Abstract
We released nearly 1.0 million 1-day post-hatch (dph) and 5-dph pallid sturgeon (Scaphirhynchus albus) free embryos in the Missouri River on 1 July 2019 and sequentially captured survivors at multiple sites through a 240-km river reach to quantify daily growth and survival rates during the early life stages. Genetic analysis was used to assign captured fish to released family lots and known ages. Growth rate was similar (0.74–0.75 mm day−1) between the 1- and 5-dph age groups during the 3–4-day dispersal period when water temperature averaged 16.8 °C. Daily survival rate was 0.64 during 1–4 dph for the original 1-dph age group and 0.80 during 5–7 dph for the original 5-dph age group. Total survival during free embryo dispersal (hatch to 9 dph) was estimated as 0.0437. The transition from dispersing as free embryos to settling as benthic larvae was verified for fish originally released as 5 dph. Growth of settled larvae was quantified with a Gompertz model through 75 dph (9 September; 112 mm) when water temperature was 18.8–21.0 °C in the rearing areas. Settled larvae had an estimated daily survival rate of 0.96, and estimated total survival during 9–75 dph was 0.0714. This study provides the first empirical survival estimates for pallid sturgeon early life stages in natural settings and is one of few studies reporting similar information for other sturgeon species. Applications of this work extend to pallid sturgeon restoration programs where population models are being developed to predict recruitment potential and population responses to river management alternatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sturgeons (family Acipenseridae) are a globally imperiled group of fishes threatened by many factors including dams and associated flow regulation, fragmentation, degraded habitat, pollution, by-catch, poaching, and overfishing (Pikitch et al. 2005; Jarić et al. 2018). Restoring sturgeon stocks from a critically imperiled status to a nominally functional status and ultimately to a fully functional population can be challenging owing to multiple stressors that affect the various life stages. Furthermore, restoration is viewed as a long-term process due to the slow rates of population growth in this late-maturing, long-lived group of fishes (Pine et al. 2001; Bruch et al. 2016).
The use of basic population models (e.g., Beamesderfer et al. 2007) or more intensive highly parameterized models (e.g., Jager et al. 2002) has increased over the last two decades (Jarić et al. 2014) to discern the influence of biological attributes, existing anthropogenic stressors, and proposed restoration alternatives on sturgeon stocks or populations. Models sufficiently parameterized can address multiple factors hypothesized to affect various life stages, and when coupled with sensitivity or uncertainty analysis, modeling provides insight into one or more factors that have the greatest influence on population change (Jager et al. 2002; Heppell 2007; Wildhaber et al. 2017). Growth and mortality during the first year of life are critical processes affecting recruitment in fishes (Houde 1987), and these vital rates are similarly critical input parameters to population models. For example, simulation studies have shown that sturgeon population growth is sensitive to slight changes in vital rates during the initial year of life (Pine et al. 2001; Gross et al. 2002). Thus, accurate vital rate estimates are needed as model inputs; otherwise, population change projections may be inaccurate.
Empirical data on vital rates during the early life stages are lacking for most sturgeon species, and these rates represent areas of much uncertainty and research need (Secor et al. 2002; Pollock et al. 2015). Laboratory studies are useful to discern the effects of different temperature regimes on growth and development during the free embryo (i.e., endogenous feeding) life stage, owing that development progressions during this life stage are primarily driven by temperature (Mrnak et al. 2020). Laboratory inferences on natural mortality for endogenous- and exogenous-feeding life stages and growth following the transition to exogenous feeding are less certain for applications to natural settings. The controlled laboratory environments lack most physical (e.g., diurnal temperature fluctuations, habitat heterogeneity, hydraulic conditions) and biological (e.g., broad-scale dispersal, food patchiness, predation risk) complexities that likely have a strong effect on growth and mortality. Growth of sturgeons during the early life stages has been assessed in limited field investigations (e.g., Benson et al. 2006; Phelps et al. 2010; Braaten et al. 2012a), but field studies examining early life stage mortality in sturgeons are rare (i.e., Usova 2009; Caroffino et al. 2010). Lacking empirical species-specific growth and mortality criteria for early life stages, population modeling for sturgeon may use a variety of methods to estimate vital rates. For example, mortality rate may be modeled based on constant recruitment (Pine et al. 2001), estimated at a rate that calibrates stable population growth (Jager et al. 2002; Doukakis et al. 2010; Steffensen et al. 2013; Wildhaber et al. 2017), or estimated for the target species using rates from other species (Blackburn et al. 2019). For the latter case, the extent to which early life stage vital rates can be applied within species between systems or between species is an area of needed research (Pollock et al. 2015).
The pallid sturgeon Scaphirhynchus albus is a federally endangered species in the USA (Dryer and Sandvol 1993) that inhabits the Missouri River, lower Mississippi River, and associated tributaries (Bailey and Cross 1954). Stocks are depressed, limited spawning occurs, and early life stages are rarely collected (USFWS 2014; Jordan et al. 2016). Consequently, little information on growth and mortality of early life stages is available. Restoration alternatives to enhance pallid sturgeon spawning and recruitment have been proposed in the Missouri River basin, and population modeling is a fundamental tool to evaluate population responses to different restoration alternatives (Fischenich et al. 2018a,b); however, population models lack empirical data for pallid sturgeon vital rates during the early life stages. Early life stage vital rates empirically determined from one species (e.g., lake sturgeon Acipenser fulvescens; Caroffino et al. 2010) or adapted from other species may not be applicable to pallid sturgeon owing to differences in the early life history among sturgeon species. For example, lake sturgeon remain associated with the substrate for 3–5 days following hatch and then disperse primarily at dusk when they transition to exogenously feeding larvae (Auer and Baker 2002, 2020). Exhibiting a different behavior, endogenous-feeding pallid sturgeon free embryos emerge from the substrate immediately at hatch (Kynard et al. 2002, 2007; DeLonay et al. 2016a) and then exhibit diel dispersal for several days prior to settling as exogenous-feeding benthic larvae (Kynard et al. 2007; Braaten et al. 2012a, b). Thus, whereas different sturgeon species are subject to common mortality agents during ontogenetic development (e.g., thermal changes, predation, river channel hydraulic conditions), their exposure to mortality agents occurs at different locations—and potentially different intensities—in habitat space.
The objectives of this study were to estimate growth and survival of dispersing pallid sturgeon free embryos and settled (benthic) larvae. The objectives were accomplished by releasing known numbers of 1- and 5-day post-hatch (dph) free embryos in the mainstem Missouri River and quantifying changes in length and abundance of free embryos as they dispersed several days through nearly 240 km of river. Estimates of growth and survival for settled larvae were accomplished by quantifying changes in length and relative abundance over a 9-week sampling period spanning 22–75 dph. Results from the analysis were subsequently used to estimate total survival of pallid sturgeon from hatch through the first growing season.
Materials and methods
Study area
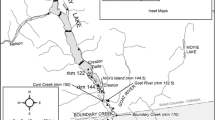
The study was conducted in a 241-km reach of the Missouri River in Montana and North Dakota, USA (Fig. 1). The upstream portion of the reach (192 km) extended from the free embryo release location (see below) at river km 2739.0 (rkm; distance upstream from the confluence of the Missouri River and Mississippi River) downstream to rkm 2547.0 (Yellowstone River confluence). This reach is positioned within a river segment (Frissell et al. 1986) that is hydrologically influenced by operations of Fort Peck Dam (rkm 2852.0). Regulated, hypolimnetic water releases through Fort Peck Dam alter flow timing and magnitude (Bowen et al. 2003) and reduce water temperatures (Erwin et al. 2018). Although hydrologically regulated, the study reach in this segment exhibits habitat heterogeneity including sand bars, islands, backwaters, main channel and channel border habitat, and varied hydraulic conditions (Shields et al. 2000; Bowen et al. 2003; Erwin et al. 2018).
The downstream portion of the study reach (49 km) was contained in a river segment extending from the Yellowstone River confluence downstream to rkm 2498.0 within the designated full-pool headwaters of Lake Sakakawea (Fig. 1). Although full pool is designated near rkm 2523.0 (Galat et al. 1996), elevated velocities (e.g., > 0.40 m s−1; Erwin et al. 2018) and riverine conditions extend downstream of the full-pool designation location beyond rkm 2500.0. The Yellowstone River contributes relatively natural flow and temperature regimes to the Missouri River (Bowen et al. 2003).
Free embryos
The elevated discharge volume of the Missouri River and the need to capture sufficient numbers of free embryos through the extended study reach required the production of large numbers of free embryos for release. We used mass hatchery production of two age groups of free embryos for release in the river, centered on 1- and 5-dph pallid sturgeon. Logistics of the study required a target implementation date; 1 July 2019 was the target date, and hatchery spawning events and incubation schedules were designed to deliver the 1- and 5-dph free embryos to the river on 1 July.
The 1- and 5-dph free embryos were sourced from a combination of F1 captive brood stock and wild-caught pallid sturgeon spawned in hatcheries (Table 1). The 1-dph age group represented 16 families, including five families produced from wild fish collected in the Missouri and Yellowstone rivers during spring 2019 and 11 families produced from captive brood stock. Hatchery spawning to produce the 1-dph free embryos occurred on 25 June 2019, and hatch initiated during the evening of 30 June. The 5-dph free embryos represented 11 families produced from captive brood stock; hatchery spawning occurred on 20 June 2019, and hatch occurred from 25 to 27 June. Free embryos produced from captive brood stock were spawned at the U.S. Fish and Wildlife Service Gavins Point National Fish Hatchery (GPNFH; Yankton, South Dakota), transported as developing embryos to the U.S. Fish and Wildlife Service Garrison Dam National Fish Hatchery (GDNFH; Riverdale, North Dakota), then reared through hatch and early development at GDNFH. Hatchery production yielded an estimated 771,707 free embryos that were approximately 1 dph (hereafter referred to as 1 dph). The 1-dph free embryos were exposed to 18.5 cumulative thermal units (CTU; calculated as the sum of mean daily water temperature exposure) prior to release. Hatchery production also yielded an estimated 200,786 free embryos that were 5 to 6 dph (hereafter referred to as 5 dph; pre-release thermal exposure 90–108 CTU). The free embryos were packaged in plastic bags at GDNFH on the morning of 1 July 2019, transported in coolers to the Missouri River release location (rkm 2739.0, Fig. 1) where they arrived at 13:00 h and were loaded on boats. Water temperature in the Missouri River was unseasonably cool (14.2–14.3 °C) owing to a cold-front that moved through northeastern Montana. Thus, the free embryos were acclimated to the cool water temperatures from 13:15 to 14:00 h by repeatedly adding river water to bagged fish in the coolers. At 14:00 h on 1 July, multiple boats spread across the river channel released the free embryos at the water surface. The release process took about 2–3 min. A portion of free embryos from the same family lots was also retained at GDNFH to assess survival under hatchery conditions and to determine age when the free embryos transitioned from swimming in the water column to settling as benthic larvae on the bottom of rearing tanks.
Sampling design and protocols for dispersing free embryos
Sampling for the dispersing free embryos was conducted at six sites (1, 2, 3, 4, 5, 6) located 15.5, 34.7, 131.0, 178.0, 194.0, and 236.0 km, respectively, downstream of the release site (Fig. 1). Sampling at each site was implemented to quantify pre-arrival of the dispersing free embryos to each site (i.e., period of zero catch prior to arrival), dispersion of the free embryos (i.e., period from the first catch to last catch), and post-passage (e.g., period of zeros extending after the last catch). Depending on downstream site distance and free embryo dispersal rates, sampling was conducted during day, night, or a combination of day and night. Collectively, sampling was conducted continuously or nearly continuously from 1 to 8 July, spanning 2.7–7.1 h post-release (HPR) at site 1, 7.5–39.8 HPR at site 2, 24.0–85.8 HPR at site 3, 42.7–125.8 HPR at site 4, 68.2–168.2 HPR at site 5, and 116.6–167.8 HPR at site 6.
Sampling at each site was conducted using 1–3 boats (2–6 crew members per boat) depending on needs for continuous day-night sampling at the sites to quantify free embryo dispersal rates and the need for crew rest following 12-h day or night deployments. Boats were positioned primarily in the main channel flow field (i.e., thalweg) where concentrations of dispersing pallid sturgeon free embryos are greatest (Braaten et al. 2010). Boats were equipped with paired rectangular frame nets (0.5-m height, 0.75-m width, 3.0-m length, 1.0-mm square mesh, terminating in a removable cod-end collecting cup with 1.0-mm mesh) weighted with either downrigger weights or sounding weights. Nets were fitted with a flow meter to estimate water velocity and volume filtered (m3) by each net. The frame-net bridle was attached to cable or rope, and nets were deployed off the port and starboard sides of the boat near the bow using paired winches.
Two sets of sampling protocols were implemented. The first protocol was designed to quantify anticipated high concentrations of free embryos at sites 1 and 2 during the initial dispersal period. Using anchored boats, we deployed two nets for 5-min samples; one net was lowered to the river bed (hereafter bottom net) to sample the lower 0.5 m of the water column, and the other net was fished in the mid-water column (hereafter midwater net). After the 5-min sampling regime, the nets were retrieved, and contents of the cod-end collecting cups were emptied into black rubber pans. Contrasting against the black rubber, the gray-white Acipenseriformes free embryos (potentially pallid sturgeon, shovelnose sturgeon Scaphirhynchus platorynchus, or paddlefish Polyodon spathula) were extracted from the detritus using forceps and placed in individually numbered vials containing 95% non-denatured ethanol (ETOH) for later genetic analysis. If the sample contained large numbers of free embryos, the entire sample was preserved in a 10% formalin solution for later processing. Formalin rather than ETOH use occurred only at site 1, and formalin was necessary to ensure adequate preservation of free embryos in the large detrital loads. After on-boat processing of the samples, nets were deployed as quickly as possible for another 5-min sampling event.
The second set of protocols was implemented after 17.0 HPR at site 2 and for all other downstream sampling at sites. Under this protocol, sampling duration was extended to 10 min to increase sample volume and improve the likelihood of capturing free embryos under the expectation that free embryo concentrations would diminish through time and space. As above, a pair of nets was deployed where one net was always deployed to the bottom, but the second net alternated between deployment to the bottom, midwater, or river surface (hereafter surface net) locations. Although concentrations of dispersing pallid sturgeon free embryos are greatest near the river bed (Braaten et al. 2008; 2010), midwater and surface net samples were used to provide an additional test of these findings and to further explore potential changes in vertical dispersal location during ontogenetic development. After the 10-min sample, Acipenseriformes free embryos were placed in individual vials containing ETOH, then the next sample was initiated. Although multiple boats sampling at individual sites had the common objectives of capturing and quantifying dispersing free embryos, net deployments were not synchronous between boats. For example, one crew might be in the process of extracting free embryos from a sample, while another crew was in the process of obtaining a 10-min sample.
Sampling design and protocols for settled larvae
Sampling for pallid sturgeon that had transitioned from dispersing free embryos to settled benthic larvae (e.g., age 0) was conducted at eight sites in the Missouri River between rkm 2499.0 and rkm 2563.0 (Fig. 1); four sites were located upstream from the Yellowstone River confluence, and four sites were located downstream from the Yellowstone River confluence. The eight sites were individual river bends sampled for nine consecutive weeks from 16 July to 11 September 2019. A benthic beam trawl (2.0-m width, 0.5-m height, 5.5-m length) was deployed in three habitats associated with each bend, including low-velocity water along the inside bend (ISB), higher-velocity water in the thalweg of the outside bend (OSB), and the channel crossover (CHXO) region of the channel where the main flow field transitions from one river bank to the other (see Braaten and Fuller 2007). Trawl area (m2) was estimated as the product of trawl deployment length (m) and trawl width (m). All Scaphirhynchus sp. captured (potentially pallid sturgeon or shovelnose sturgeon) were measured to the nearest 1.0 mm total length (TL, excluding the caudal filament), and a fin clip for genetic analysis from each fish was stored in vial containing ETOH. If one or more larval Scaphirhynchus sp. was found in the first trawl sample conducted in each habitat (hereafter standard trawl), then additional trawls (hereafter targeted trawls) were conducted in the same habitat. Targeted trawls were conducted in an effort to collect additional settled larvae and to increase sample size for growth analysis, but larvae only from standard trawls were used for mortality estimates (see below). Trawling of the eight sites was completed in 2–3 days each week.
Laboratory and genetic processing
Free embryos preserved in ETOH and formalin (subsequently transferred to ETOH in the laboratory) were measured to the nearest 0.1 mm total length (TL). Sampling in high-velocity river environments causes damage to free embryos (e.g., torn or missing portions of the body, deformities), and as such, length measurements from only intact, high-quality specimens were used in the growth analyses. Length measurements from free embryos originally preserved in formalin from site 1 then later transferred to ETOH were excluded from the analyses as these lengths could not be directly compared to ETOH-only samples due to different preservation methods (Bayer and Counihan 2001).
Dispersing free embryos sampled from the mainstem Missouri River potentially included the experimentally released 1- and 5-dph pallid sturgeon and wild-produced progeny of pallid sturgeon, shovelnose sturgeon, and paddlefish in varying stages of ontogenetic development. Free embryos preserved in ETOH were genetically screened using single-nucleotide polymorphism markers (SNP; Eichelberger et al. 2014) to differentiate specimens as definitive paddlefish, definitive shovelnose sturgeon, or potential pallid sturgeon. Specimens classified as potential pallid sturgeon included pallid sturgeon and a small percentage of shovelnose sturgeon (Eichelberger et al. 2014) that share the pallid sturgeon SNP genotypes. Additional genetic analysis to unequivocally identify shovelnose sturgeon and pallid sturgeon for all SNP-determined potential pallid sturgeon was cost-prohibitive; however, a subsample (N = 174) of potential pallid sturgeon was further analyzed at 19 microsatellite loci (McQuown et al. 2000) to definitively differentiate pallid sturgeon and shovelnose sturgeon.
Although the SNP and complementary microsatellite analysis verified free embryos as pallid sturgeon, the analysis did not differentiate individuals as the original 1- and 5-dph released age groups. Three techniques were used to assign the free embryos to age groups. First, genetic parentage analysis (DeHaan et al. 2008) was performed on the microsatellite subsample to match free embryo genetics to parental genetics (Table 1). The second technique used differences in free embryo lengths between the released age groups from the onset of release to termination of the study. Length frequency distributions were bimodal at all sites indicating two age groups; mean length of ETOH fish differed between the two age groups by an average of 5.3 mm (range 4.9–5.7 mm depending on site) and the length gap between the largest presumed 1-dph free embryo and smallest presumed 5-dph free embryo averaged 1.2 mm (range 0.4–3.0 mm depending on site). Third, and complementary to the length differences, differences in morphological development between the age groups were used to partition formalin-preserved free embryos collected at site 1 to age groups; the released 5-dph free embryos had a heavily black-pigmented tail region (see Snyder 2002) that was not present in the released 1-dph free embryos. Thus, presence or absence of the black tail pigmentation was used to partition free embryos from site 1 formalin samples into the 1- or 5-dph age groups. Fin clips from settled Scaphirhynchus sp. larvae caught with the beam trawl were analyzed using SNP procedures, microsatellite analysis, and parentage analysis as outlined above for free embryos.
Discharge and water temperature
Water temperature loggers (HOBO Pro V2, Onset Computer Corporation, Bourne, Massachusetts, USA) were deployed at four sites to quantify water temperature during the free embryo dispersal and larval life stages. Three loggers were deployed in the Missouri River segment upstream from the Yellowstone River confluence (rkm 2706.9, 2604.0, 2560.2). One logger was positioned at rkm 2538.2 in the Missouri River segment downstream from the Yellowstone River confluence to quantify temperature regimes influenced by Yellowstone River additions. The loggers recorded water temperature at 1-h intervals. A logger deployed in GDNFH recorded rearing water temperatures at 4-h intervals. Discharge data for the Missouri River segment upstream from the Yellowstone River confluence were obtained from a gage station at rkm 2739.0 (USGS gage number 06177000). For the Missouri River segment downstream from the Yellowstone River confluence, discharge was estimated by adding daily Missouri River gage data to daily Yellowstone River data obtained from a gage at rkm 47.0 (USGS gage number 06329500).
Data analysis
Free embryos were summed by age group for individual net deployments within each boat and divided by volume of water filtered during deployment to estimate free embryo concentration (free embryos m−3). Paired nets simultaneously deployed to the bottom were subsamples and averaged to produce a mean concentration for the HPR bottom sample. Paired nets deployed simultaneously to different water column locations (i.e., bottom and midwater, bottom and surface) were treated as individual samples. Wilcoxon two-sample tests (Proc NPAR1WAY; SAS 2019) were used to compare free embryo concentrations for the 1- and 5-dph age groups between bottom and midwater nets simultaneously deployed and between bottom and surface nets simultaneously deployed. Although not a specified objective of the study, comparisons of free embryo concentrations between net-sampling locations were necessary for the concentration-based mortality analysis to determine whether free embryo concentrations from different nets could be pooled. Free embryo concentrations of zero obtained during pre-arrival and post-passage sampling were not included in the two-sample tests.
Growth for the original 1- and 5-dph released free embryos was quantified by the following process. Free embryos sampled during each net deployment at each site were assigned an age by converting sample HPR to days (HPR/24 h), then adding 1 day (for the original 1-dph free embryos) or 5 days (for the original 5-dph free embryos) to the converted HPR. Thus, age was infrequently an integer but rather a fractional age (i.e., 3.2 dph). Free embryos collected during each net deployment were subsamples, and lengths were averaged to yield a free embryo mean length for each net deployment. Free embryo length was regressed against age (Proc REG; SAS 2019), and analysis of covariance (Proc GLM; SAS 2019) was used to compare growth rate slopes and intercepts between the original 1- and 5-dph free embryos.
Mortality of dispersing free embryos was estimated independently for the original 1- and 5-dph released age groups using a multi-step process to calculate the numbers of free embryos at each site to include the effects of dispersion (i.e., declining concentrations and broadening catch distributions during longitudinal transport; Erwin et al. 2018). Mortality estimates were based exclusively on catches from bottom net samples owing that free embryo concentrations were greater in bottom than midwater or surface nets (see “Results”). Median free embryo concentrations were calculated from bottom net deployments at each site for the timeframe encompassing free embryo dispersion (i.e., time in hours from the first catch to the last catch). Median velocity (m s−1) from bottom nets was multiplied by site width (m) and net height (0.5 m) to estimate cross-sectional discharge (m3 s−1) for the lower 0.5 m of the water column. Cross-sectional discharge was multiplied by free embryo dispersion time (i.e., hours converted to seconds) to estimate the total volume of water (m3) passing through the lower 0.5 m of the water column during dispersion. The total number of original 1- and 5-dph free embryos passing each site was estimated as the product of median free embryo concentration from bottom nets and total volume of water passing through the lower 0.5 m of the water column during dispersion. The estimated total number of free embryos and corresponding mean free embryo age (weighted by HPR-specific concentrations) at each site were used to estimate the instantaneous mortality rate (Z) for the original 1- and 5-dph cohorts between successive sites following Bailey et al. (1995):
where Nx is the estimated number of free embryos in the cohort at site x, Nx+1 is the estimated number of free embryos in the cohort at site x + 1, agex is cohort age (dph) at site x, and agex+1 is cohort age at site x + 1. The daily survival rate (S) was calculated as e−z, and daily mortality rate (M) was calculated as 1-e−z (Miranda and Bettoli 2007). Survival in the hatchery was also assessed by dividing daily counts of dead free embryos by the estimated number of free embryos on the previous date.
Beam trawl catches of settled pallid sturgeon larvae by date and their genetically verified original age at release provided known age-at-capture to facilitate estimating growth and mortality. Growth of settled larvae collected in standard and targeted trawls was quantified using a Gompertz growth model (Campana and Jones 1992) of the form:
where L∞ is the asymptotic length (mm), G is the instantaneous growth rate at Age0, and Age0 is the inflection point of the curve and age at which growth rate begins to decline. The Gompertz model was fitted using Proc NLIN (SAS 2019).
Mortality for the settled larvae was estimated using catch-curve analysis (Miranda and Bettoli 2007) based exclusively on catch from standard trawl efforts. Individual beam trawl catches at a river bend (e.g., ISB, OSB, CHXO) were subsamples for the river bend replicate and averaged to obtain a mean catch-per-unit-area (CPUA; number of larvae m−2) for each river bend during each sampling week. For mortality estimation, mean CPUA across bends for each week was regressed against mean age of larvae (e.g., captured during the 2–3-day sampling interval each week). The slope of the relationship estimates Z, from which daily S and daily M were calculated as described above for free embryos.
Water temperature during free embryo dispersal was quantified using hourly data from loggers, but a longitudinal approach was required to link the changing location of the dispersing population through time and distance to loggers at successive downstream sites. The quantification approach used the following for each logger (dates and HPR in parentheses): rkm 2706.9 (1–3 July, 7–36 HPR), rkm 2604.0 (2–4 July, 27–80 HPR), and rkm 2560.2 (3–6 July, 54–120 HPR). The overlapping HPR among sites quantified average thermal exposure for free embryos between loggers and sampling sites. In addition to logger data, spot measurements of water temperature early in the study (e.g., at release, site 1) augmented logger data. Hourly temperature data were averaged across HPR for all sites to estimate the average water temperature during dispersal. Water temperature during the growing season for settled larvae was quantified from 4 July (initiation of settlement) to 9 September (final collection of a pallid sturgeon larvae) with loggers positioned at rkm 2560.2 and rkm 2538.2. Hourly water temperatures for larvae were averaged by day and across dates to obtain an average water temperature during the growing season.
Results
Growth and mortality of dispersing free embryos
Sampling for the released free embryos across all sites yielded 3357 Acipenseriformes free embryos, of which 2603 were preserved in ETOH and 754 in formalin (Braaten and Holley 2021). The SNP analysis of ETOH-preserved specimens identified 2034 free embryos as potential pallid sturgeon and 561 specimens as shovelnose sturgeon or paddlefish. Microsatellite analysis on the subsample of 174 potential pallid sturgeon identified 165 definitive pallid sturgeon and nine shovelnose sturgeon. Species identification based on genetics could not be conducted for the remaining 754 formalin-preserved free embryos sampled at site 1; however, these fish were almost exclusively pallid sturgeon based on the finding that 99.2% of 488 ETOH-preserved free embryos from site 1 were identified as pallid sturgeon from SNP analysis. Collectively, a total of 2779 pallid sturgeon from the released age groups were designated from catches at the six sites (i.e., 2025 from genetic analysis, 754 preserved in formalin). Only three pallid sturgeon were identified from site 6, and these fish were used only in the free embryo growth analysis.
Free embryo concentrations at sites 1 through 5 (15.5–194.0 km downstream) quantified patterns of pre-arrival, passage, and post-passage of fish that were diagnostic of the mass-release of large number of fish exhibiting downstream dispersal (Fig. 2). Free embryo concentration was greater in bottom nets than surface nets (1 dph: P = 0.0003, N = 28; 5 dph: P < 0.0001, N = 50) or midwater nets (1 dph: P = 0.034, N = 76; 5 dph: P = 0.003, N = 98), so concentrations from the different net locations were not pooled; only bottom nets were used to quantify free embryo concentrations for mortality analysis. Both free embryo age groups passed through site 1 over a brief timeframe and exhibited high concentrations during mass arrival. Continuous 24-h sampling regimes through site 3 characterized full dispersion of the free embryos. Sampling at sites 4 and 5 was not fully continuous on a diel cycle due to sampling crew demands at other sites. Sampling at site 4 spanned an extended period of dispersion (i.e., 67–100 HPR, 115–125 HPR) to quantify elevated concentrations during mass arrival and declining concentrations late in dispersion, but initial arrival of the free embryos was not assessed. Concentrations of free embryos at site 5 quantified mass arrival, but the complete range of dispersion was not assessed. Whereas continued dispersal was evident for the original 1-dph free embryos between sites 4 and 5, absolute numbers and concentrations for the original 5-dph free embryos diminished between sites 4 and 5 after about 70 HPR (about 4 July) to suggest (and verified in Larval section below) that the older released age group had initiated the transition from dispersing free embryos to settled larvae between the sites. Observations from the hatchery also supported field observations in that initial settlement of larvae on the bottom of rearing tanks occurred on 3 July, and 100% settlement was documented on July 6. Comparatively, settlement for the original 1 dph in the hatchery spanned from 7 July (initial settlement) to 10 July (100% settlement).
Concentrations (number m−3) of pallid sturgeon free embryos originally released as 1-day post-hatch (dph; filled circles) and 5 dph (open squares) by hours post-release at five sampling sites 15.5–194.0 km downstream from the release location. Numbers collected at each site are listed by dph. Sampling was conducted during 2.7–7.1 h post-release (HPR) at site 1, 7.5–39.8 HPR at site 2, 24.0–85.8 HPR at site 3, 42.7–125.8 HPR at site 4, and 68.2–168.2 HPR at site 5. Prolonged periods of zero catch at sites have been omitted from graphs to emphasize periods when concentrations were greater than 0 free embryos m.−3. Note the change in abscissa and ordinate values among sampling sites
Median concentrations of the original 1- and 5-dph free embryos declined, and dispersion increased as free embryos dispersed among successive downstream sampling sites (Table 2). Median concentration declined to zero for the original 1-dph free embryos at site 5 and for the original 5-dph free embryos at sites 4 and 5; therefore, these sites were not used in mortality estimation. The estimated number of pallid sturgeon free embryos from the 1-dph cohort declined from site 1 (59,807 fish, mean age 1.2 dph) to site 2 (29,745 fish, mean age 1.5 dph) to yield an initially elevated Z of 2.33 and low daily S of 0.10 (Table 2). Instantaneous mortality for the 1-dph cohort declined to 0.45 (daily S = 0.64) as the free embryos dispersed from site 2 (1.5 dph) to site 3 (3.2 dph) and from site 3 to site 4 (4.0 dph). For the 5-dph cohort, the estimated number of free embryos declined from 29,513 fish at site 1 to 16,660 fish at site 2, and Z was 1.91 to indicate a low initial survival rate of 0.15 during 5.2–5.5 dph (Table 2). From site 2 (5.5 dph) to site 3 (7.4 dph), Z declined to 0.22 and daily survival increased to 0.80. The reason for the elevated Z and low daily S common to both age group shortly after release is not known but may have included short-term mortality associated with river acclimation following release from hatchery bags. In the hatchery, daily S averaged 0.92 for the 1-dph age group during 1–4 dph and 0.95 for the 5-dph age group during 5–9 dph.
Growth was quantified for ages 1 to 5 dph for free embryos originally released as 1 dph and for ages 5 to 10 dph for free embryos originally released as 5 dph. Length was significantly related to age for both free embryo age groups (Fig. 3). Slopes were similar between age groups (0.75 versus 0.74 mm d−1; ANCOVA, P = 0.84); however, intercepts differed between age groups (P < 0.0001).
Mean hourly water temperature during free embryo dispersal (1–6 July, 120 HPR) was 16.8 °C (SD = 0.9, CV = 5.0%, N = 117 hourly measurements averaged among loggers in the Missouri River upstream from the Yellowstone River confluence; Fig. 4). The dispersal thermal regime included a pronounced initial decline in temperature due to cold-front conditions followed by gradual warming (Fig. 4). Diel water temperature variation (midnight to midnight at each logger) averaged 1.2 °C (range 0.7 to 1.5 °C). In addition to diel variations, dispersing free embryos were exposed to a longitudinal warming gradient as mean water temperature increased about 2.2 °C between loggers positioned in the Missouri River upstream from the Yellowstone River confluence (Fig. 4). Mean daily discharge during the 1–6 July free embryo dispersal period was 372 m3 s−1 (SD = 19.0, CV = 5.0%); the dispersal flow regime was about 1.3 times greater than long-term discharge for these dates (291 m3 s−1, 1942–2018; USGS gage number 06177000).
Water temperature (°C) in the Missouri River during free embryo dispersal (left of vertical line) and growth period of benthic larvae (right of vertical line). Temperature at the time of free embryo release (open circle) and at sampling site 1 (rkm 2723.5; open triangles) were point measurements; other measurements were recorded at hourly intervals using temperature loggers. The most downstream logger (rkm 2538.2) was located downstream from the Yellowstone River confluence, and measurements illustrate increased temperatures resulting from warm inputs from the Yellowstone River
Growth and mortality of settled larvae
Standard and targeted trawling across the eight river bends from 16 July to 11 September 2019 resulted in the collection of 703 larval Scaphirhynchus sp. The SNP and microsatellite analyses verified 83 larvae as pallid sturgeon that were sampled during 16 July–9 September. Parentage analysis confirmed the 83 larvae as survivors from the original 5-dph released free embryos; no larvae representative of the original 1-dph free embryos were collected. Catches of larvae identified that the transition from dispersing as free embryos to settling on benthic habitats as larvae was initiated in bends upstream from the Yellowstone River confluence; the majority of pallid sturgeon larvae (84%) were caught in the upstream bends. Of these, 37% and 47% were collected during standard and targeted trawling efforts, respectively. For the 16% of pallid sturgeon larvae sampled in bends downstream from the Yellowstone River confluence, 10% and 6% were collected during standard and targeted sampling, respectively.
The Gompertz growth model provided a strong fit to the length-at-age data for known-age pallid sturgeon through the initial 75 dph (Fig. 5). As an estimate for length at the end of the initial growing season, the L∞ (159 mm) was greater than the maximum observed length of 112 mm; however, sampling concluded in mid-September, and it is likely that additional growth continued beyond our sampling. Mean daily water temperature was 18.8 °C (SD = 1.8 °C, CV = 9.6, N = 68 days) from 4 July (~ initiation of settlement) to 9 September in bends upstream from the Yellowstone River confluence where most larvae were captured. Discharge during the same timeframe averaged 436 m3 s−1 (SD = 27.0, CV = 6%). Warmer temperatures (mean daily = 21.0 °C, SD = 2.1 °C, CV = 10.1%; Fig. 4) and greater discharges (mean = 935 m3 s−1, SD = 313, CV = 34.0%) during the same period occurred in bends downstream from the Yellowstone River confluence.
Gompertz growth model for pallid sturgeon through the initial 75 days post-hatch (dph). Values in parentheses are 95% CI for the parameter estimates. The model R2 and P-value are approximate based on nonlinear model fitting. Data for points ≤ 10 dph were predicted from models in Fig. 3 and added to the Gompertz model to enhance curve-fit at the youngest ages. The growth model also contains one additional fish caught on 9 September 2019 (112 mm, 75 dph) as part of a separate project; this one fish was also genetically verified as a survivor from the original 5-dph free embryo release group
The CPUA from standard trawls was highly variable among the eight replicate river bends; nonetheless, CPUA exhibited a declining trend with increasing age of pallid sturgeon larvae (Fig. 6). Excluding CPUA = 0 that occurred 26–28 August (mean age = 62 dph), the catch-curve model quantified a significant slope (Fig. 6) to estimate daily S = 0.96 for settled larvae during 22–75 dph (16 July–9 September).
Mean trawl catch-per-unit-area (CPUA; larvae m.−2 ± 1SE) by age (days post-hatch; dph) for settled pallid sturgeon larvae (top panel) and the catch-curve model estimating instantaneous mortality (Z, ± 95% CI in parentheses; bottom panel). Note that the zero CPUA observed at 62 dph (top panel) was excluded from the catch-curve model (bottom panel)
Estimates of total survival from hatch through the first growing season
Survival rates from the dispersing free embryos and settled larvae were used to estimate total survival from hatch to 75 dph. The initially elevated Z and reduced daily S quantified for both age groups shortly after release were excluded under the assumption that rates determined after river acclimation more accurately quantified free embryo survival during dispersal. Survival from hatch through the 9-dph dispersal life stage was estimated as 0.0437 (Table 3). Incorporating free embryo dispersal survival and survival of benthic larvae from 9 to 75 dph (0.0714), estimated total survival from hatch to 75 dph was estimated as 0.0031 (Table 3).
Discussion
The release of nearly one million known-age pallid sturgeon free embryos into the Missouri River, followed by captures during ontogeny, enabled us to estimate early life stage growth and mortality in a quasi-natural river setting. Although hydrologically and thermally altered, the Missouri River downstream from Fort Peck Dam retains physical habitat heterogeneity (e.g., bars, islands, side channels) remnant of natural conditions. Thus, the dispersing free embryos and settled larvae were exposed to a range of hydraulic attributes (depth, velocity, turbulence, substrate) and experienced diel water temperature fluctuations, food patchiness, and a diverse predator assemblage—multiple factors that affect growth and mortality in early life stages.
The importance of early life stage growth and mortality in fish recruitment processes is well-established (Werner and Gilliam 1984; Houde 1987). For pallid sturgeon, the significance of early life stage growth applies not only to controls on natural recruitment processes but also to anthropogenic factors that disrupt life-stage processes and contribute to recruitment failure in thermally altered and reservoir-fragmented river systems. Pallid sturgeon enter the flow field immediately after hatch (Kynard et al. 2007; DeLonay et al. 2016a, b), disperse for several days, then initiate the transition to benthic habitats as larvae when length is about 18 mm (Braaten et al. 2008, 2012b; Mrnak et al. 2020). Ontogenetic development during the endogenous-feeding dispersal period is thermally regulated, and although all aspects of the free embryo to larval transition process are not known, free embryo dispersal may persist for 5 days (23.3 °C) to 13 days (16.0 °C) prior to larval settlement (Braaten et al. 2012b; Mrnak et al. 2020). Reduced water temperatures from hypolimnetic releases slow growth and ontogenetic development compared to natural temperature regimes (Song et al. 2018), extend dispersal duration (Raventós et al. 2021), and thereby necessitate a longer extent of free-flowing river to transition to the benthic life stage (Braaten et al. 2012b; Mrnak et al. 2020). Reservoirs such as Lake Sakakawea in the current study area and other reservoirs on the Missouri River system impose additive recruitment stressors; these slow-moving or lentic systems truncate dispersal distance prior to completion of ontogenetic development, and reservoir survival is likely poor (Kynard et al. 2007). Thus, reservoirs may be recruitment sinks for riverine-obligate early life stages of pallid sturgeon (Guy et al. 2015). Following Houde’s (1987) assertion, a deviation in ecosystem properties from the norm could have catastrophic effects on recruitment. In the case for pallid sturgeon, cold hypolimnetic releases combined with regulated flows and fragmentation could be viewed as ecosystem deviations that have contributed to recruitment failure for decades (Braaten et al. 2015). Restoration of pallid sturgeon stocks in thermally altered and fragmented river segments focuses on increasing dispersal-stage survival by increasing water temperature, thereby increasing growth and the rate of ontogenetic development (Fischenich et al. 2018a, b). If these efforts are successful, free embryos would transition from water column dispersal to benthic settling in riverine habitat prior to reaching reservoirs.
Both age groups of released free embryos had similar growth rates during dispersal (0.74–0.75 mm day−1) under the cool (mean = 16.8 °C) and diel-fluctuating (mean daily range = 1.2 °C) temperature regimes during this study. These results indicate a common growth response to the thermal regime during the endogenous-feeding period despite differences in initial length at release. Higher growth rates have been observed under warmer, stable thermal regimes in laboratory studies where pallid sturgeon growth from hatch through 9 dph was 1.05 mm day−1 (18.7 °C), 1.17 mm day−1 (20.4 °C), and 2.2 mm day−1 (23.3 °C, Mrnak et al. 2020). Dispersing pallid sturgeon in the Mrnak et al. (2020) study also expressed a growth rate of 0.97 mm day−1 at 16.3 °C that exceeded growth rate observed under the warmer but diel-fluctuating thermal regime in the present study. Growth differences between studies may reflect different parental-source genotypes of the free embryos or growth variation expressed under constant versus diel-fluctuating water temperature regimes (Dammerman et al. 2016). In addition, energetic demands associated with active dispersal in high-velocity environments may shunt energy from growth and partially account for differences in growth between high-velocity field and lower-velocity laboratory investigations. Whereas the 1- and 5-dph free embryos expressed a common growth rate, the growth model based on the original 5-dph free embryos should not be used for length at age comparisons to other studies or for predicting length at age in modeling studies. The 5-dph free embryos were moderately large at release (14.0 mm), contributing to a length at hatch estimate (10.6 mm, model intercept) that is larger than typical lengths at hatch (7–9 mm, Snyder 2002). Thus, use of the 5-dph model would overestimate length for each age. In comparison, the 1-dph model more accurately predicted length at hatch (8.3 mm) to improve length at age predictions under the 16.8 °C temperature regime. Growth models presented in Mrnak et al. (2020) would similarly be useful for estimating length at age for dispersing free embryos through a broader range of temperatures, but estimates would not include the effects of diel temperature fluctuations or high velocities that occur in natural systems.
Estimations of cohort mortality and survival rates rely on the assumptions that (1) the samples are representative of the entire population, (2) the cohorts can be identified, and (3) the population is not subject to migration or differential gear selection with age (i.e., constant catchability; Campana and Jones 1992). If catchability changes, mortality must be estimated separately for different life stages (Campana and Jones 1992). The extended sampling regime for dispersing free embryos quantified pre-arrival, dispersion, and post-passage of the dispersing populations at sites 1–3 to satisfy the first assumption. Although sampling at site 4 quantified much of the free embryo dispersion, estimates of Z and daily S based on catches from this site are less certain owing that the full range of dispersion (primarily concentrations for initial arrivals) was not quantified. The study also included a broad (64 km) and extended (9 week) beam trawl regime to sample for the population of settled larvae in multiple areas of the river. The genetic analysis and supporting length and morphometric criteria facilitated recognition of the 1- and 5-dph cohorts towards satisfying the second assumption. For the third assumption, there was little evidence to indicate changes in frame-net catchability or gear selection for the 1- and 5-dph free embryos during dispersal through site 5 (1 dph) and site 4 (5 dph). This conclusion is based on the finding that free embryo catches at these sites maintained a pattern of pre-arrival, passage, and post-passage consistent with results expected for a mass release of free embryos dispersing through time and space. In addition, growth plots did not lack large fish that would be indicative of gear avoidance. Moreover, although dispersing free embryos are active swimmers, they would have little ability to avoid nets in elevated velocities of the Missouri River. For example, Kynard et al. (2007) observed that pallid sturgeon free embryos exposed to 0.30 m s−1 velocity treatments at 16.0–18.0 °C were unable to hold position through 10 dph, and dispersal was controlled by water velocity. Water velocity from bottom-net samples in the present study exceeded rates tested in the Kynard et al. study to suggest that both age groups of pallid sturgeon free embryos had extremely limited to no net-avoidance capabilities. Under these considerations, the assumption of constant catchability in frame nets is supported for the dispersing 1-dph and 5-dph free embryos. The assumption of constant catchability is uncertain for the beam trawl that targeted the settled benthic larvae. This gear captured a broad size (age) range of settled larvae in this study and earlier investigations of pallid sturgeon (Braaten et al. 2012a) and shovelnose sturgeon (Braaten and Fuller 2007); however, potential size-related gear selection for the beam trawl has not been evaluated. If older and larger larvae were less susceptible to capture in the beam trawl, then mortality rate would have been overestimated (Leak and Houde 1987) and larval survival greater than estimated in the current study (daily S = 0.96).
Pallid sturgeon free embryos experienced initially elevated, then reduced mortality rates as dispersal progressed through time and space. The initial 44-50% decline in the estimated number of free embryos between sites 1 and 2 most likely resulted from mortality associated with transitioning from quiescent hatchery conditions to high-velocity river channel conditions, but other factors are also possible. Although the mechanism for the suspected short-term decline in concentrations is unknown, both age groups experienced elevated short-term losses to suggest the lack of an age-related (or size-related) factor. Earlier experiments involving the release of pallid sturgeon free embryos into the mainstem Missouri River also identified elevated (80–90%) short-term reductions in free embryo concentrations (Braaten et al. 2012b) most likely associated with river acclimation processes. On this basis, mortality and survival estimated following the initially elevated losses of free embryos likely quantify natural processes more accurately as these free embryos survived the hatchery-river transition process.
Survival during free embryo dispersal was slightly less for the younger (1.5–4.0 dph, daily S = 0.64) than older (5.5–7.4 dph, daily S = 0.80) pallid sturgeon. Dispersal-stage mortality is typically greater in smaller individuals and younger life stages than older life stages (Dahlberg 1979; Houde 1987; Perez and Munch 2010). These results suggest that survival modeling during the pallid sturgeon dispersal stage should include different rates corresponding to the younger and older free embryos. Additionally, survival results from the field contrasted with those from the hatchery where daily S was higher (0.92–0.95) for both age groups. Thus, survival rates estimated in controlled settings should be viewed cautiously when considered population model inputs, as survival may be overestimated. Inferences on survival and mortality from this study are restricted to the cool temperature regime and free embryo growth rates that occurred during this study. Pallid sturgeon in the Upper Missouri River basin spawn at 18.0–22.0 °C (DeLonay et al. 2014, 2016a, b, c), and free embryo dispersal initiates and continues at warmer water temperatures than observed in this study. Different temperatures and corresponding growth rates can affect survival and have a large impact on recruitment (Houde 1987; Rice et al. 1993).
Water temperature is the primary factor driving the life stage transition from dispersing as free embryos to settling as benthic larvae (Raventós et al. 2021). For pallid sturgeon, the behavioral transition from water column dispersal to benthic settlement is initiated when free embryos reach about 18.0 mm, but the settling transition may persist through about 20.0 mm (Braaten et al. 2008, 2012b; Mrnak et al. 2020). Early investigations suggested the transition from dispersal to settlement was initiated at 10–11 dph following thermal exposure of about 200 CTU (Kynard et al. 2007; Braaten et al. 2012b). Shortcomings of a constant CTU (or the near-equivalent day-degree summation) noted by Kamler (2002) have recently been documented in pallid sturgeon, in that CTU exposure as a prerequisite to settling is not constant but differs across temperatures. Specifically, settlement of pallid sturgeon in laboratory settings may occur after about 5 days of dispersal at 23.3 °C (112 CTU exposure) to about 9 days of dispersal at 18.7 °C (161 CTU; Mrnak et al. 2020). Free embryos retained in the hatchery as part of this study transitioned from dispersal to rearing-tank settling at 7–10 dph for the 1-dph age group (128–182 CTU exposure at 18.2–18.3 °C) and at 7–11 dph for the 5-dph age group (144–199 CTU exposure at 18.0–18.1 °C). Settlement processes verified in the river setting were similar to laboratory, and hatchery verifications as oldest individuals in the 5-dph age group initiated settlement at 9–10 dph following exposure to 158–175 CTU (pre-release 108 CTU plus 50–67 CTU post-release exposure). In contrast, the original 1-dph age group incurred only 69–86 CTU inclusive of rearing and the initial 3–4 days of dispersal and were only 11.0–12.0 mm. Warmer water downstream from the Yellowstone River confluence (~ 20.0 °C) likely enhanced growth over the next few days of dispersal, but settlement for this age group was not confirmed based on trawl catches.
Following the transition to settled benthic larvae, segments of the Missouri River upstream and downstream from the Yellowstone River confluence provided habitat conditions and food resources supporting the initiation of exogenous feeding and continued growth of larval pallid sturgeon through their first growing season. Lengths of pallid sturgeon estimated from the Gompertz model at 25 dph (34.7 mm) and 75 dph (107.8 mm) were slightly less than previously quantified for pallid sturgeon in the same segments of the Missouri River (25 dph = 44.1 mm, 75 dph = 116.3 mm; Braaten et al. 2012a); however, the growth rate between age increments was nearly identical between studies (1.4–1.5 mm day−1) indicating that the empirical and modeled growth are representative of typical pallid sturgeon growth across years in this portion of the Missouri River basin. In both studies, however, growth information was based on surviving larvae that had been sourced from hatchery-produced free embryos released in the river. No wild-produced larvae have been captured for growth comparisons in this portion of the species range despite verified natural spawning and production of free embryos (DeLonay et al. 2016a, b).
Although larval growth was evident, the extent to which growth rates compare to pre-regulation timeframes is uncertain. Based on bioenergetic modeling, growth potential in young pallid sturgeon is greatest at about 24.0 °C (Heironimus 2014). Most data for the Gompertz model were based on larval pallid sturgeon that used habitat and food resources in the cool river segment (mean = 18.8 °C from settlement to near the end of the growing season) upstream from the warmer inputs of the Yellowstone River. Maximum water temperature in this segment of the river attained 22.6 °C during a few hourly measurements on 15 July, and temperatures greater than 22.0 °C occurred during a few hourly measurements on 14–16 July and 3–4 August (Fig. 5). The remaining larvae used in the Gompertz model represented a combination of fish, including some individuals that initially settled in the cooler river upstream from the Yellowstone River confluence then dispersed downstream, and some individuals that initially settled and remained in the warmer waters (mean = 21.0 °C) downstream from the Yellowstone River confluence. Water temperature in this river segment equaled or exceeded 24.0 °C for only a few days in 2019 (14–17 July, 3–6 August). Thus, optimal growth temperature in the contemporary river may be diminished relative to pre-regulation times owing to the suppressed thermal regimes throughout summer. Rapid growth and larger size during the initial year of life may reduce predation on young sturgeon (Crossman et al. 2018) and reduce the likelihood of size-dependent overwinter mortality. Overwinter mortality has not been evaluated in pallid sturgeon but has been reported at 40–60% in lake sturgeon (Crossman et al. 2009; Deslauriers et al. 2018). The relationship between first growth and overwinter mortality was not evaluated in this study; however, our netting surveys within the study area during summer 2020 yielded 11 yearling pallid sturgeon that were genetically identified as survivors from the original 5-dph age group (J. Kalie, U.S. Fish and Wildlife Service, Conservation Genetics Laboratory, email communications on 30 September 2020 and 15 December 2020). Thus, accrued growth and energy reserves through the 2019 growing season (likely > 112 mm attained beyond 9 September) contributed to survival for at least some pallid sturgeon larvae through winter.
The daily survival rate of 22–75 dph benthic pallid sturgeon larvae estimated in this study (daily S = 0.96) tends to be greater than rates for similar life stages in other sturgeons; however, direct comparisons are difficult as other studies did not utilize known-age fish. For example, based on catch-curves, shovelnose sturgeon larvae through an estimated 85 dph had instantaneous mortality rates varying from − 0.64 to − 0.25 (daily S = 0.527–0.779) over a 4-year study in the Mississippi River (Phelps et al. 2010). Comparisons to other studies are based on stage-duration estimates as daily survival was not reported. For example, in a 2-year study of lake sturgeon in the Peshtigo River, Wisconsin, Caroffino et al. (2010) estimated mortality rates of 0.9046–0.9826 (survival = 0.0174–0.0954) between the dispersive larval life stage and the age-0 life stage (early August). For stellate sturgeon Acipenser stellatus in the Volga River, larval survival averaged 0.025 from about 5 to 60 dph over a 12-year period with maximum survival exceeding 0.080 in one year (Usova 2009). The instantaneous mortality rate of pallid sturgeon larvae in the present study (Z = − 0.04) expanded to the 53-day benthic period between 22 and 75 dph (approximating the larval duration of Caroffino et al. 2010 and Usova 2009) estimated a larval stage interval survival rate of 0.12. Annual variations in pallid sturgeon survival rates would be expected under different environmental conditions, but this hypothesis could not be addressed in our single-year study. In addition, survival rates may differ among sturgeon species due to species-specific larval stage behaviors, as well as to differences in growth, predation intensity, food resources, and habitat that occur among rivers. Although survival and mortality rates of settled pallid sturgeon larvae were quantified for much of the growing season, our study lacked quantification of these vital rates between initial settlement (~ 9–10 dph) and 22 dph. The transition from dispersing to settling and associated initiation of exogenous feeding can be a period of elevated mortality in sturgeons (Szczepkowski et al. 2000; Hardy and Litvak 2004; Boucher et al. 2014; Mrnak et al. 2020).
Population modeling in sturgeons commonly compartmentalizes all mortality occurring during the initial year of life into a single egg to age-1 survival metric. For example, across several sturgeon species, a broad range of estimated egg to age-1 survival rates has been used as model inputs (e.g., 0.000000756–0.002, Pine et al. 2001; Gross et al. 2002; Jager et al. 2002; Bajer and Wildhaber 2007; Heppell 2007; Doukakis et al. 2010; Jarić and Gessner 2013; Steffensen et al. 2013; Jarić et al. 2015; Wildhaber et al. 2017; Blackburn et al. 2019). Specific to pallid sturgeon, an egg to age-1 survival rate of 0.00011 has been used in population modeling (Steffensen et al. 2013; Wildhaber et al. 2017). Data from the present study provided a survival estimate of 0.0031 for pallid sturgeon from hatch to near completion of the first growing season. Survival to hatch for incubating pallid sturgeon embryos in the wild is not known, but if estimates from lake sturgeon (~ 0.10; Forsythe et al. 2013) are combined with dispersal and settlement survival in the present study, pallid sturgeon survival from egg to the end of the first growing season would be estimated as 0.00031. Additional information for incubation success and overwinter survival in pallid sturgeon is needed to fully quantify the egg to age-1 survival metric and test existing population model estimates. In other work, Caroffino et al. (2010) estimated that the total egg to age-0 survival rate was 0.00016–0.00075 for lake sturgeon in a 2-year study. Usova (2009) estimated a mean survival rate of 0.0075 (range 0.001–0.0256 over 12-year study) for the egg to age-0 stage (50–60 dph) in stellate sturgeon.
Obtaining vital rate estimates for pallid sturgeon early life stages under natural conditions is exceptionally challenging. The long-distance dispersal exhibited by free embryos requires multi-day longitudinal assessments throughout the dispersal reach to quantify changes in free embryo concentrations and estimate mortality during dispersal. Furthermore, naturally produced pallid sturgeon free embryos are extremely rare (Eichelberger et al. 2014), thus preventing estimation of growth and survival in wild-produced fish. The use of nearly 1.0 million hatchery-produced pallid sturgeon free embryos combined with multi-day assessments provided fundamental knowledge of early life stage dynamics. In addition, results extend to parameterization of pallid sturgeon population models that are used to evaluate restoration alternatives in the upper Missouri River basin (Fischenich et al. 2018a, b). Multiple years of conducting similar large-scale free embryo release studies would be required to discern variations in growth and survival under different environmental conditions. In this context, free embryo growth and survival rates estimated in this 1-year study likely represent minimum or near-minimum values that would occur in the upper Missouri River basin owing to the cool-water conditions under which the study was conducted.
Data availability
Data sets for this project are available in Braaten and Holley (2021) through the U.S. Geological Survey ScienceBase.gov. https://doi.org/10.5066/P9N2MFV8.
Code availability
Statistical package and programs are cited in the manuscript.
References
Auer NA, Baker EA (2002) Duration and drift of larval lake sturgeon in the Sturgeon River, Michigan. J Appl Ichthyol 18:557–564. https://doi.org/10.1046/j.1439-0426.2002.00393.x
Auer NA, Baker EA (2020) New insights into larval lake sturgeon daytime drift dynamics. J Great Lakes Res 46:339–346. https://doi.org/10.1016/j.jglr.2019.12.010
Bailey RM, Cross FB (1954) River sturgeons of the American genus Scaphirhynchus: characters, distribution, and synonymy. Pap Mich Acad Sci Arts Lett 39:169–207 (http://www.nativefishlab.net/library/textpdf/17253.pdf) Accessed 8 Aug 2022
Bailey KM, Canino MF, Napp JM, Spring SM, Brown AL (1995) Contrasting years of prey levels, feeding conditions and mortality of walleye pollock Theragra chalcogramma in the western Gulf of Alaska. Mar Ecol Prog Ser 119:11–23 (https://www.int-res.com/abstracts/meps/v119/p11-23/#:~:text=doi%3A10.3354/meps119011) Accessed 8 Aug 2022
Bajer P, Wildhaber M (2007) Population viability analysis of Lower Missouri River shovelnose sturgeon with initial application to the pallid sturgeon. J Appl Ichthyol 23:457–464. https://doi.org/10.1111/j.1439-0426.2007.00879.x
Bayer JM, Counihan TD (2001) Length changes in white sturgeon larvae preserved in ethanol or formaldehyde. Collect Forum 15(1–2):57–64
Beamesderfer RC, Simpson ML, Kopp GJ (2007) Use of life history information in a population model for Sacramento green sturgeon. Environ Biol Fishes 79:315–337. https://doi.org/10.1007/s10641-006-9145-x
Benson A, Sutton T, Elliott R, Meronek T (2006) Biological attributes of age-0 lake sturgeon in the lower Peshtigo River, Wisconsin. J Appl Ichthyol 22:103–108. https://doi.org/10.1111/j.1439-0426.2006.00707.x
Blackburn SE, Gingras ML, DuBois J, Jackson ZJ, Quist MC (2019) Population dynamics and evaluation of management scenarios for white sturgeon in the Sacramento-San Joaquin River Basin. N Am J Fish Manage 39:896–912. https://doi.org/10.1002/nafm.10316
Boucher MA, McAdam SO, Shrimpton JM (2014) The effect of temperature and substrate on the growth, development and survival of larval white sturgeon. Aquaculture 430:139–148. https://doi.org/10.1016/j.aquaculture.2014.03.011
Bowen ZH, Bovee KD, Waddle TJ (2003) Effects of flow regulation on shallow-water habitat dynamics and floodplain connectivity. Trans Am Fish Soc 132:809–823. https://doi.org/10.1577/T02-079
Braaten P, Fuller DB (2007) Growth rates of young-of-year shovelnose sturgeon in the upper Missouri River. J Appl Ichthyol 23:506–515. https://doi.org/10.1111/j.1439-0426.2006.00821.x
Braaten PJ, Fuller DB, Holte LD, Lott RD, Viste W, Brandt TF, Legare RG (2008) Drift dynamics of larval pallid sturgeon and shovelnose sturgeon in a natural side channel of the upper Missouri River, Montana. N Am J Fish Manag 28:808–826. https://doi.org/10.1577/M06-285.1
Braaten PJ, Fuller DB, Lott RD, Ruggles MP, Holm RJ (2010) Spatial distribution of drifting pallid sturgeon larvae in the Missouri River inferred from two net designs and multiple sampling locations. N Am J Fish Manage 30:1062–1074. https://doi.org/10.1577/M09-149.1
Braaten PJ, Fuller DB, Lott RD, Haddix TM, Holte LD, Wilson RH, Bartron ML, Kalie JA, DeHaan PW, Ardren WR, Holm RJ, Jaeger ME (2012a) Natural growth and diet of known-age pallid sturgeon (Scaphirhynchus albus) early life stages in the upper Missouri River basin, Montana and North Dakota. J Appl Ichthyol 28:496–504. https://doi.org/10.1111/j.1439-0426.2012.01964.x
Braaten PJ, Fuller DB, Lott RD, Ruggles MP, Brandt TF, Legare RG, Holm RJ (2012b) An experimental test and models of drift and dispersal processes of pallid sturgeon (Scaphirhynchus albus) free embryos in the Missouri River. Environ Biol Fishes 93:377–392. https://doi.org/10.1007/s10641-011-9925-9
Braaten PJ, Campana SE, Fuller DB, Lott RD, Bruch RM, Jordan GRJ (2015) Age estimations of wild pallid sturgeon (Scaphirhynchus albus, Forbes & Richardson 1905) based on pectoral fin spines, otoliths and bomb radiocarbon: Inferences on recruitment in the dam-fragmented Missouri River. J Appl Ichthyol 31:821–829. https://doi.org/10.1111/jai.12873
Braaten PJ, Holley CT (2021) Pallid sturgeon free embryo drift and dispersal experiment data from the Upper Missouri River, Montana and North Dakota, 2019. U.S. Geological Survey data release. https://doi.org/10.5066/P9N2MFV8
Bruch RM, Haxton TJ, Koenigs R, Welsh A, Kerr SJ (2016) Status of lake sturgeon (Acipenser fulvescens Rafinesque 1817) in North America. J Appl Ichthyol 32:162–190. https://doi.org/10.1111/jai.13240
Campana SE, Jones CM (1992) Analysis of otolith microstructure data. In: Stevenson KD and Campana SE (eds) Otolith microstructure examination and analysis. Can Spec Publ Fish Aquat Sci 117:73–100
Caroffino DC, Sutton TM, Elliott RF, Donofrio MC (2010) Early life stage mortality rates of lake sturgeon in the Peshtigo River, Wisconsin. N Am J Fish Manage 30:295–304. https://doi.org/10.1577/M09-082.1
Crossman JA, Forsythe PS, Baker EA, Scribner KT (2009) Overwinter survival of stocked age-0 lake sturgeon. J Appl Ichthyol 25:516–521. https://doi.org/10.1111/j.1439-0426.2009.01310.x
Crossman JA, Scribner KT, Forsythe PS, Baker EA (2018) Lethal and non-lethal effects of predation by native fish and an invasive crayfish on hatchery-reared age-0 lake sturgeon (Acipenser fulvescens Rafinesque, 1817). J Appl Ichthyol 34:322–330. https://doi.org/10.1111/jai.13558
Dahlberg MD (1979) A review of survival rates of fish eggs and larvae in relation to impact assessments. Mar Fish Rev 41:1–12
Dammerman KJ, Steibel JP, Scribner KT (2016) Increases in the mean and variability of thermal regimes result in differential phenotypic responses among genotypes during early ontogenetic stages of lake sturgeon (Acipenser fulvescens). Evol Appl 9:1258–1270. https://doi.org/10.1111/eva.12409
DeHaan P, Jordan G, Ardren W (2008) Use of genetic tags to identify captive-bred pallid sturgeon (Scaphirhynchus albus) in the wild: Improving abundance estimates for an endangered species. Conserv Genet 9:691–697. https://doi.org/10.1007/s10592-007-9374-3
DeLonay AJ, Jacobson RB, Chojnacki KA, Annis ML, Braaten PJ, Elliott CM, Fuller DB, Haas JD, Haddix TM, Ladd HLA, McElroy BJ, Mestl GE, Papoulias DM, Rhoten JC, Wildhaber ML (2014) Ecological requirements for pallid sturgeon reproduction and recruitment in the Missouri River: annual report 2011. U.S. Geological Survey Open-File Report 2014–1106. https://doi.org/10.3133/ofr20141106
DeLonay AJ, Chojnacki KA, Jacobson RB, Albers JL, Braaten PJ, Bulliner EA, Elliott CM, Erwin SO, Fuller DB, Haas JD (2016a) Ecological requirements for pallid sturgeon reproduction and recruitment in the Missouri River—a synthesis of science, 2005 to 2012. U.S. Geological Survey Scientific Investigations Report 2015–5145. https://doi.org/10.3133/sir20155145
DeLonay AJ, Jacobson RB, Chojnacki KA, Braaten PJ, Buhl KJ, Eder BL, Elliott CM, Erwin SO, Fuller DB, Haddix TM, Ladd HLA, Mestl GE, Papoulias DM, Rhoten JC, Wesolek CJ, Wildhaber ML (2016b) Ecological requirements for pallid sturgeon reproduction and recruitment in the Missouri River: annual report 2013. U.S. Geological Survey Open-File Report 2015–1197. https://doi.org/10.3133/ofr20151197
DeLonay AJ, Chojnacki KA, Jacobson RB, Braaten PJ, Buhl KJ, Elliott CM, Erwin SO, Faulkner JDA, Candrl JS, Fuller DB, Backes KM, Haddix TM, Rugg ML, Wesolek CJ, Eder BL, Mestl GE (2016c) Ecological requirements for pallid sturgeon reproduction and recruitment in the Missouri River: annual report 2014. U.S. Geological Survey Open-File Report 2016c–1013. https://doi.org/10.3133/ofr2016c1013
Deslauriers D, Yoon GR, Earhart ML, Long C, Klassen CN, Anderson WG (2018) Over-wintering physiology of age-0 lake sturgeon (Acipenser fulvescens) and its implications for conservation stocking programs. Environ Biol Fishes 101:623–637. https://doi.org/10.1007/s10641-018-0724-4
Doukakis P, Babcock EA, Pikitch EK, Sharov AR, Baimukhanov M, Erbulekov S, Bokova Y, Nimatov A (2010) Management and recovery options for Ural River beluga sturgeon. Conserv Biol 24:769–777. https://doi.org/10.1111/j.1523-1739.2010.01458.x
Dryer M, Sandvol A (1993) Pallid sturgeon recovery plan. US Fish and Wildlife Service, Bismarck, North Dakota. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1034&context=endangeredspeciesbull. Accessed 8 Aug 2022
Eichelberger JS, Braaten PJ, Fuller DB, Krampe MS, Heist EJ (2014) Novel single-nucleotide polymorphism markers confirm successful spawning of endangered Pallid Sturgeon in the upper Missouri River basin. Trans Am Fish Soc 143:1373–1385. https://doi.org/10.1080/00028487.2014.935479
Erwin SO, Bulliner EA, Fischenich JC, Jacobson RB, Braaten PJ, DeLonay AJ (2018) Evaluating flow management as a strategy to recover an endangered sturgeon species in the Upper Missouri River, USA. River Res Appl 34:1254–1266. https://doi.org/10.1002/rra.3371
Fischenich JC, Buenau KE, Bonneau JL, Fleming CA, Marmorek DR, Nelitz MA, Pickard D, Ma B, Gemeinhardt TR (2018a) Science and adaptive management plan appendices and attachments. Missouri River Recovery Program, U.S. Army Corps of Engineers. https://usace.contentdm.oclc.org/utils/getfile/collection/p16021coll7/id/8071. Accessed 8 Aug 2022
Fischenich JC, Marmorek DR, Nelitz MA, Murray CL, Ma BO, Buenau KE, Long G, Bonneau JL, Fleming CA, Schwarz CJ (2018b) Science and adaptive management plan. Missouri River Recovery Program, U.S. Army Corps of Engineers. https://usace.contentdm.oclc.org/utils/getfile/collection/p16021coll7/id/8070. Accessed 8 Aug 2022
Forsythe PS, Scribner KT, Crossman JA, Ragavendran A, Baker EA (2013) Experimental assessment of the magnitude and sources of lake sturgeon egg mortality. Trans Am Fish Soc 142:1005–1011. https://doi.org/10.1080/00028487.2013.790847
Frissell CA, Liss WJ, Warren CE, Hurley MD (1986) A hierarchical framework for stream habitat classification: viewing streams in a watershed context. Environ Manage 10:199–214. https://doi.org/10.1007/BF01867358
Galat DL, Robinson JW, Hesse LW (1996) Restoring aquatic resources to the lower Missouri River: issues and initiatives. In: Galat DL, Frazier AG (eds) Science for Floodplain Management into the 21st Century, vol 3. US Government Printing Office, Washington, DC, pp 49–71
Gross MR, Repka J, Robertson CT, Secor DH, Van Winkle W (2002) Sturgeon conservation: insights from elasticity analysis. In: Van Winkle W, Anders P, Secor DH, Dixon D (eds) Biology, management, and protection of North American sturgeon, American Fisheries Society Symposium 28. American Fisheries Society, Bethesda, pp 13–30
Guy CS, Treanor HB, Kappenman KM, Scholl EA, Ilgen JE, Webb MA (2015) Broadening the regulated-river management paradigm: a case study of the forgotten dead zone hindering Pallid Sturgeon recovery. Fisheries 40:6–14. https://doi.org/10.1080/03632415.2014.987236
Hardy RS, Litvak MK (2004) Effects of temperature on the early development, growth, and survival of shortnose sturgeon, Acipenser brevirostrum, and Atlantic sturgeon, Acipenser oxyrhynchus, yolk-sac larvae. Environ Biol Fishes 70:145–154. https://doi.org/10.1023/B:EBFI.0000029345.97187.5b
Heironimus LB (2014) The development and application of a larval Pallid Sturgeon (Scaphirhynchus albus) bioenergetics model. Thesis, South Dakota State University. https://openprairie.sdstate.edu/etd/463. Accessed 8 Aug 2022
Heppell SS (2007) Elasticity analysis of green sturgeon life history. Environ Biol Fishes 79:357–368. https://doi.org/10.1007/s10641-006-9052-1
Houde ED (1987) Fish early life dynamics and recruitment variability. In: Hoyt RD (ed) 10th Annual larval fish conference. American Fisheries Society Symposium 2, Bethesda, pp 17–29
Jager HI, Van Winkle W, Chandler JA, Lepla KB, Bates P, Counihan TD (2002) A simulation study of factors controlling white sturgeon recruitment in the Snake River. In: Van Winkle W, Anders P, Secor DH, Dixon D (eds) Biology, management, and protection of North American sturgeon, American Fisheries Society Symposium 28. American Fisheries Society, Bethesda, pp 127–150
Jarić I, Gessner J (2013) A life-stage population model of the European sturgeon (Acipenser sturio) in the Elbe River. Part I: General model outline and potential applications. J Appl Ichthyol 29:483–493. https://doi.org/10.1111/jai.12419
Jarić I, Gessner J, Acolas ML, Lambert P, Rochard E (2014) Modelling attempts utilized in sturgeon research: a review of the state-of-the art. J Appl Ichthyol 30:1379–1386. https://doi.org/10.1111/jai.12572
Jarić I, Gessner J, Lenhardt M (2015) A life-table metamodel to support the management of data deficient species, exemplified in sturgeons and shads. Environ Biol Fishes 98:2337–2352. https://doi.org/10.1007/s10641-015-0439-8
Jarić I, Riepe C, Gessner J (2018) Sturgeon and paddlefish life history and management: Experts’ knowledge and beliefs. J Appl Ichthyol 34:244–257. https://doi.org/10.1111/jai.13563
Jordan GR, Heist EJ, Braaten PJ, DeLonay AJ, Hartfield P, Herzog DP, Kappenman KM, Webb MAH (2016) Status of knowledge of the pallid sturgeon (Scaphirhynchus albus Forbes and Richardson, 1905). J Appl Ichthyol 32(Suppl S1):191–207. https://doi.org/10.1111/jai.13239
Kamler E (2002) Ontogeny of yolk-feeding fish: an ecological perspective. Rev Fish Biol Fish 12:79–103. https://doi.org/10.1023/A:1022603204337
Kynard B, Henyey E, Horgan M (2002) Ontogenetic behavior, migration, and social behavior of pallid sturgeon, Scaphirhynchus albus, and shovelnose sturgeon, S. platorynchus, with notes on the adaptive significance of body color. Environ Biol Fishes 63:389–403. https://doi.org/10.1023/A:1014950202783
Kynard B, Parker E, Pugh D, Parker T (2007) Use of laboratory studies to develop a dispersal model for Missouri River pallid sturgeon early life intervals. J Appl Ichthyol 23:365–374. https://doi.org/10.1111/j.1439-0426.2007.00908.x
Leak JC, Houde ED (1987) Cohort growth and survival of bay anchovy Anchoa mitchilli larvae in the Biscayne Bay, Florida. Mar Ecol Prog Ser 37:109–122 (https://www.jstor.org/stable/24824685) Accessed 8 Aug 2022
McQuown EC, Sloss BL, Sheehan RJ, Rodzen J, Tranah GJ, May B (2000) Microsatellite analysis of genetic variation in sturgeon: new primer sequences for Scaphirhynchus and Acipenser. Trans Am Fish Soc 129:1380–1388. https://doi.org/10.1577/1548-8659(2000)129%3c1380:MAOGVI%3e2.0.CO;2
Miranda LE, Bettoli PW (2007) Mortality. In: Guy CS, Brown ML (eds) Analysis and interpretation of freshwater fisheries data. American Fisheries Society, Bethesda, pp 229–277
Mrnak JT, Heironimus LB, James DA, Chipps SR (2020) Effect of water velocity and temperature on energy use, behaviour and mortality of pallid sturgeon Scaphirhynchus albus larvae. J Fish Biol 97:1690–1700. https://doi.org/10.1111/jfb.14532
Perez KO, Munch SB (2010) Extreme selection on size in the early lives of fish. Evol 64:2450–2457. https://doi.org/10.1111/j.1558-5646.2010.00994.x
Phelps QE, Tripp SJ, Hintz WD, Garvey JE, Herzog DP, Ostendorf DE, Ridings JW, Crites JW, Hrabik RA (2010) Water temperature and river stage influence mortality and abundance of naturally occurring Mississippi River Scaphirhynchus sturgeon. N Am J Fish Manage 30:767–775. https://doi.org/10.1577/M09-176.1
Pikitch EK, Doukakis P, Lauck L, Chakrabarty P, Erickson DL (2005) Status, trends and management of sturgeon and paddlefish fisheries. Fish Fish 6:233–265. https://doi.org/10.1111/j.1467-2979.2005.00190.x
Pine WE III, Allen MS, Dreitz VJ (2001) Population viability of the Gulf of Mexico sturgeon: inferences from capture–recapture and age-structured models. Trans Am Fish Soc 130:1164–1174. https://doi.org/10.1577/1548-8659(2001)130%3c1164:PVOTGO%3e2.0.CO;2
Pollock MS, Carr M, Kreitals NM, Phillips ID (2015) Review of a species in peril: what we do not know about lake sturgeon may kill them. Environ Rev 23:30–43. https://doi.org/10.1139/er-2014-0037
Raventós N, Torrado H, Arthur R, Alcoverro T, Macpherson E (2021) Temperature reduces fish dispersal as larvae grow faster to their settlement size. J Anim Ecol 90:1419–1432. https://doi.org/10.1111/1365-2656.13435.10.1111/1365-2656.13435
Rice JA, Miller TJ, Rose KA, Crowder LB, Marschall EA, Trebitz AS, DeAngelis DL (1993) Growth rate variation and larval survival: inferences from an individual-based size-dependent predation model. Can J Fish Aquat Sci 50:133–142. https://doi.org/10.1139/f93-015
SAS (2019) SAS/STAT user’s guide, release 15.2. SAS Institue Inc, Cary
Secor DH, Anders PJ, Van Winkle W, Dixon DA (2002) Can we study sturgeons to extinction? What we do and don’t know about the conservation of North American sturgeons. In: Van Winkle W, Anders P, Secor DH, Dixon D (eds) Biology, management, and protection of North American sturgeon, American Fisheries Society Symposium 28. American Fisheries Society, Bethesda, pp 3–10
Shields FD Jr, Simon A, Steffen LJ (2000) Reservoir effects on downstream river channel migration. Environ Conserv 27:54–66. https://doi.org/10.1017/S0376892900000072
Snyder DE (2002) Pallid and shovelnose sturgeon larvae–morphological description and identification. J Appl Ichthyol 18:240–265. https://doi.org/10.1046/j.1439-0426.2002.00429.x
Song Y, Cheng F, Murphy BR, Xie S (2018) Downstream effects of the Three Gorges Dam on larval dispersal, spatial distribution, and growth of the four major Chinese carps call for reprioritizing conservation measures. Can J Fish Aquat Sci 75:141–151. https://doi.org/10.1139/cjfas-2016-0278
Steffensen KD, Pegg MA, Mestl G (2013) Population prediction and viability model for pallid sturgeon (Scaphirhynchus albus, Forbes and Richardson, 1905) in the lower Missouri River. J Appl Ichthyol 29:984–989. https://doi.org/10.1111/jai.12277
Szczepkowski M, Kolman R, Szczepkowska B (2000) Postembryonic development, survival and growth rate of Siberian sturgeon (Acipenser baeri Brandt) larvae. Arch Pol Fish 8:193–204
U.S. Fish and Wildlife Service (2014) Revised recovery plan for the pallid sturgeon (Scaphirhynchus albus). US Fish and Wildlife Service, Denver, p 115. https://ecos.fws.gov/docs/recovery_plan/Pallid%20Sturgeon%20Recovery%20Plan%20First%20Revision%20signed%20version%20012914_3.pdf. Accessed 8 Aug 2022
Usova T (2009) Survival of naturally spawned stellate sturgeon fry during downstream migration in the Volga river. Russ J Ecol 40:374–376. https://doi.org/10.1134/S1067413609050117
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425. https://doi.org/10.1146/annurev.es.15.110184.002141
Wildhaber ML, Albers JL, Green NS, Moran EH (2017) A fully-stochasticized, age-structured population model for population viability analysis of fish: Lower Missouri River endangered pallid sturgeon example. Ecol Model 359:434–448. https://doi.org/10.1016/j.ecolmodel.2015.07.019
Acknowledgements
The authors extend appreciation to individuals who provided field, laboratory, and logistical support including personnel from Montana Fish, Wildlife and Parks (A. Annear, R. Benson, D. Fuller, A. Gaffney, J. Hunziker, J. Janecek, B. Marotz, J. Pesik, M. Rugg, D. Schott, Z. Shattuck, K. Stagmiller), U.S. Bureau of Reclamation (E. Best, T. Gust), Southern Illinois University (R. Flamio, N. Smith), U.S. Army Corps of Engineers (J. Bonneau, N. Herriage, J. Hintz, D. Latka, B. McPhee, A. Shorter), U.S. Fish and Wildlife Service (M. Bartron, S. Cole, C. Hooley, K. Huber, S. Hultberg, D. James, J. Kalie, L. Pierce, D. Turner, W. Nelson-Stastny, L. Romance), and the U.S. Geological Survey (B. Anderson, E. Bulliner, R. Burlbaw, B. Call, D. Combs, S. Davenport, S. Erwin, D. Flesch, C. Green, T. Helmuth, C. Hickcox, Kathryn Kelly, Killian Kelly, C. Slaugh, J. Staggs). Reviews from S. Chipps and three anonymous reviewers on earlier versions of the manuscript contributed to a much-improved final version. The study was funded by the U.S. Army Corps of Engineers Missouri River Integrated Science Program, U.S. Department of Energy Western Area Power Administration, and U.S. Bureau of Reclamation and supported through the U.S. Geological Survey Ecosystems Mission Area. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Funding
Support for the study was provided by the U.S. Army Corps of Engineers Missouri River Integrated Science Program, U.S. Department of Energy Western Area Power Administration, and the U.S. Bureau of Reclamation.
Author information
Authors and Affiliations
Contributions
All authors provided significant expertise and contributions to warrant authorship including study conception (PJB), study design (PJB, RJH, JP, TMH, AJD, RBJ), hatchery production and fish handling (RJH, JP, RHW), field crew leads and data collection (PJB, CTH, AJD, RBJ, TMH, RHW), genetic processing and analyses (EJH, ACB), laboratory fish processing (PJB, CTH, AJD), and data analysis (PJB, CTH). PJB led the writing of the manuscript; co-authors contributed to drafts and approved the final for publication.
Corresponding author
Ethics declarations
Ethics approval
Pallid sturgeon broodstock and spawning followed Biological procedures and protocols for researchers and managers handling pallid sturgeon (USFWS 2012). Handling of free embryos and larvae collected in the field followed the Columbia Environmental Research Center (CERC), Institutional Animal Care and Use Committee (IACUC) Policy (Issuance Number 1401), and IACUC-approved study plan (CERC IACUC Number 19–011).
Consent to participate
Not applicable.
Consent for publication
All authors agree with content of the manuscript, agree to submission of the manuscript for publication, and have permission from their respective agencies to submit the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Braaten, P.J., Holm, R.J., Powell, J. et al. Growth and survival rates of dispersing free embryos and settled larvae of pallid sturgeon (Scaphirhynchus albus) in the Missouri River, Montana and North Dakota. Environ Biol Fish 105, 993–1014 (2022). https://doi.org/10.1007/s10641-022-01294-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01294-w