Abstract
Atlantic salmon post-spawners from a population in northern Norway were tagged with data storage tags (N = 773), and the depth use and diving behaviour of recaptured individuals within the coastal zone were examined, both on their outward migration to sea (N = 44) and their return to the natal river after overwintering at sea (N = 34). In addition, the stomach contents of 909 returning adults caught in the fjord were examined to determine the extent to which, how recently and on what species they had fed. The tagged individuals migrated through surface waters and performed aperiodic dives, regardless of whether they were leaving the fjord as post-spawners (kelts) or returning after a winter or more at sea. However, diving behaviour differed between the fjord and outer coast. During both outward and return migration, dives when fish were likely in the fjord were shallower than on the outer coast. Deep dives of longer duration were more frequent on the outer coast than in the fjord. The stomach content analysis of salmon captured in the fjord did not show strong evidence of recent feeding: 58% of the salmon had empty stomachs, and most stomach contents were highly digested fish (mainly herring, but some capelin and unidentifiable species). We conclude that the inferred diving behaviour in the coastal zone, both on outward migration to sea and on return to the natal river, did not provide sufficient evidence of foraging within the water column, and hypothesize that diving in search for navigation cues is a more likely explanation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Atlantic salmon (Salmo salar L.) is a culturally and economically important cold-water fish species (Liu et al. 2011; Ignatius and Haapasaari 2018) that serves as an indicator of freshwater and coastal ecosystem health (Lien et al. 2016). In contrast to Pacific salmon (Oncorhynchus sp.), which display a semelparous life-history strategy with individuals dying shortly after spawning, Atlantic salmon are facultatively iteroparous and may spawn multiple times during their lifespan (Klemetsen et al. 2003). This bet-hedging strategy spreads the risk of reproductive failure over time and may enhance population resilience to environmental perturbations because the presence of larger-sized repeat spawners may sustain recruitment in years with low abundance of maiden spawners (Bordeleau et al. 2020). For Atlantic salmon, the success of iteroparity is dependent on the ability to transit fjords/estuaries to migrate between fresh water and marine feeding grounds.
Coastal areas are challenging environments for Atlantic salmon. Firstly, aggregations of marine predators may cause high mortality in both post-smolts (Thorstad et al. 2007) and larger adults (Lacroix 2014; Strøm et al. 2019). Secondly, there is increasing human activity in the coastal zone. Pathogen spill from open-net pen Atlantic salmon farming has been shown to impact post-smolt survival (Kristoffersen et al. 2018; Shephard and Gargan 2021). Anthropogenic stressors such as boat traffic (Codarin et al. 2009; Becker et al. 2013), pollution (Meador 2014), and the construction of infrastructure such as harbours or tidal and wind power plants (Bergstrom et al. 2014) impact marine fish, and it is possible that these factors may impact Atlantic salmon during their coastal migration. Being iteroparous, Atlantic salmon will be exposed to these conditions several times throughout their lifespan. Therefore, detailed knowledge about the behaviour of Atlantic salmon in coastal areas is essential for evaluating the impacts of different stressors and developing and evaluating mitigation measures designed to protect Atlantic salmon during migration through this challenging environment.
Diving and feeding are important behaviours within the coastal zone. However, there have been relatively few studies examining these behaviours in Atlantic salmon adults. A pop-up satellite tag (PSAT) study by Strøm et al. (2017) of outward migrating adults within the Gulf of St Lawrence found that they mainly migrated in near-surface waters with frequent shallow dives and that deep dives to > 50 m were rare. A PSAT study of returning adults in Scottish waters by Godfrey et al. (2015) found a similar preference for surface waters, alongside diving to an average depth of 64 m, and to a maximum of > 100 m. The reasons for diving by adult Atlantic salmon at sea are not fully understood, but it may be that diving behaviour is associated with searching for homing cues when returning through fjords (Davidsen et al. 2013), and feeding in more open waters (Strøm et al. 2017) and oceanic fronts (Rikardsen et al. 2021). Stomach contents of recaptured fish may provide information on whether they have fed and on what prey types they have selected, and may be used to infer reasons for diving behaviour (Rikardsen and Dempson 2011). Obtaining information on diets from stomach contents requires killing fish for sampling, and adult Atlantic salmon typically occur in low numbers so killing them for sampling prior to spawning is often not feasible or desirable. However, in Northern Norway, there are still areas with abundant Atlantic salmon populations, supporting a regular sea fishery and enabling the collection of fish stomach samples from the catch of local fishers.
The main aim of this study was to investigate the behaviour of adult Atlantic salmon during migration through the coastal zone. Specifically, we examined the coastal diving behaviour of Atlantic salmon adults, tagged with archival tags, during the initial and final phase of their ocean migration through a Norwegian fjord. In addition, a stomach content analysis of untagged adult Atlantic salmon captured by bag net fishers in the fjord during their return migration towards the river was conducted to determine if the prevalence and type of prey items could be used to infer the underlying causes for the observed diving behaviour of the tagged fish.
Method
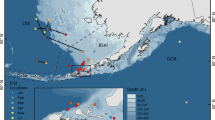
The study was conducted in the coastal zone surrounding and including the Alta fjord on the northern coast of Norway (70.13°N, 23.08°E) (Fig. 1). The Alta fjord is a large sill fjord (length = 30 km, mean width = 7 km, maximum depth = 488 m, tidal range = 1.5–2.5 m) that flows into the Barents Sea ≈60 km from the mouth of its largest river (the River Alta) via the Stjernsund, Rognsund and Vargsund straits. The surface water layer of the fjord is strongly influenced by river discharge and may become brackish during summer (Skarðhamar et al. 2018). The fjord becomes thermally stratified in summer with a warm surface layer of ≈12–13 °C overlying a colder layer of < 10 °C at depths > 10 m in June/July. Surface temperature patterns within the fjord are complex and suggest horizontal recirculation.

source: Kartverket (https://dybdedata.kartverket.no)
Map of study area, showing the Alta Fjord. Bathymetry data
The study consisted of two parts: (1) a tagging study using data storage tags which archived information on depth use and diving behaviour of Atlantic salmon adults migrating through the Alta fjord coastal zone both on outrun and return and (2) a stomach contents study of untagged adults captured in the fjord that were returning for spawning, which provided information on the prevalence of feeding and the prey species.
Tagging study
Capture, tagging, release and recapture
From 2008 to 2015, 773 post-spawned adult Atlantic salmon from the River Alta were tagged with data storage tags. Post-spawners were captured in the river after ice break-up in May of each year by angling using spoon bait (two hook lures with the barbs removed so as to minimize fish damage). Larger individuals (mean body length = 92 cm, range = 55–121 cm; mean body mass = 6.0 kg, range = 1.2–13.8 kg; mean condition factor, K = 0.75, range = 0.49–1.59) were selected for tagging to minimize the tagging effects on the probabilities of survival and subsequent recovery, so tagged individuals were predominantly female (93% of those tagged) due to their larger size relative to males in this river (Halttunen et al. 2010).
Each post-spawner selected for tagging was placed in a holding tank and anesthetized in an aqueous solution of 0.5 ml 2-phenoxy ethanol l−1 (EC No 204–589-7, Sigma Chemical Co., St Louis, Missouri, USA). Once anesthetized, it was placed in a tagging tube that was partly filled with freshwater so that the mouth and gills were submerged, a lateral cut (≈2–2.5 cm long) was made through its ventral surface posterior to the pelvic girdle, and a tag was inserted into its body cavity. Several tag types were used. A total of 576 post-spawners were tagged with data storage tags supplied by Star Oddi, Reykjavik, Iceland (www.star-oddi.com): either (1) DST milli: 39.4 × 13 mm, 9.2 g in air; or (2) DST centi: 46 × 15 mm, 19 g in air. These tags recorded depths at intervals of 1, 5, 10 or 30 min, depending upon year of tagging, and recorded body temperatures concurrently with depth, except for the tags measuring depth at a 1-min interval which recorded temperature every 5 min. An additional 197 individuals were tagged with Geo data storage tags supplied by Lotek Wireless Inc., Ontario, Canada (www.lotek.com): LAT2810L, 44 × 13 mm, 13 g in air. These recorded depth and temperature (both the internal body temperature of the fish and the external temperature of the surrounding water) at a 30-s interval. Fish were also tagged externally with Carlin identification tags (Floy tag, Mervin Manufacturing Inc., Seattle, Washington, USA) to ensure easier identification on return. The handling and tagging procedure for each post-spawner took approximately six minutes. After tagging, post-spawners were released into the River Alta, except for in 2009 when post-spawners were released directly into the fjord at the river mouth to avoid potential negative effects of an expected large flood in the river (Table 1).
Recapture of tagged Atlantic salmon was done by fishers (bag net fishing in Alta fjord or angling in the Alta River) who had been informed of the tagging project and offered a monetary reward for each returned tag. Recaptures occurred in both the fjord and along the nearby coast (Note, recapture location in one returning adult was not registered due to error). Of the 773 tagged Atlantic salmon released, 45 were recaptured: three on out migration and 42 after a winter or more at sea. One tag had failed to record temperature, hindering identification of time of sea entry, so data from this tag were discarded. This left tag data for 44 post-spawners. Of the 42 tags captured after a winter or more at sea, eight tags were discarded due to infrequent or incomplete measurements of depth and temperature before recapture, leaving tag data for 34 returning adults (Supplementary table 1).
Data analyses
Parts of the data series when the tagged Atlantic salmon were not in sea water (before release or after recapture, or within the River Alta) were identified and removed. Tag registrations within the river were identified by a consistent diurnal temperature pattern of daytime warming and night-time cooling of ≈1–3 °C, as opposed to those in sea water which did not show such a pattern. Tags were calibrated at a 10 m depth from several hours before tagging. However, some tags “drifted” in their depth recordings, and depths were adjusted by subtracting the shallowest depth recorded on the assumption that this depth represented the fish surfacing.
Depth use and diving behaviour of the post-spawners and returning adults were analyzed according to time since release (on outward migration for post-spawners) or time before recapture (for returning adults) on the rationale that this would indicate their probable location relative to the river mouth and fjord. Previous studies have shown rapid migration through the fjord. Halttunen et al. (2009) found a mean post-spawner transit time of 33 h on outmigration through the 30 km length of the fjord, and Davidsen et al. (2013) identified a mean migration speed of returning adults through the fjord of 9.7 km day−1 (range 0.7–33.1 km day−1) corresponding to a transit time of several days through the fjord. Based on the progression speeds from these studies, fish on day 1 after entry into the sea were assumed to be mainly in the inner fjord (0–15 km from river mouth), fish on day 2–3 were assumed to be mainly in the outer fjord (15–60 km from river mouth, ending at the outer mouth of the straits), and fish on day 4–14 were assumed to be mainly on the outer coast (> 60 km from river mouth, Fig. 1). Time-series data further than 14 days outside of sea entry or before recapture were omitted from further analysis as they most likely represented times when the fish were in the deep sea, and outside the scope of this study (see Strøm et al. 2018).
For all post-spawners, trends in depth use and diving were examined as a function of time since sea entry. Data from all tags (N = 44) were used to obtain depth composition and maximum daily depth, providing information on what parts of the water column the fish were using, and the approximate maximum depths to which fish dived. For the fish tagged with high-resolution tags (N = 13, measurement interval ≤ 1 min), it was possible to obtain more detailed metrics of the diving behaviour (dives being defined as a movement from the surface ≤ 5 m to a deeper depth with a subsequent return to the surface), even for short dives that may have been missed with a low sample resolution. Trends were examined using linear mixed-effects models (R function lmer{lmerTest}). Four models were fitted with the following response variables: (1) maximum daily depth (m) (all tags); (2) dive depth (m) (high-resolution tags); (3) dive frequency (N day−1) (high-resolution tags); and (4) dive duration (min) (high-resolution tags). Individual fish were used as the random effect, and models were fitted using random slopes and fixed intercepts. Random slopes allowed analysis of whether trends were consistent among individuals; fixed intercepts were used to ensure convergence for models that included random slopes. To ensure appropriate residual distributions, response variables were log-transformed prior to model fitting. For maximum daily depth and dive frequency, the predictor variable was day after sea entry (1, 2, 3,… 14). For dive depth and dive duration, the predictor variable was the fractional day after sea entry on initiation of the dive. Dives of the fish tagged with high-resolution tags were further analyzed by comparing descent and ascent speeds using a Wilcoxon signed-rank test (R function wilcox-test{stats}) as differences in speed might suggest underlying behavioural mechanisms. Finally, registered temperatures (both internal and external) of the six Geo tags were examined to determine if the tagged fish were diving into colder waters (from the external temperatures) and if the temperature change on diving was great enough to affect internal body temperature.
For all returning adults where capture location had been recorded (N = 33), depth distributions and maximum recorded depths in the final 24 h at sea were analyzed with respect to recapture zone—(1) outer coast or (2) fjord or river—using Wilcoxon tests. Longitudinal trends in maximum daily depth, dive depth, dive frequency and dive duration were examined in the same way as for post-spawners using linear mixed-effects models, but with regard to time before recapture rather than after sea entry. Descent and ascent speeds were also compared for the high resolution tags, and registered temperatures of the Geo tags were examined in the same way as for post-spawners.
Stomach contents study
Feeding by adult Atlantic salmon in and proximate to the fjord during return migration was investigated by examining the stomach contents of 909 individuals which had been captured in the fjord by local bag net fishers (May to September, 2008–2010). Captured individuals had a median body length of 76 cm (range = 50–130 cm), a median body mass of 4.2 kg (range = 1.0–20.2 kg) and a median condition factor of 1.0 (range = 0.49–1.66). Individuals captured in May and June tended to be larger (median body length = 95.5 cm, median body mass = 9.4 kg, N = 309) than those captured in July to September (median body length = 62 cm, median body mass = 2.5 kg, N = 585): note, capture month was unavailable for 15 fish. To determine a potential effect of fish characteristics on whether they had recently fed, the probability of a stomach containing one or more prey items was modelled as a function of body mass, body length, condition factor, and day of year of capture using generalized linear modelling (R function glm{stats}) with a binomial family (N = 884; 25 fish were omitted due to an unregistered day of year of capture). A range of models was fitted including either body mass (M) or length (L) and different additive combinations with condition factor (K) and/or day of year of capture (D): (1) M, (2) M + K, (3) M + D, (4) M + K + D, (5) L, (6) L + K, (7) L + D, (8) L + K + D. The optimal model was selected based on the AIC value. Stomach contents were also analyzed with regard to the degree of digestion of prey items (N = 810 prey items across 374 Atlantic salmon), and the prey type. The degree of digestion was examined according to Atlantic salmon body length to see if there was a size effect on how recently the fish had fed. Degree of digestion was characterized as (1) undigested; (2) partly digested, where some skin was digested but the head was mostly intact; (3) mostly digested, where most skin and flesh was digested and the head was partially digested; (4) highly digested, where only vertebrae and some flesh remained; or (5) almost completely digested. A test of equal proportions (R function prop.test{stats}) was done to compare small (body length < 80 cm) and large (≥ 80 cm) Atlantic salmon in terms of stomach content digestion (undigested to mostly digested versus highly to almost completely digested).
Results
Post-spawners released in the river entered the fjord during early summer (median date of entry = June 5, range = May 7–July 29, N = 44). Returning adults with full tag data records were recaptured in the fjord and river during mid- to late summer (median date of recapture = June 27, range = June 6–July 30, N = 34) 1 year after release.
Depth use and diving behaviour of post-spawners during outward migration
Post-spawners migrated through surface waters in the fjord and outer coast, spending > 90% of their time at depths < 5 m on the first day after sea entry, and between ≈75 and > 90% of their time at these depths on subsequent days (Fig. 2A). The average of the individual median depths was 1.5 m (range = 0.3–6.0 m, N = 44). Maximum daily depths generally increased with time at sea (Fig. 2B; Table 2). However, this relationship varied according to the individual, and nearly 20% of post-spawners (8 out of 44) showed a reduction in maximum daily depth with time at sea (Supplementary Fig. 1). On day 1, when post-spawners were likely in the inner fjord, the median of their daily maximum depths recorded was ≈16 m; this increased to ≈18 m (day 2–3), and ≈28 m (day 3–14).
Dive depth increased with time across the 14 days following sea entry for fish tagged with high-resolution tags (N = 13) (Table 2). This relationship was shown by 92% of fish (12 out of 13) (Supplementary Fig. 1). Dive frequency was greatest on fjord entry (day 1, median = 34 dives day−1, range = 0–171, N = 13), and declined far offshore on day 14 (median = 5 dive day−1, range = 0–134, N = 13) (Fig. 2C). However, there was not a significant trend over the 14 days (Table 2). Dives were generally of short duration (mean = 2–3 min) throughout the 14 days monitored (Fig. 2D), and there was not a consistent change in dive duration with time after sea entry (Table 2). Post-spawners showed a range of diving behaviours, both during the first 3 days of sea entry, when they were likely in the fjord (Fig. 3) and later when they were more likely to be in the outer coast (Supplementary Fig. 2). For example the salmon with tag B1093 remained near the surface and did not dive deeper than 100 m until 9 days after sea entry, whereas the salmon with tag Geo1574 dived deeper than 100 m on the first day after entry. There was no difference between vertical speed on descent (mean = 0.07 m s−1) and vertical speed on ascent (mean = 0.06 m s−1) (Wilcoxon signed-rank test, p = 0.25). Measurements of water temperature by the Geo tags within the first 14 days after entry showed that fish were generally diving into colder waters (mean reduction = 0.46 °C from surface to dive trough). There was a negligible change in body temperature on diving registered by these tags (mean reduction = 0.01 °C), so dives were not of a sufficient duration to cool the fish to ambient water temperature.
Depths and internal temperatures of post-spawners tagged with high-resolution tags in the first 3 days after sea entry. Note, the fish with tag number Geo1565, which did not dive in this period, has been omitted. Depths shown have a cut-off at 65 m, and exclude parts of some dives by the fish with tag number Geo1574 and Geo1302
Depth use and diving behaviour of returning adults
Returning adults recaptured outside the fjord had occupied greater depths in the final 24 h at sea than those recaptured within the fjord or river, spending less time in surface waters (depth < 5 m) and having greater maximum depths (Fig. 4). However, there was a large individual variation; therefore, differences in depth distribution according to recapture zone were not significant (Wilcoxon tests, p > 0.05). Returning adults, similar to post-spawners migrated through surface waters in the outer coast and fjord, spending > 75% of their time at depths < 5 m (Fig. 5A). The average of the individual median depths was 2.6 m (range = 0.5–22.6 m, N = 34). Maximum daily depths registered tended to decline with proximity to capture in the sea or return to the river (Fig. 5B; Table 2): 85% of fish (29 out of 34) showed this pattern (Supplementary Fig. 1). For example, from day 4–14, when returning adults were likely offshore, the median of daily maximum depths was ≈38 m, with one individual diving to ≈650 m. In contrast, on day 2–3, the median had declined to 33 m, and on day 1 (within 24 h before recapture or return), the median was ≈16 m with the deepest dive registered being ≈100 m.
Depth patterns of returning adults according to time before recapture/return to river: A depth composition, B maximum depth, C dive frequency, and D mean dive duration. A and B show data for all returning adults (N = 34); C and D show data for returning adults tagged with high-resolution tags (N = 13)
For returning adults, tagged with high-resolution tags (N = 13), dive depth decreased with proximity to recapture/return (Table 2), a relationship shown by 92% of fish (12 out of 13) (Supplementary Fig. 1). Similar to during out migration, there was no significant temporal trend in dive frequency on return (Fig. 5C; Table 2). However, the average diving rate in the last 14 days at sea (mean = 38.4 dives day−1, range = 10.6–74.7, N = 13) was approximately twice that on out migration during the first 14 days after sea entry (mean = 18.82 dives day−1, range = 2.1–59.4, N = 13). Diving behaviour was characterized by shorter dives with proximity to recapture/return (Fig. 5D; Table 2) for all individuals with high-resolution tags (Supplementary Fig. 1). Diving behaviour on return also showed wide individual variation (Fig. 6; Supplementary Fig. 3). For example, B1093 did not dive deeper than 8 m in the 14 days before recapture, whereas the other 12 returning adults showed multiple deeper dives. There was no difference between speed on descent (mean = 0.11 m s−1) and speed on ascent (mean = 0.11 m s−1) (Wilcoxon signed-rank test, p = 0.97). Similar to post-spawners on out migration, returning adults were diving into slightly colder waters (mean reduction = 0.49 °C for the 14 days at sea preceding recapture/return for the fish tagged with Geo tags), but dives were short enough that there was a negligible change in body temperature on diving (mean reduction = 0.01 °C).
Depths and internal temperatures of returning adults tagged with high-resolution tags in the last 3 days before recapture/return to river. Note, the fish with tag number B1093, which did not dive in this period, has been omitted. Depths shown have a cut-off at 65 m, and exclude parts of some dives by the fish with tag number B1113, Geo1257, Geo1565, and Geo1574
Stomach contents of returning adults
Of 909 captured Atlantic salmon, 58% (N = 530) had empty stomachs, and only 5% (N = 50) had stomachs > 90% full (Fig. 7A). Most stomachs that contained prey only had one or two prey items (50% and 21% of stomachs with prey, respectively), but one stomach contained 17 items. Models of the probability of an Atlantic salmon having prey in its stomach fitted to body length had lower AIC values (AIC = 1075.7–1080.6) than those fitted to body mass (AIC = 1089.2–1095.5). The model with body length and day of year as predictors had the lowest AIC value (Supplementary table 2). The probability of an Atlantic salmon stomach containing prey decreased with increasing salmon body length (Fig. 7B) (p < 0.001), ranging from ≈0.71 at a body length of 50 cm to ≈0.05 at a body length of 130 cm for individuals captured on day of year 192 (the median day of capture). The probability of the stomach containing prey also decreased with day of year, but the relationship was only marginally significant (p = 0.021).
Stomach contents of Atlantic salmon captured in Alta fjord: A Stomach volume occupied by food (%) (N = 909); B probability of stomach containing one or more prey items as a function of Atlantic salmon body length, as modelled by a generalized linear model (model 7, Supplementary table 2); C digestion rating of stomach prey items (N = 810) as a function of Atlantic salmon body length. In B, the curve shown is for median date of capture (day of year = 192)
Most prey items were at least partly digested (> 98%). The stomach contents of larger Atlantic salmon tended to be more digested than those of smaller Atlantic salmon (Fig. 7C). For example, ≈70% of prey items were highly or almost completely digested in Atlantic salmon with a body length of ≥ 80 cm, as opposed to ≈24% of prey items in Atlantic salmon < 80 cm in body length (Test of equal proportions, p < 0.001). Among the prey items, the two identifiable fish species were herring (Clupea harengus) (46% of prey items) and capelin (Mallotus villosus) (6% of prey items). The remaining prey items (48%) were too digested for fish species to be identifiable.
Discussion
The tagged adult Atlantic salmon migrated through surface waters and performed aperiodic dives, both on outward migration and return. Dives were generally shallow (≈10–40 m) and of short duration (2–3 min). This pattern is consistent with previous studies, both for post-spawners (Halttunen et al. 2009) and returning adults (Godfrey et al. 2015). However, diving behaviour changed over time, during both outward and return migration, and dives within the first day after entering the fjord and in the day before recapture/return (likely mainly within the fjord) were generally shallower than those further away from entry or recapture/return (likely further out to sea).
Post-spawners are energy-depleted fish (Jonsson et al. 1991; Halttunen et al. 2013) that likely need to start feeding soon after sea entry, but also appear to be motivated to migrate rapidly through fjords (see Halttunen et al. 2009). Atlantic salmon are opportunistic feeders that may feed on a large variety of food items (Rikardsen and Dempson 2011), and adults may feed both close to the surface and in deeper water. In general, sea productivity is greater near the surface where primary production is concentrated; therefore, the likelihood of encountering food during migration without actively foraging may be greater near the surface than in deeper waters. The short-dive times observed within the fjord in the current study were not consistent with the longer-lasting ‘U’ shaped dives that have been hypothesized to indicate foraging by fish at sea (Wilson and Block 2010; Hedger et al. 2017). Hence, swimming mostly near the surface, as recorded in this study, may be a beneficial strategy for rapid migration while increasing the likelihood of finding the occasional prey without expending time and energy foraging within the water column.
The stomach contents of adult Atlantic salmon captured in the fjord indicated little feeding during the last phase of the return migration, which would concur with a lack of foraging through diving within the fjord. Nearly two-thirds of fish had empty stomachs. The stomach evacuation rate in Atlantic salmon is in the order of 1–3 days. Storebakken et al. (1999) reported an 88% evacuation within 24 h for small salmon individuals (body mass = 0.15–0.30 kg) at a temperature of 9 °C. A near 100% evacuation within 3 days has been reported for smolts at temperatures of 6–18 °C (Handeland et al. 2008) and adults at temperatures of 4–5 °C (Waagbo et al. 2017). Available temperature data from the fjord, measured daily at a fish farm at a depth of 3 m in June–August 2008 and 2009, were comparable with these temperatures: median = 9.8 °C (range = 5.5–13.6 °C) (note, data from 2010 were unavailable). Therefore, similar stomach evacuation rates can be expected for the captured Atlantic salmon in the current study. Given this, it can be inferred that nearly two-thirds of captured individuals did not feed within a period of several days before recapture.
The likelihood of an Atlantic salmon individual having prey in its stomach decreased with body size and the digestion level of prey items increased with its body size, suggesting that larger individuals may have stopped feeding earlier during the return migration than smaller individuals. The fact that the size of individuals captured in the fjord later in the year (July–September) was smaller than those captured earlier (May–June) suggests that larger individuals were swimming through the fjord to return to the river earlier in the year. Early return to rivers may be beneficial, because the ocean may be a more dangerous place to stay than rivers due to predators (Strøm et al. 2019). At the same time, early return implies a longer period without food intake since adult Atlantic salmon largely stop feeding when they enter rivers. It might be that large individuals already have sufficient energy reserves for spawning and survival by early-spring, whereas smaller individuals have more to gain by continued feeding at sea: smaller individuals have lower energy reserves and higher maintenance requirements per unit mass, so have greater need to gain mass for survival and reproduction on return to freshwater.
For the Atlantic salmon that did have food remains in their stomachs, identified prey types were exclusively fish species (mainly herring) rather than other potential prey types (e.g. crustaceans, squids, amphipods). The absence of any other prey types implies that they were not major recent sources of prey. Atlantic salmon feed opportunistically at sea but become more specialized as they return (MacKenzie et al. 2012) and Atlantic salmon may select fish over other prey types (Jacobsen and Hansen 2000; Rikardsen and Dempson 2011). Identified prey species in the current study were herring and capelin (89% and 11% of identified prey items, respectively). Herring has been commonly observed as a food source for Atlantic salmon in the NE Atlantic, in Icelandic waters (Sturlaugsson 2000), in the Norwegian Sea off Northern Norway (Grønvik and Klemetsen 1987; Aykanat et al. 2020) and in the Northern Baltic (Salminen et al. 2001), but capelin has also been observed as a prey type for Atlantic salmon (Hansen and Pethon 1985; Aykanat et al. 2020). Herring is more commonly found in the Norwegian Sea (Vogel et al. 2021), while the larger capelin commonly feeds in the more northern Barents Sea, except during spawning on the Norwegian continental shelf in March–April. Capelin die soon after spawning, so their importance as prey for Atlantic salmon diminishes throughout summer (Rasmussen 2012). Predation on mainly herring by returning Atlantic salmon was consistent with the salmon feeding on them when migrating through surface or near-surface waters, or during their longer-lasting dives on the outer coast towards the periphery of the Norwegian Sea. The absence of benthic species such as crustaceans or sand eels, Ammodytes marinus, which Atlantic salmon are known to feed on, coupled with the fact that maximum recorded depths were generally shallow (≈10–40 m), suggests that they were not diving to the seabed (depths typically ≈ 100–400 m within the fjord) for foraging.
Alternative reasons for diving may be energy conservation, thermoregulation or navigation. It is possible for fish to conserve energy by gently gliding during descents before rapid active swimming on ascents to the surface (Kawabe et al. 2004; Gleiss et al. 2011). However, the Atlantic salmon of the current study showed a wide range of diving patterns, and average vertical speeds of descent were similar to those of ascent, so there was no strong evidence to suggest that this was occurring, either on out migration or return. Thermoregulation has been suggested as an influence on diving (Reddin et al. 2004). However, this is also not likely to be a primary cause of diving in the current study because depth-dependent variation in temperature was too small for this to provide a thermoregulatory advantage, evident from the fact that changes in internal body temperature during dives were negligible. Diving for the purpose of navigation is possible, involving individuals exploring different layers within the water column to find cues for navigating out of the fjord and back to the natal river. Atlantic salmon return to their natal river with a high precision, and seemingly use cues sequentially learned during outward migration to orient through near-coastal and fjord areas to locate the natal river (Hansen et al. 1993). The Atlantic salmon did not dive to depths consistent with them interacting with the fjord bottom. However, we speculate that diving may be associated with exploring water layers. This may be particularly important for the type of fjord considered, which is influenced by wind-induced surface currents that may affect longitudinal gradients in surface water properties, and which becomes stratified in summer such that there is a warmer, lower salinity 5–10 m surface layer overlying cooler and more saline waters (Skarðhamar et al. 2018).
Conclusion
Adult Atlantic salmon migrating through a fjord showed a wide range of individual behaviours, both on out migration as post-spawners and on return to the natal river. The diving behaviour of post-spawners was characterized by frequent, shallow dives on entering the fjord, which changed to deeper dives further out in coastal waters. That of returning adults was characterized by shorter and shallower dives as they approached the fjord and river. Most adults captured in the fjord and nearby coast by bag net fishers had not fed in the days immediately before recapture, suggesting that feeding in the fjord and nearby coastal waters was rare. Those which had fed preyed exclusively on epipelagic fish species (mainly herring, and some capelin). Given the general lack of feeding, and lack of evidence of feeding on demersal species, it can be inferred that the short, frequent diving is not strong evidence of feeding within fjords. Based on the lack of alternative explanations for diving, it can be hypothesized that the diving recorded during entry into the fjord from the river, and during return from sea, may be mainly for the purpose of finding navigation cues within a vertically stratified fjord that is characterized by complex horizontal circulation patterns.
Availability of data and material
Data are available on request.
Code availability
R code is available on request.
References
Aykanat T, Rasmussen M, Ozerov M, Niemela E, Paulin L, Vaha JP, Hindar K, Wennevik V, Pedersen T, Svenning MA, Primmer CR (2020) Life-history genomic regions explain differences in Atlantic salmon marine diet specialization. J Anim Ecol 89(11):2677–2691. https://doi.org/10.1111/1365-2656.13324
Becker A, Whitfield AK, Cowley PD, Jarnegren J, Naesje TF (2013) Does boat traffic cause displacement of fish in estuaries? Mar Pollut Bull 75(1–2):168–173. https://doi.org/10.1016/j.marpolbul.2013.07.043
Bergstrom L, Kautsky L, Malm T, Rosenberg R, Wahlberg M, Capetillo NA, Wilhelmsson D (2014) Effects of offshore wind farms on marine wildlife-a generalized impact assessment. Environ Res Lett 9(3):12. https://doi.org/10.1088/1748-9326/9/3/034012
Bordeleau X, Pardo SA, Chaput G, April J, Dempson B, Robertson M, Levy A, Jones R, Hutchings JA, Whoriskey FG, Crossin GT (2020) Spatio-temporal trends in the importance of iteroparity across Atlantic salmon populations of the northwest Atlantic. ICES J Mar Sci 77(1):326–344. https://doi.org/10.1093/icesjms/fsz188
Codarin A, Wysocki LE, Ladich F, Picciulin M (2009) Effects of ambient and boat noise on hearing and communication in three fish species living in a marine protected area (Miramare, Italy). Mar Pollut Bull 58(12):1880–1887. https://doi.org/10.1016/j.marpolbul.2009.07.011
Davidsen JG, Rikardsen AH, Thorstad EB, Halttunen E, Mitamura H, Praebel K, Skardhamar J, Naesje TF (2013) Homing behaviour of Atlantic salmon (Salmo salar) during final phase of marine migration and river entry. Can J Fish Aquat Sci 70(5):794–802. https://doi.org/10.1139/cjfas-2012-0352
Gleiss AC, Norman B, Wilson RP (2011) Moved by that sinking feeling: variable diving geometry underlies movement strategies in whale sharks. Funct Ecol 25:595–607. https://doi.org/10.1111/j.1365-2435.2010.01801.x
Godfrey JD, Stewart DC, Middlemas SJ, Armstrong JD (2015) Depth use and migratory behaviour of homing Atlantic salmon (Salmo salar) in Scottish coastal waters. ICES J Mar Sci Doi:doi. https://doi.org/10.1093/icesjms/fsu118
Grønvik S, Klemetsen A (1987) Marine food and diet overlap of cooccurring arctic charr Salvelinus alpinus (L.), brown trout Salmo trutta (L.) and Atlantic salmon S. salar (L.) off Senja. N. Norway. Polar Biol 7(3):173–177. https://doi.org/10.1007/bf00259205
Halttunen E, Jensen JLA, Naesje TF, Davidsen JG, Thorstad EB, Chittenden CM, Hamel S, Primicerio R, Rikardsen AH (2013) State-dependent migratory timing of postspawned Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 70(7):1063–1071. https://doi.org/10.1139/cjfas-2012-0525
Halttunen E, Rikardsen A, Davidsen J, Thorstad E, Dempson J (2009) Survival, migration speed and swimming depth of Atlantic salmon kelts during sea entry and fjord migration Tagging and Tracking of Marine Animals with Electronic Devices. Springer, Netherlands, pp 35–49
Halttunen E, Rikardsen AH, Thorstad EB, Naesje TF, Jensen JLA, Aas O (2010) Impact of catch-and-release practices on behavior and mortality of Atlantic salmon (Salmo salar L.) kelts. Fish Res 105(3):141–147. https://doi.org/10.1016/j.fishres.2010.03.017
Handeland SO, Imsland AK, Stefansson SO (2008) The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283(1–4):36–42. https://doi.org/10.1016/j.aquaculture.2008.06.042
Hansen LP, Jonsson N, Jonsson B (1993) Oceanic migration in homing Atlantic salmon. Anim Behav 45(5):927–941. https://doi.org/10.1006/anbe.1993.1112
Hansen LP, Pethon P (1985) The food of Atlantic salmon, Salmo salar L., caught by long-line in northern Norwegian waters. J Fish Biol 26(5):553–562. https://doi.org/10.1111/j.1095-8649.1985.tb04296.x
Hedger RD, Rikardsen AH, Strom JF, Righton DA, Thorstad EB, Naesje TF (2017) Diving behaviour of Atlantic salmon at sea: effects of light regimes and temperature stratification. Mar Ecol Prog Ser 574:127–140. https://doi.org/10.3354/meps12180
Ignatius S, Haapasaari P (2018) Justification theory for the analysis of the socio-cultural value of fish and fisheries: the case of Baltic salmon. Mar Policy 88:167–173. https://doi.org/10.1016/j.marpol.2017.11.007
Jacobsen JA, Hansen LP (2000) Feeding habits of Atlantic salmon at different life stages at sea. In: Mills D (ed) The Ocean Life of Atlantic Salmon: Environmental and Biological Factors Influencing Survival. Wiley-Blackwell, Oxford, pp 170–192
Jonsson N, Jonsson B, Hansen LP (1991) Energetic cost of spawning in male and female Atlantic salmon (Salmo salar L.). J Fish Biol 39(5):739–744. https://doi.org/10.1111/j.1095-8649.1991.tb04403.x
Klemetsen A, Amundsen PA, Dempson JB, Jonsson B, Jonsson N, O’Connell MF, Mortensen E (2003) Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol Freshw Fish 12(1):1–59. https://doi.org/10.1034/j.1600-0633.2003.00010.x
Kawabe R, Naito Y, Sato K, Miyashita K, Yamashita N (2004) Direct measurement of the swimming speed, tailbeat, and body angle of Japanese flounder (Paralichthys olivaceus). ICES J Mar Sci 61:1080–1087. https://doi.org/10.1016/j.icesjms.2004.07.014
Kristoffersen AB, Qviller L, Helgesen KO, Vollset KW, Viljugrein H, Jansen PA (2018) Quantitative risk assessment of salmon louse-induced mortality of seaward-migrating post-smolt Atlantic salmon. Epidemics 23:19–33. https://doi.org/10.1016/j.epidem.2017.11.001
Lacroix GL (2014) Large pelagic predators could jeopardize the recovery of endangered Atlantic salmon. Can J Fish Aquat Sci 71(3):343–350. https://doi.org/10.1139/cjfas-2013-0458
Lien S, Koop BF, Sandve SR, Miller JR, Kent MP, Nome T, Hvidsten TR, Leong JS, Minkley DR, Zimin A, Grammes F, Grove H, Gjuvsland A, Walenz B, Hermansen RA, von Schalburg K, Rondeau EB, Di Genova A, Samy JKA, Vik JO, Vigeland MD, Caler L, Grimholt U, Jentoft S, Vage DI, de Jong P, Moen T, Baranski M, Palti Y, Smith DR, Yorke JA, Nederbragt AJ, Tooming-Klunderud A, Jakobsen KS, Jiang XT, Fan DD, Liberles DA, Vidal R, Iturra P, Jones SJM, Jonassen I, Maass A, Omholt SW, Davidson WS (2016) The Atlantic salmon genome provides insights into rediploidization. Nature 533(7602):200. https://doi.org/10.1038/nature17164
Liu YJ, Olaussen JO, Skonhoft A (2011) Wild and farmed salmon in Norway-a review. Mar Policy 35(3):413–418. https://doi.org/10.1016/j.marpol.2010.11.007
MacKenzie KM, Trueman CN, Palmer MR, Moore A, Ibbotson AT, Beaumont WRC, Davidson IC (2012) Stable isotopes reveal age-dependent trophic level and spatial segregation during adult marine feeding in populations of salmon. ICES J Mar Sci 69(9):1637–1645. https://doi.org/10.1093/icesjms/fss074
Meador JP (2014) Do chemically contaminated river estuaries in Puget Sound (Washington, USA) affect the survival rate of hatchery-reared Chinook salmon? Can J Fish Aquat Sci 71(1):162–180. https://doi.org/10.1139/cjfas-2013-0130
Rasmussen M (2012) Diet of Atlantic salmon Salmo salar along the coast of Finnmark. Universitet i Tromsø UIT
Reddin DG, Friedland KD, Downton P, Dempson JB, Mullins CC (2004) Thermal habitat experienced by Atlantic salmon (Salmo salar L.) kelts in coastal Newfoundland waters. Fish Oceanogr 13:24–35. https://doi.org/10.1111/j.1365-2419.2004.00237.x
Rikardsen AH, Dempson JB (2011) Dietary life-support: the food and feeding of Atlantic salmon at sea. Wiley-Blackwell
Rikardsen AH, Righton D, Strøm JF, Thorstad EB, Gargan PG, Sheehan TF, Økland F, Chittenden C, Hedger RD, Næsje T, Renkawitz M, Sturlaugsson J, Caballero P, Baktoft H, Davidsen JG, Halttunen E, Wright S, Finstad B, Aarestrup, K (2021) Redefining the oceanic distribution of Atlantic salmon. Sci Rep 11 s
Salminen M, Erkamo E, Salmi J (2001) Diet of post-smolt and one-sea-winter Atlantic salmon in the Bothnian Sea. Northern Baltic J Fish Biol 58(1):16–35. https://doi.org/10.1111/j.1095-8649.2001.tb00496.x
Shephard S, Gargan P (2021) Wild Atlantic salmon exposed to sea lice from aquaculture show reduced marine survival and modified response to ocean climate. ICES J Mar Sci 78(1):368–376. https://doi.org/10.1093/icesjms/fsaa079
Skarðhamar J, Albretsen J, Sandvik AD, Lien VS, Myksvoll MS, Johnsen IA, Asplin L, Ådlandsvik B, Halttunen E, Bjorn PA (2018) Modelled salmon lice dispersion and infestation patterns in a sub-arctic fjord. ICES J Mar Sci 75(5):1733–1747. https://doi.org/10.1093/icesjms/fsy035
Storebakken T, Kvien IS, Shearer KD, Grisdale-Helland B, Helland SJ (1999) Estimation of gastrointestinal evacuation rate in Atlantic salmon (Salmo salar) using inert markers and collection of faeces by sieving: evacuation of diets with fish meal, soybean meal or bacterial meal. Aquaculture 172(3–4):291–299. https://doi.org/10.1016/s0044-8486(98)00501-8
Strøm J, Thorstad E, Chafe G, Sørbye S, Righton D, Carr J (2017) Ocean migration of pop-up satellite archival tagged Atlantic salmon from the Miramichi River in Canada. ICES J Mar Sci
Strøm JF, Thorstad E, Chafe G, Sørbye S, Righton D, Carr J (2019) Ocean predation and mortality of adult Atlantic salmon. Sci Rep 9:11. https://doi.org/10.1038/s41598-019-44041-5
Strøm JF, Thorstad EB, Hedger RD, Rikardsen AH (2018) Revealing the full ocean migration of individual Atlantic salmon. Anim Biotelemetry 6
Sturlaugsson J (2000) The food and feeding of Atlantic salmon (Salmo salar L.) during feeding and spawning migrations in Icelandic coastal waters The Ocean Life of Atlantic salmon - Environmental and Biological Factors influencing Survival. Fishing News Books, Oxford, p 193–209
Thorstad EB, Økland F, Finstad B, Sivertsgard R, Plantalech N, Bjørn PA, McKinley RS (2007) Fjord migration and survival of wild and hatchery-reared Atlantic salmon and wild brown trout post-smolts. Hydrobiologia 582:99–107. https://doi.org/10.1007/s10750-006-0548-7
Vogel EF, Biuw M, Blanchet MA, Jonsen ID, Mul E, Johnsen E, Hjollo SS, Olsen MT, Dietz R, Rikardsen A (2021) Killer whale movements on the Norwegian shelf are associated with herring density. Mar Ecol Prog Series 665:217–231. https://doi.org/10.3354/meps13685
Wilson SG, Block BA (2010) Habitat use in Atlantic bluefin tuna Thunnus thynnus inferred from diving behavior. Endanger Species Res 10(1–3):355–367
Waagbo R, Jorgensen SM, Timmerhaus G, Breck O, Olsvik PA (2017) Short-term starvation at low temperature prior to harvest does not impact the health and acute stress response of adult Atlantic salmon. PeerJ 5:22. https://doi.org/10.7717/peerj.3273
Acknowledgements
We thank Alta Laksefiskeri Interessentskap for providing facilities and field assistance during the study. We also thank all the salmon bag net fishermen in the Alta fjord and the students related to the Salmotrack project at UiT The Arctic University of Norway that contributed during the fieldwork. Karin Strand Johannessen, Cesilie Bye and Jens Olav Østerdal are thanked for doing the laboratory analyses of stomach samples.
Funding
Open access funding provided by Norwegian institute for nature research This study was funded by the Research Council of Norway (project 280308 SeaSalar), and the Tromsø Research Foundation and Alta Laksefiskeri Interessentskap.
Author information
Authors and Affiliations
Contributions
AH Rikardsen was responsible for project initiation and management. M Kjellman, EB Thorstad, JF Strøm and AH Rikardsen performed field work. All authors were involved in data analysis and manuscript production.
Corresponding author
Ethics declarations
Ethics approval
All handling and tagging was conducted according to the Norwegian regulations on treatment and welfare of animals (Directive 2010/63/EU of the European Parliament).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hedger, R.D., Kjellman, M., Thorstad, E.B. et al. Diving and feeding of adult Atlantic salmon when migrating through the coastal zone in Norway. Environ Biol Fish 105, 589–604 (2022). https://doi.org/10.1007/s10641-022-01269-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01269-x