Abstract
This research aimed to explore the variability in the distribution of muscle, perivisceral, and retroperitoneal fat in Diplodus sargus during the pre-spawning period (the period of maximum accumulation of fat reserves) and its possible relationship with the abnormally tough specimen syndrome (ATS) affecting this species. Since pollutants accumulate in adipose tissue, trace metal load in the fattest specimens were also analyzed. Muscle fat content and abdominal fat were highly correlated, and larger individuals had more abundant abdominal fat. However, given its great variability (26% of the specimens had no abdominal fat at all), abdominal fat is likely a transitional fat to be quickly transferred to other tissues. The liver appeared to have a minor role as a fat deposit in D. sargus since HSI varied relatively little. None of the fishes without lipidic reserves were ATS individuals; by contrast, the only ATS individual found, a 28 cm male, showed a muscle fat content above the observed average and one of the highest amounts of perivisceral and retroperitoneal fat. Therefore, we conclude that flesh hardness is not directly related to the low lipid reserves. However, this ATS individual showed a very high liver copper concentration, suggesting a potential link between fat content, copper concentration, and ATS syndrome that should be explored in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The white seabream, Diplodus sargus (Linnaeus, 1758), is an omnivorous species of the Family Sparidae, found in the Mediterranean Sea as well as in the eastern Atlantic Ocean, including the archipelagos of Madeira, Cape Verde, and the Canary Islands (Fisher et al. 1987). It is a demersal fish, living in rocky infralittoral and circalittoral habitats at 0–50 m of depth, where it dominates fish assemblages. Individuals are mid-sized with a relatively long lifespan and an omnivorous benthic diet (Sala and Ballesteros 1997). It is the target of artisanal and recreational fisheries and constitutes a valuable resource to the former because of its elevated price in local fish markets.
This species is affected by a strange phenomenon observed mainly in the Mediterranean and occasionally reported in the European Atlantic and Macaronesia (Casadevall et al. 2020). This phenomenon, called “abnormally tough specimens” syndrome (ATS) (Casadevall et al. op. cit.), has been seldom found in other species. ATS is usually detected after cooking (not in fresh fish) when the flesh of affected individuals becomes very chewy and inedible.
The first hypotheses proposed about the drivers of this phenomenon pointed to lipid metabolism disturbances. Several studies (Felline et al. 2012, 2014; Gorbi et al. 2014; Terlizzi et al. 2011) noted that the uptake of lipophilic secondary metabolites from an invasive alga, Caulerpa cylindracea Sonder (Bryopsidales, Chlorophyta), may cause a depletion of energetic reserves in D. sargus. Vitale et al. (2018) identified the alkaloid caulerpin as a causal factor of the metabolic disorders observed in D. sargus. Moreover, Del Coco et al. (2018) observed that diet-borne caulerpin is directly responsible for changes observed in the metabolic profile of fish muscle, including the alteration of lipid metabolism, in particular with a reduction of Omega-3 PUFAs (polyunsaturated fatty acids) content. Nevertheless, the relationship between ATS and the introduction of exotic Caulerpa spp. appears unclear in those sites where ATS was detected before the alga (Casadevall et al. 2020). Indeed, no exotic species of Caulerpa are present in the continental Atlantic European coasts where ATS has been reported, whereas the native C. prolifera is only found in the southwestern region of Portugal and Spain, next to the Mediterranean (Guiry and Guiry 2021). In addition, Merciai et al. (2018) analyzed the diet of D. sargus on the northern Catalan coast (Northeastern Spain), but, although macrophytes were very common, they never found any species of Caulerpa in the stomachs. Also, in a recent study from the Balearic Islands, Santamaría et al. (2021) showed that the contribution of C. cylindracea to the total stomach content of D. sargus was low.
Casadevall et al. (2020) found a correlation between the occurrence of ATS and the proximity of sea fish farms and/or commercial ports and suggested that some kind of pollution may affect species, being the most affected individuals the ones with the highest flesh chewiness. Metals, for instance, are known to trigger oxidative stress in fish, affecting, in turn, their flesh quality (Fernandes et al. 2008; Livingstone 2001; Türkmen et al. 2008). D. sargus is prone to accumulation of mercury and other potentially harmful elements (Casadevall and Rodríguez-Prieto 2017; Merciai et al. 2018; Tramati et al. 2011).
Cases of ATS are usually detected after cooking, but the phenomenon is not related to the cooking method (Casadevall et al. 2020). A similar syndrome was described in cooked saddletail snapper, Lutjanus malabaricus (Bloch and Schneider 1801), and crimson snapper, L. erythropterus Bloch 1790, in Australia (Forrest et al. 2014). Those authors analyzed the saddletail snapper and concluded that fish age accounted for 75.6% of the observed variation in cooked muscle texture.
It is known that Diplodus sargus accumulates lipids in the gonads and muscle during the pre-spawning period, i.e., from November to March (Pérez et al. 2007). Pérez et al. (2007) demonstrated the importance of those lipid types during gonadal maturation, forming an energy reserve and a temporary reservoir for future embryonic development. Moreover, energy deposits as perivisceral fat are described for this and other species of the same family. A perivisceral lipid accumulation and utilization during the production and emission of gametes has been observed for D. sargus from the north of Spain (Martínez Pastor and Villegas Cuadros 1996). The main contribution of perivisceral fat energy was also observed during the pre-spawning period in other sparids such as Pagrus pagrus (Aristizabal 2007) or Sparus aurata (Santinha et al. 1999).
Some recreational fishers have reported that D. sargus is particularly tough during the reproductive period, when the fish invest their fat reserves in the maturation of gonads and eggs (Casadevall et al. 2020). Man-Wai (1985) observed a post-spawn slimming of D. sargus individuals from the Gulf of Lion. As the pre-spawn period corresponds to a greater accumulation of fat and supposing ATS syndrome is driven by the depletion of muscle fat reserves, finding ATS individuals in that period would be a paradox.
Consequently, in this study, we aim to explore the variability in the accumulation of muscle, perivisceral, and retroperitoneal fat in D. sargus during the pre-spawning period and to challenge the hypothesis of a relationship between fat accumulation and ATS syndrome. Moreover, Casadevall et al. (2020) hypothesized a link between ATS and some kind of pollutants; since pollutants accumulate in adipose tissues, trace metal loads in the fattest specimens were analyzed too.
Material and methods
Samples were obtained from professional fishers in the area of Cap de Creus (Gulf of Lion, NW Mediterranean; Fig. 1) from November 2019 to January 2020. The fish were purchased at the fish market in the ports where they were landed, so they were already dead. Only mature individuals were chosen, to analyze how lipid reserves accumulate for reproduction purposes. The total number of individuals analyzed was 42, including 28 mature males, 11 mature females, and 3 juvenile females in the first maturation process (Table 1).
Map showing the area (circle) from where the specimens were sampled from professional fishers (Gulf of Lion, NW Mediterranean). CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=814106
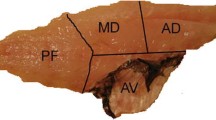
In the laboratory, the total length of fish was measured to the nearest 0.5 cm and the total body weight to the nearest 1 g. Sex and reproductive status were determined by visual inspection of the gonads. Fish were dissected and eviscerated, and the carcass was weighed to the nearest 1 g. Liver and gonads were weighted, and hepatosomatic index (HSI) and gonadosomatic index (GSI) were calculated as HSI = 100 (LW/EW) and GSI = 100(GW/EW), where LW, GW, and EW represent liver, gonad, and somatic (eviscerated) wet weights, respectively. The ratio of liver weight to total body weight is used as a measure of fat (energy) reserves, whereas GSI is a good indicator of reproductive activity. Finally, the deposit of fat in the peritoneal cavity (perivisceral fat) and under the peritoneum (retroperitoneal fat) was also weighted when present, to the nearest 1 mg, as another measure of fat reserve. Hereafter, we define abdominal fat as the sum of perivisceral and retroperitoneal fat.
Determination of muscle total lipid content was carried out from all muscle samples. From each fish, one sample of ca. 5 g of muscle tissue was taken, dorsally, directly under the anterior dorsal fin. Tissue samples were cleaned of scales, skin, and bones and immediately frozen at − 30 °C for total lipid analysis. The total lipid content in muscle was determined following the Soxhlet method described by Shahidi (2003) as a percentage of lipid content in dry mass.
For trace elements, a portion of liver tissue, about 400 mg wet weight, was extracted, frozen stored, and lyophilized to analyze the liver concentration of several elements (Cu, Zn, Se, Cd, Hg, Pb, As). These samples were digested in quartz vessels with HNO3 (4 ml) (Panreac AppliChem, Hiperpur) and H2O2 (1 ml) (Sigma-Aldrich, for trace analysis) at 210 °C in an MW oven (Milestone, Ethos Sel). Digestion was done by raising the temperature to 210 °C from steps 1 to 4 as detailed in Table 2.
Transfer quantitatively all the solution to a weighted plastic container, cleaning the vessel quartz with water (Milli-Q), until a solution of about 15 mL is obtained.
Total concentrations of trace elements were quantified by inductively coupled plasma-mass spectrometry (ICP-MS Agilent 7500c and ICP-OES Agilent 5100). Several analytical blanks were prepared and analyzed, along with samples, to determine the quantification limits. Limits of detection were lower than 5 ng mL−1 for all elements (Table 3). The analytical process was performed at the STR UdG “Serveis tècnics de recerca de la Universitat de Girona.”
Spearman correlations were carried out to explore the relationships between reproduction and condition indices: GSI, HSI, fish length, and weight, muscle and liver fat content, and abdominal fat. A t-test was performed to compare Cu mean values between ATS and non-ATS individuals. Statistical analyses were performed using SPSS 22.
Finally, once tissue samples were collected, the rest of the fish was baked between 15 and 20 minutes, depending on the size, and the meat was tasted to detect possible ATS individuals.
Results
The smallest mature individual (a male) had a total length of 19 cm (Table 1). Juveniles in the first maturation process in our sample were between 20 and 21 cm and had a part of the gonad maturing as ovary, so they would become females.
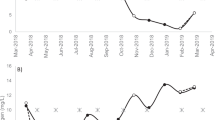
The amount of muscle fat (dry weight) fluctuated widely, being between 1.2 and 14.3% in the pre-spawning period (Fig. 2). The lipid dry weight percentage mean for the period was 4.88 (± 2.4).
The role of the liver appeared to be marginal, in terms of participation as an energy reserve organ, since HSI varied little across months (Fig. 3), especially in males.
The mean value of GSI for females was 0.63 (± 0.27) and 0.25 (± 0.39) for males. Figure 4 shows the changes observed across the 3 months.
Despite the similar sizes of the sampled individuals, abdominal fat deposits had high variability, from 0 to 16.02 g (Table 4). The results obtained after the cooking process allowed us to find a single ATS individual, a male with 28 cm TL, 5.2% of muscle dry fat (above the mean), and 16.02 g of abdominal fat (the second fattest) (Fig. 5). This specimen was captured in December and was in the process of gonadal maturation. Remarkably, all individuals predicted as possible ATS specimens, i.e., with very low or no fat content, were not ATS.
Ten males and one female (26% of the sample) had no abdominal fat at all. Muscle and abdominal fat were strongly correlated (Table 4).
Apart from the relationship size-weight, Spearman correlations show a strong positive relationship between muscle fat content and abdominal fat. There is also a positive correlation between gonadosomatic index and abdominal fat and between gonadosomatic index and eviscerated weight (Table 5).
The results of trace metal analyses are shown in Table 6. Liver copper concentration in two individuals, including the only ATS found, was strikingly higher than in the others. Although there are only two individuals with a very high copper load, the difference between the ATS and the other individuals was statistically significant (t = 3.251; P = 0.009).
Discussion
We have analyzed the amount of fat reserves during the D. sargus pre-spawning period to explore possible relationships between lipid reserves and flesh hardness as an indicator of the ATS syndrome. The values of the GSI, in both males and females (< 1%), demonstrated that all the individuals were in the pre-spawning period because the values of this index in the spawning phase are always close to 10% (Lloret & Planes 2003; Martínez Pastor and Villegas Cuadros 1996).
The expectations of finding an ATS specimen were low because ATS only appears from time to time on the Catalan coast (Casadevall et al. 2020). Among the possible drivers of the ATS phenomenon, Casadevall et al. (2020) excluded the stress produced by the capture system, as well as any mechanism of contagion, since ATS individuals appeared singularly in time in different places. The variation of textural quality of fish muscle mainly depends on the structure of muscle tissue, which is attributed to internal factors related to the structure of contractile proteins, the framework of connective tissue, lipid oxidation, and some external factors, such as methods of sample handling and storage (Aussanasuwannakul et al. 2012). It is also known that retroperitoneal and perivisceral adipose tissues influence carcass and filet composition, whereas muscle fat deposits modulate flesh organoleptic quality (Weil et al. 2013).
From these possible ATS drivers, we must reject sampling and handling, since all individuals were caught and treated the same way. Effects due to other variables (e.g., age and size, food sources, sample heterogeneity, gaping, tissue water, fat and collagen content, and distribution), and their interactions (Cheng et al. 2014), cannot be excluded.
Regarding the total amount of fat in the pre-spawning period, we observed a higher degree of variability of muscle fat content than in previous works, from 1.2 up to 14.3% in dry muscle fat with a mean of 4.88 (± 2.4). Lloret et al. (2005) analyzed D. sargus spawners and post-spawners from the same area and lipids constituted between 0.01 and 2.01% of dry muscle fat in pre-spawners and between 0.29 and 4.41% in post-spawners. The lower values indicate spawning as a cause of the depletion of fat reserves, which are invested in gonads. At the end of the reproductive period (spring), Saoud et al. (2008) found muscle lipid contents of white seabream within 0.35 and 1.85%, and similar results were found by Kouropakis et al. (2019) for wild individuals. Özyurt et al. (2005) observed a mean of 2.37% (± 0.99) in spring, 2.70% (± 0.11) in summer, and 0.97% (± 0.37) in winter. Hornung et al. (1994) reported higher values (between 2.3 and 7.7%) in August, a very post-spawning period. Comparing all those results together with the present ones, it becomes evident that pre-spawn is the period of highest muscle fat accumulation in most D. sargus individuals.
Taşbozan and Gökçe (2017) stated that, although the lipid contents of fish depend on many factors, they are generally split into three categories based on their muscle fat composition: lean (less than 5% fat), mid-fat (5–10% fat), and fatty fish (10–25% fat), whereas the ones below 2% should be considered very lean fish. The white seabream, like other Mediterranean Sparidae, has been considered a mid-fat species (Grigorakis 2017), but, according to our data, only some individuals in the pre-spawning period could be included in this category, as most individuals should be classified as lean.
Abdominal adipose tissues in this period were present in many individuals, sometimes in high amounts, although a few had none. In fact, 26% of the specimens had no abdominal fat at all, but none of these fishes were ATS. Pre-spawning individuals with more abundant reserves of abdominal fat were fatter, as observed by Man-Wai (1985), who reported a slimming of D. sargus after spawning. The use of perivisceral lipid during the production and emission of gametes was also observed in individuals from the north of Spain (Martínez Pastor and Villegas Cuadros 1996). Moreover, a primary contribution of perivisceral fat energy was also observed during the pre-spawning period in other sparids such as Pagrus pagrus (Aristizabal 2007). Nevertheless, retroperitoneal fat was not mentioned in that study. The ATS individual who appeared in our sample, a 28 cm male, showed a load of muscle fat above the observed average and one of the highest amounts of abdominal fat. According to Taşbozan and Gökçe (2017), that individual should be classified as “fatty.”
A strong relationship between muscle fat content and abdominal fat was found in our study. However, given its high variability, abdominal fat probably corresponds to a transitional fat that can be quickly metabolized for reproductive purposes. This is reinforced by the fact that the only other correlation of abdominal fat is with the gonadosomatic index. In fact, PUFAs increase in the muscle of male and female D. sargus during gonadal maturation and decrease during the gonadal recession, which also confirms the importance of muscle as a reserve of essential fatty acids in support of reproductive effort in this species (Pérez et al. 2007). Also, Batičić et al. (2009) found that total unsaturated fatty acids of D. sargus, in all analyzed lipid fractions, were the highest in winter (pre-spawning), while saturated fatty acids were the highest in spring in all lipid fractions. Those authors (Batičić et al. 2009) suggested that saturated fatty acids are used in winter to ensure the additional metabolic energy required in that period and the production of PUFAs required for gonad development and spawning.
It must be concluded that flesh hardness is not directly related to the low content of lipid reserves. Therefore, other variables have to be found as drivers of the ATS syndrome. Forrest et al. (2012) suggested age as a possible driver of that syndrome in saddletail snapper. Indeed, in the case of white seabream, affected individuals appear only from a certain size (Casadevall et al. 2020), which coincides moreover with two things, the size of sexual maturation and also a change in diet (Merciai et al. 2018). As shown by Figueiredo et al. (2005) and Merciai et al. (2018), there is a size-related diet shift in D. sargus, from approximately 25 cm onwards, which implies a wider home range in larger specimens, which start to feed also in the circalittoral zone, instead of remaining in the infralittoral zones like younger individuals.
The liver appears to have a minor role as a fat-reserve organ in D. sargus, since HSI varied relatively little; only females showed slightly higher values. The liver plays a primary role in processing fatty acids mobilized from muscle before their transfer to the ovary (Wiegand, 1996). Therefore, high-fat amounts probably account for this transient increase in female liver weight before the quick ovary development. In agreement with this hypothesis, Pérez et al. (2007) observed no pre-spawn changes in males’ liver lipid content or in HSI, whereas females’ liver registered an increase in both. Therefore, muscle and abdominal fat appear to have a more relevant role than the liver as an energy store for reproduction in D. sargus.
An unexpected result of this work is a striking concentration of copper in the liver of two specimens, one of which was the ATS specimen. Little information is available about Cu liver loads in D. sargus, but the few data published show that values are much lower than the highest values found in our study. Kress et al. (1999) found 7.08 (± 2.05) mg/Kg for individuals from Jaffa and 7.82 ± 2.01 mg/Kg from the Haifa Bay, with the heaviest value of 11.00 mg/Kg. On the Atlantic coast of the Canary Islands, Afonso et al. (2018) found some higher values (23.66 ± 10.25 mg/Kg). The variability in our sample is high, ranging usually from 8.39 to 125.9 mg/kg but reaching 748.5 and 838.0 mg/kg in the two individuals with exceptional Cu loads. We have not found examples of species with a hepatic concentration of copper higher than 100 mg/Kg.
In a recent review, Richir et al. (2021) suggested that several rarely studied sources could be significant contributors to Cu, Ni, and Zn sediment concentration evolution, such as antifouling paints, scrubbers, and anodes, reinforced by the fact that 88% of the sites with increasing Cu concentrations (P < 0.05) were transitional/coastal waters, close to urban areas with substantial vessel numbers.
Of course, our finding does not demonstrate that copper plays a role in the ATS etiology, but this should be followed up. Peaking Cu concentrations in the same species and area were already detected (Merciai et al., 2018). Should it ever be demonstrated a relationship between ATS syndrome and any contaminants accumulated in adipose tissue, then a correlation between ATS incidence and body fat would be positive.
In summary, our findings indicate that the poor quality of D. sargus flesh (muscle), i.e., the gumminess that some individuals present once cooked (ATS), is not caused by any lack of fat. On the contrary, the only ATS individual detected showed a very high-fat content and a high Cu load, suggesting a potential positive relationship between fat content, Cu load, and ATS syndrome that should be explored in the future, analyzing a larger sample of ATS specimens. In this sense, considering that a lot of pollutants are accumulated in the adipose tissue, which acts as a vehicle for their entry, the greater fat reserves of larger individuals might expose them to higher toxicological risk and to a higher probability of developing ATS syndrome. Moreover, it must be noted that organic compounds of anthropic origin are also associated with fats and could be other potential factors of this syndrome.
Data availability
Data for this study are available on request from the authors but belong to the University of Girona.
References
Afonso A, Gutiérrez ÁJ, Lozano G, González-Weller D, Lozano-Bilbao E, Rubio C, Caballero JM, Revert C, Hardisson A (2018) Metals in Diplodus sargus cadenati and Sparisoma cretense - a risk assessment for consumers. Environ Sci Pollut Res 25(3):2630–2642
Aristizabal EO (2007) Energy investment in the annual reproduction cycle of female red porgy, Pagrus pagrus (L.). Marine Biol 152(3):713–724. https://doi.org/10.1007/s00227-007-0729-6
Aussanasuwannakul A, Slider SD, Salem M, Yao J, Brett Kenney P (2012) Comparison of variable-blade to Allo-Kramer shear method in assessing rainbow trout (Oncorhynchus mykiss) fillet firmness. J Food Sci 77(9). https://doi.org/10.1111/j.1750-3841.2012.02879.x
Batičić L, Varljen N, Butorac MŽ, Kapović M, Varljen J (2009) Potential value of hepatic lipids from white seabream (Diplodus sargus, L) as a good source of biomedical components: seasonal variations. Food Technol Biotechnol 47(3):260–268
Casadevall M, Rodríguez-Prieto C (2017) The importance of the age when evaluating mercury pollution in fishes: the case of Diplodus sargus (Pisces, Sparidae) in the NW Mediterranean. AIMS Environ Sci 4(1):17–26. https://doi.org/10.3934/environsci.2017.1.17
Casadevall M, Rodríguez-Prieto C, Pueyo J, Martí C, Merciai R, Verlaque M, Real E, Torres J, Richir J (2020) The strange case of tough white seabream (Diplodus sargus, Teleostei: Sparidae): a first approach to the extent of the phenomenon in the Mediterranean. Front Mar Sci 7:1–13. https://doi.org/10.3389/fmars.2020.00387
Cheng JH, Sun DW, Han Z, Zeng XA (2014) Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: a review. Compr Rev Food Sci Food Saf 13(1):52–61. https://doi.org/10.1111/1541-4337.12043
Del Coco L, Felline S, Girelli C, Angilè F, Magliozzi L, Almada F, D’Aniello B, Mollo E, Terlizzi A, Fanizzi F (2018) 1H NMR spectroscopy and MVA to evaluate the effects of caulerpin-based diet on Diplodus sargus lipid profiles. Mar Drugs 16(10):390. https://doi.org/10.3390/md16100390
Felline S, Caricato R, Cutignano A, Gorbi S, Lionetto MG, Mollo E, Regoli F, Terlizzi A (2012) Subtle effects of biological invasions: cellular and physiological responses of fish-eating the exotic pest Caulerpa racemosa. PLoS ONE 7(6). https://doi.org/10.1371/journal.pone.0038763
Felline S, Mollo E, Ferramosca A, Zara V, Regoli F, Gorbi S, Terlizzi A (2014) Can a marine pest reduce the nutritional value of Mediterranean fish flesh? Mar Biol 161(6):1275–1283. https://doi.org/10.1007/s00227-014-2417-7
Fernandes D, Zanuy S, Bebianno MJ, Porte C (2008) Chemical and biochemical tools to assess pollution exposure in cultured fish. Environ Pollut 152(1):138–146. https://doi.org/10.1016/j.envpol.2007.05.012
Figueiredo M, Morato T, Barreiros JP, Afonso P, Santos RS (2005) Feeding ecology of the white seabream, Diplodus sargus, and the ballan wrasse, Labrus bergylta, in the Azores. Fish Res 75(1–3):107–119. https://doi.org/10.1016/j.fishres.2005.04.013
Fisher W, Schneider M, Bauchot ML (1987) Fiches FAO d’identification des espèces pour les besoins de la pèche. Méditerranée et Mer Noire (Zone De Pêche 37). Vertébrés Rome, FAO 2:761–1530
Forrest AJ, Exley P, Mayze J, Paulo C, Williams D, Sikes A, Poole SE (2014) Physiological factors influencing toughness in cooked saddletail snapper (Lutjanus malabaricus). J Food Sci 79(10):1877–1885. https://doi.org/10.1111/1750-3841.12586
Forrest A, Poole S, Exley P, Mayze J, Paulo C (2012) Management of “tough fish syndrome” in tropical Saddletail snapper (Lutjanus malabaricus) to re-instill market confidence. Agriculture, Fisheries and Forestry. Queensland Government 96 pp
Gorbi S, Giuliani ME, Pittura L, d’Errico G, Terlizzi A, Felline S, Grauso L, Mollo E, Cutignano A, Regoli F (2014) Could molecular effects of Caulerpa racemosa metabolites modulate the impact on fish populations of Diplodus sargus? Mar Environ Res 96:2–11. https://doi.org/10.1016/j.marenvres.2014.01.010
Grigorakis K (2017) Fillet proximate composition, lipid quality, yields, and organoleptic quality of Mediterranean-farmed marine fish: a review with emphasis on new species. Crit Rev Food Sci Nutr 57(14):2956–2969. https://doi.org/10.1080/10408398.2015.1081145
Guiry MD, Guiry GM (2021). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; last searched on 9 December 2021
Hornung H, Sukenik A, Gabrielides GP (1994) Distribution and composition of fatty acids in muscle lipids of inshore fish and deep water sharks from the eastern Mediterranean. Mar Pollut Bull 28(7):448–450. https://doi.org/10.1016/0025-326X(94)90131-7
Kouropakis E, Grigorakis K, Vardali S, Ilia V, Batjakas I, Kotzamanis I (2019) Evaluation of the fillet quality of wild-caught white seabream (Diplodus sargus L.) and brown meagre (Sciaena umbra L.) captured from the Aegean Sea. Mediterr Mar Sci 20(2) https://doi.org/10.12681/mms.18878
Kress N, Herut B, Shefer E, Hornung H (1999) Trace element levels in fish from clean and polluted coastal marine sites in the Mediterranean Sea, Red Sea and North Sea. Helgol Mar Res 53(3):163–170
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666. https://doi.org/10.1016/S0025-326X(01)00060-1
Lloret J, Planes S (2003) Condition, feeding and reproductive potential of white seabream Diplodus sargus as indicators of habitat quality and the effect of reserve protection in the northwestern Mediterranean. Mar Ecol Prog Ser 248:197–208. https://doi.org/10.3354/meps248197
Lloret J, Galzin R, Gil De Sola L, Souplet A, Demestre M (2005) Habitat related differences in lipid reserves of some exploited fish species in the north-western Mediterranean continental shelf. J Fish Biol 67(1):51–65. https://doi.org/10.1111/j.0022-1112.2005.00708.x
Man-Wai R (1985) Les sars du Golfe du Lion: Diplodus sargus, D. vulgaris, D. annularis (Pisces, Sparidae). PhD Thesis. Université des Sciences et Techniques du Languedoc, Montpellier
Martínez Pastor C, Villegas Cuadros ML (1996) Edad, crecimiento y reproducción de Diplodus sargus Linnaeus, 1758 (Sparidae) en aguas asturianas (norte de España). Boletín Instituto Español de Oceanografia 12(1):65–76
Merciai R, Rodríguez-Prieto C, Torres J, Casadevall M (2018) Bioaccumulation of mercury and other trace elements in bottom-dwelling omnivorous fishes: the case of Diplodus sargus (L.) (Osteichthyes: Sparidae). Mar Pollut Bull 136:10–21. https://doi.org/10.1016/j.marpolbul.2018.08.061
Özyurt G, Polat A, Özkütük S (2005) Seasonal changes in the fatty acids of gilthead sea bream (Sparus aurata) and white seabream (Diplodus sargus) captured in Iskenderun Bay, eastern Mediterranean coast of Turkey. Eur Food Res Technol 220(2):120–124. https://doi.org/10.1007/s00217-004-1060-9
Pérez MJ, Rodríguez C, Cejas JR, Martín MV, Jerez S, Lorenzo A (2007) Lipid and fatty acid content in wild white seabream (Diplodus sargus) broodstock at different stages of the reproductive cycle. Comp Biochem Physiol - B Biochem Mole Biol 146(2):187–196. https://doi.org/10.1016/j.cbpb.2006.10.097
Richir J, Bray S, McAleese T, Watson GJ (2021) Three decades of trace element sediment contamination: the mining of governmental databases and the need to address hidden sources for clean and healthy seas. Environ Int 149:106362. https://doi.org/10.1016/j.envint.2020.106362
Sala E, Ballesteros E (1997) Partitioning of space and food resources by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem. Mar Ecol Prog Ser 152(1–3):273–283. https://doi.org/10.3354/meps152273
Saoud I, Batal M, Ghanawi J, Lebbos N (2008) Seasonal evaluation of nutritional benefits of two fish species in the eastern Mediterranean Sea. Int J Food Sci Technol 43(3):538–542. https://doi.org/10.1111/j.1365-2621.2006.01491.x
Santamaría J, Tomas F, Ballesteros E, Cebrian E (2021) Herbivory on the Invasive Alga Caulerpa cylindracea: The Role of Omnivorous Fishes. Front Mar Sci 8:1–10. https://doi.org/10.3389/fmars.2021.702492
Santinha P, Medale F, Corraze G, Gomes ESF (1999) Effects of the dietary protein: lipid ratio on growth and nutrient utilization in gilthead seabream Sparus aurata L. Aquac Nutr 5:147–156
Shahidi F (2003) Extraction and measurement of total lipids. Curr Protoc Food Anal Chem 7(1). https://doi.org/10.1002/0471142913.fad0101s07
Taşbozan O, Gökçe MA (2017) Fatty acids in fish. In: Catala A (ed) Fatty acids. IntechOpen book series, London. https://doi.org/10.5772/68048
Terlizzi A, Felline S, Lionetto MG, Caricato R, Perfetti V, Cutignano A, Mollo E (2011) Detrimental physiological effects of the invasive alga Caulerpa racemosa on the Mediterranean white seabream Diplodus sargus. Aquat Biol. https://doi.org/10.3354/ab00330
Tramati C, Vizzini S, Maci S, Basset A, Mazzola A (2011) Trace metal contamination in a Mediterranean coastal pond (Acquatina, Puglia). Trans Waters Bullet 5(2):124–137. https://doi.org/10.1285/i1825229Xv5n2p124
Türkmen M, Türkmen A, Tepe Y, Ateş A, Gökkuş K (2008) Determination of metal contaminations in seafood from Marmara, Aegean and Mediterranean seas: twelve fish species. Food Chem 108(2):794–800. https://doi.org/10.1016/j.foodchem.2007.11.025
Vitale RM, D’aniello E, Gorbi S, Martella A, Silvestri C, Giuliani ME, Fellous T, Gentile A, Carbone M, Cutignano A, Grauso L, Magliozzi L, Polese G, D’Aniello B, Defranoux F, Felline S, Terlizzi A. Calignano A, Regoli F, DiMarzo V, Amodeo P, Mollo E (2018) Fishing for targets of alien metabolites: a novel peroxisome proliferator-activated receptor (PPAR) agonist from a marine pest. Marine Drugs 16(11). https://doi.org/10.3390/md16110431
Weil C, Lefèvre F, Bugeon J (2013) Characteristics and metabolism of different adipose tissues in fish. Rev Fish Biol Fisheries 23(2):157–173. https://doi.org/10.1007/s11160-012-9288-0
Wiegand MD (1996) Composition, accumulation, and utilization of yolk lipids in teleost fish. Rev Fish Biol Fisheries 6(3):259–286. https://doi.org/10.1007/BF00122583
Acknowledgements
We are grateful to all the colleagues who helped us with tasting the seabream samples and collaborated to identify their quality: Josep M Bas, Maria Lorenzo, Marta Muñoz, and Núria Roura.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was carried out as part of a project funded by the University of Girona (UdG/3/Ref. MPCUdG2016-071).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No ethical approval was required for fish provided dead by local fishermen.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Casadevall, M., Merciai, R., Lloret, J. et al. Pre-spawning fat and trace metals in Diplodus sargus (Pisces, Sparidae) and possible relationship with the abnormally tough syndrome (ATS). Environ Biol Fish 105, 729–739 (2022). https://doi.org/10.1007/s10641-022-01284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01284-y