblages. Fisheries 41(7):346–361
Mauger S, Shaftel R, Leppi JC, Rinella DJ (2017) Summer temperature regimes in southcentral Alaska streams: watershed drivers of variation and potential implications for Pacific salmon. Can J Fish Aquat Sci 74(5):702–715
Mayer TD (2012) Controls of summer stream temperature in the Pacific Northwest. J Hydrol 475:323–335
Michel A, Brauchli T, Lehning M, Schaefli B, Huwald H (2020) Stream temperature and discharge evolution in Switzerland over the last 50 years: annual and seasonal behaviour. Hydrol Earth Syst Sc 24(1):115–142
Mochnacz NJ (2021) Thermal ecology of bull trout (Salvelinus confluentus) and potential consequences of climate warming in montane watersheds. PhD thesis, University of Manitoba, Winnipeg, MB
Mochnacz NJ, Bajno R, Reist JD, Low G, Babaluk JA (2013) Distribution and biology of Bull Trout (Salvelinus confluentus) in the Mackenzie Valley, Northwest Territories, with notes on sympatry with Dolly Varden (Salvelinus malma). Arctic 66(1):79–93
Mochnacz NJ, MacKenzie DI, Koper N, Docker MF, Isaak DJ (2021) Fringe effects: detecting bull trout (Salvelinus confluentus) at distributional boundaries in a montane watershed. Can J Fish Aquat Sci 78:1030–1044
Mohseni O, Stefan HG, Erickson TR (1998) A nonlinear regression model for weekly stream temperatures. Water Resour Res 34(10):2685–2692
Morrill JC, Bales RC, Conklin MH (2005) Estimating stream temperature from air temperature: implications for future water quality. J Environ Eng 131(1):139–146
Nafziger J, Hicks F, Thoms P, McFarlane V, Banack J, Cunjak RA (2013) Measuring supercooling prevalence on small regulated and unregulated streams in New Brunswick and Newfoundland, Canada. In: Proceedings of the 17th CGU HSE CRIPE Workshop on River Ice, Edmonton, AB, pp 21–24
Narum SR, Campbell NR, Meyer KA, Miller MR, Hardy RW (2013) Thermal adaptation and acclimation of ectotherms from differing aquatic climates. Mol Ecol 22(11):3090–3097
Neuheimer AB, MacKenzie BR (2014) Explaining life history variation in a changing climate across a species’ range. Ecology 95(12):3364–3375
Neuheimer AB, Taggart CT (2007) The growing degree-day and fish size-at-age: the overlooked metric. Can J Fish Aquat Sci 64(2):375–385
NOAA (2020) Climate Data Records | National Centers for Environmental Information (NCEI) (noaa.gov). https://www.ncei.noaa.gov/products/climate-data-records. Accessed 01 July 2020
Olden JD, Naiman RJ (2009) Incorporating thermal regimes into environmental flow assessments: modifying dam operations to restore freshwater ecosystem integrity. Freshwat Biol 55(1):86–107
Parkinson E, Lea E, Nelitz M, Knudson J, Moore R (2016) Identifying temperature thresholds associated with fish community changes in British Columbia, Canada, to support identification of temperature sensitive streams. River Res Appl 32(3):330–347
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Paul AJA, Post JR (2001) Spatial distribution of native and nonnative salmonids in streams of the eastern slopes of the Canadian Rocky Mountains. Trans Am Fish Soc 130:417–430
Peterson AT (2011) Ecological niches and geographic distributions. Princeton University Press, Princeton
Piccolroaz S, Calamita E, Majone B, Gallice A, Siviglia A, Toffolon M (2016) Prediction of river water temperature: a comparison between a new family of hybrid models and statistical approaches. Hydro Proc 30(21):3901–3917
Pinheiro J, Bates D, DebRoy S, Sarkar D (2016) R Core Team (2016) nlme: linear and nonlinear mixed effects models. R Package Version 3:1–128
Pörtner H-O, Farrell AP (2008) Physiology and climate change. Science 322(5902):690–692
Prowse TD, Wrona FJ, Reist JD, Hobbie JE, Levesque LMJ, Vincent WF (2006a) General features of the Arctic relevant to climate change in freshwater ecosystems. Ambio 35(7):330–338
Prowse TD et al (2006b) Climate change effects on hydroecology of Arctic freshwater ecosystems. Ambio 35(7):347–358
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reist JD et al (2006a) Effects of climate change and UV radiation on fisheries for Arctic freshwater and anadromous species. Ambio 35(7):402–410
Reist JD et al (2006b) General effects of climate change on Arctic fishes and fish populations. Ambio 35(7):370–380
Rieman BE et al (2007) Anticipated climate warming effects on bull trout habitats and populations across the interior Columbia River basin. Trans Am Fish Soc 136(6):1552–1565
Rieman BE, McIntyre JD (1995) Occurrence of bull trout in naturally fragmented habitat patches of varied size. Trans Am Fish Soc 124(3):285–296
Sandblom E et al. (2016) Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat Commun 7:11447
Schindler DW (2000) Aquatic problems caused by human activities in Banff National Park, Alberta. Canada Ambio 29(7):401–407
Selong JH, McMahon TE, Zale AV, Barrows FT (2001) Effect of temperature on growth and survival of bull trout, with application of an improved method for determining thermal tolerance in fishes. Trans Am Fish Soc 130:1026–1037
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits annual review of ecology evolution and systematics. Ann Rev Ecol Evol Syst 40:415–436
Shuter BJ, Finstad AG, Helland IP, Zweimüller I, Hölker F (2012) The role of winter phenology in shaping the ecology of freshwater fish and their sensitivities to climate change. Aquat Sci 74(4):637–657. https://doi.org/10.1007/s00027-012-0274-3
Sinnatamby NR, Dempson BJ, Reist JD, Power M (2015) Latitudinal variation in growth and otolith-inferred field metabolic rates of Canadian young-of-the-year Arctic charr. Ecol Freshwat Fish 24(3):478–488
Snyder CD, Hitt NP, Young JA (2015) Accounting for groundwater in stream fish thermal habitat responses to climate change. Ecol Appl 25(5):1397–1419
Somero G (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers.’ J Exp Biol 213(6):912–920
Sparks MM et al (2019) Influences of spawning timing, water temperature, and climatic warming on early life history phenology in western Alaska sockeye salmon. Can J Fish Aquat Sci 76(1):123–135
Sparks MM, Westley PA, Falke JA, Quinn TP (2017) Thermal adaptation and phenotypic plasticity in a warming world: insights from common garden experiments on Alaskan sockeye salmon. Global Change Biol 23(12):5203–5217
Stamp J, Hamilton A, Craddock M, Parker L, Roy A, Isaak D, Holden Z, Passmore M, Bierwagen B (2014) Best practices for continuous monitoring of temperature and flow in wadeable streams, Global Change Research Program, National Center for Environmental Assessment, Washington, D.C., EPA/600/R-13/170F, 2014
Steel AE, Sowder C, Peterson EE (2016) Spatial and temporal variation of water temperature regimes on the Snoqualmie River network. J Amer Water Res Assoc 52(3):769–787
Su G, Logez M, Xu J, Tao S, Villéger S, Brosse S (2021) Human impacts on global freshwater fish biodiversity. Science 371(6531):835–838. https://doi.org/10.1126/science.abd3369
Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nat Clim Change 2(9):686–690
Sunday JM et al (2014) Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc Natl Acad Sci U S A 111(15):5610–5615
Utting N, Lauriol B, Mochnacz N, Aeschbach-Hertig W, Clark I (2013) Noble gas and isotope geochemistry in western Canadian Arctic watersheds: tracing groundwater recharge in permafrost terrain. Hydrogeol J 21(1):79–91. https://doi.org/10.1007/s10040-012-0913-8
Walther G-R et al (2002) Ecological responses to recent climate change. Nature 416:389–395
Wenger SJ et al (2011) Flow regime, temperature, and biotic interactions drive differential declines of trout species under climate change. Proc Natl Acad Sci U S A 108(34):14175–14180. https://doi.org/10.1073/pnas.1103097108
Zuur A, Leno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Zuur AF, Leno EN (2016) A protocol for conducting and presenting results of regression-type analyses. Methods Ecol Evol 7(6):636–645
Acknowledgements
We thank the following people for the assistance in the field: M. Alperyn, R. Bajno, A. Bell, C. Carli, A. Chapelsky, P. Cott, K. Dunmall, K. Dyszy, M. Gillespie, E. Lea, B. Lewis, P. Marcellais, A. Okrainec, B. Robinson, P. Rodger, J. Sabourin, A. Steedman, D. Struthers, D. Tate, and A. Zier-vogel. Parks Canada Agency (Nahanni National Park, Banff National Park) provided in-kind and technical support. We thank D. Teleki for the assistance in developing the map; and D. Horan, G. Chandler, and D. Struthers for formatting the data. Comments from two anonymous reviewers substantially improved the manuscript.
Funding
Open Access provided by Fisheries & Oceans Canada. Funding was provided by Fisheries and Oceans Canada; the Government of the Northwest Territories, Cumulative Impacts Monitoring Program; the National Research Council of Canada, Program for Energy Research and Development; and Parks Canada Agency.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Neil J. Mochnacz and Mark K. Taylor. The first draft of the manuscript was written by Neil J. Mochnacz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Compliance with Ethical Standards
No human participants were involved in this research. All animals in this research were handled in compliance with the standards set forth by the Canadian Council for Animal Care.
Competing interests
Margaret F. Docker is the Editor-in-Chief of this journal, but she had no involvement in the peer review of this article and had no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to the Managing Editor and Guest Editors. The authors have no relevant financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mochnacz, N.J., Taylor, M.K., Docker, M.F. et al. An ecothermal paradox: bull trout populations diverge in response to thermal landscapes across a broad latitudinal gradient. Environ Biol Fish 106, 979–999 (2023). https://doi.org/10.1007/s10641-022-01339-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01339-0