Abstract
The huge tsunami associated with the 2011 Great East Japan Earthquake severely impacted the ecosystems on the Sanriku coast of Japan. The life history traits of ayu, Plecoglossus altivelis altivelis, which have a one-year amphidromous life history, were investigated by otolith analyses in two rivers of the Sanriku coast just after the tsunami compared with results before the tsunami to reveal the immediate impact of the tsunami. Hatching date compositions differed between the upstream migrants before and after the tsunami. The migrants after the tsunami were solely composed of fish hatched during October and November, whereas the migrants before the tsunami were mostly composed of fish hatched in September. Discrepancies between the hatching dates of the migrants and drifting larvae (hatched larvae) indicate that selective mortality of early-hatched fish occurred during the tsunami. Differences in otolith Sr:Ca ratios between upstream migrants before and after the tsunami suggest that fish surviving the tsunami inhabited saline water and early-hatched fish that inhabited the estuary decreased selectively in number because of the severe erosion around the river mouth. The oceanic growth period shortened in accordance with the change in hatching date. These results show that the tsunami drastically altered the ecology of P. altivelis altivelis on the Sanriku coast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tsunami are a recurring natural hazard that have repeatedly disturbed coastal ecosystems, such as sandy beaches, seagrass beds, coral reefs, mangrove forests and near shore terrestrial ecosystems (Cochard et al. 2008; Whanpetch et al. 2010; McAdoo et al. 2011). Considerable changes in community structure and decreases in abundance and species richness were observed after the huge tsunami associated with the 2004 Indian Ocean earthquake, the 2007 Peru earthquake and the 2010 Chile earthquake (Whanpetch et al. 2010; Lomovasky et al. 2011; Jaramillo et al. 2012). It has been suggested that tsunami have long-lasting effects on coastal ecosystems and that the restoration process is long-standing (Whanpetch et al. 2010; Jaramillo et al. 2012). It is essential to precisely estimate the impact of a tsunami on the local community to understand the ecosystem response.
The tsunami associated with the Great East Japan Earthquake, with a moment magnitude of 9.0, occurred on 11 March 2011 and struck the Pacific coast of northeastern Japan (Mori et al. 2011; Ozawa et al. 2011; Simons et al. 2011). The tsunami extensively inundated over 400 km2 of the coastal area and devastated a large area of the northeastern part of Japan together with co-seismic subsidence and eastward movement of the coast (Mori et al. 2011; Simons et al. 2011). The Sanriku coast is a ria, composed of many steep narrow bays; thus, the tsunami was amplified to about 40 m in maximum run-up height and severely destructed the coastal topography and structures (Mori et al. 2011; Tanaka et al. 2012).
Many studies conducted after the tsunami have focused on the ecological impacts caused by the 2011 tsunami and have demonstrated drastic changes in species composition and abundance of fish and benthic communities at various sites (Masuda 2012, 2013; Miura et al. 2012; Nakayama et al. 2013; Seike et al. 2013; Urabe et al. 2013). The tsunami similarly affected riverine macroinvertebrate communities far upstream from the river mouth during inundation (Watanabe et al. 2014). Some studies have reported that some species recolonized and other rare species invaded this area within several months after the tsunami (Masuda 2013; Seike et al. 2013; Watanabe et al. 2014).
Considering the physical disturbance to the coastal and riverine environments, the tsunami also affected fish ecology. However, few studies have been conducted about the impact of the tsunami on fish ecology, probably because of their mobility and the fragmented information available before the tsunami for comparative studies. We conducted a continuous ecological investigation of ayu Plecoglossus altivelis altivelis (Temminck et Schlegel, 1846) on the Sanriku coast before the tsunami. Thus, we performed a comparative study to reveal the ecological impacts of the 2011 tsunami on the P. altivelis altivelis population.
Plecoglossus altivelis altivelis are distributed throughout the Japanese archipelago and parts of China, Korea, and Vietnam (Hosoya 2002). They are one of the most important freshwater fisheries species in the Sanriku region. P.altivelis altivelis have a one-year amphidromous life history and migrate through rivers and the ocean during their life history (Tsukamoto 1988; Tsukamoto et al. 1989; Takahashi et al. 2002; Azuma et al. 2003; Otake et al. 2005); larvae hatch in the river and immediately drift downstream to the ocean during autumn and winter. Then, they migrate among habitats such as the surface offshore, the surf zone, brackish water, and the bottom of an estuary to grow until entering the river during the next spring and summer. They continue to grow in the river and eventually mature and spawn in autumn and winter. The P. altivelis altivelis larvae and juveniles were in their oceanic habitat in March, when the tsunami occurred, which was reportedly the most destroyed area along the coast (Tanaka et al. 2012). Therefore, it was presumed that the P. altivelis altivelis population in the Sanriku region suffered directly from the physical disturbance caused by the tsunami.
The aim of this study was to clarify the immediate impact of the 2011 tsunami on the local population of P. altivelis altivelis in the Sanriku region. The life history traits of P. altivelis altivelis that survived the tsunami were investigated by otolith analyses and the results were compared with those obtained from populations before the tsunami to reveal how the tsunami impacted the P. altivelis altivelis population. Daily rings were formed in P. altivelis altivelis otoliths (Tsukamoto and Kajihara 1987), and the hatch date was precisely determined by subtracting the number of otolith increments from the collection date. Furthermore, the changes in the timing of upstream migration were examined by combining the increment and otolith Sr:Ca ratio analysis. The diadromous migratory history of fish can be reconstructed based on chronological changes in the otolith Sr:Ca ratio, which is correlated with salinity and is useful for estimating past freshwater, estuarine, and marine habitats (Secor et al. 1995; Otake and Uchida 1998; Campana 1999; Secor and Rooker 2000). We integrated these results to describe the ecological changes in the P. altivelis altivelis population immediately after the tsunami.
Materials and methods
Study sites
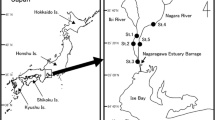
The life history traits of P. altivelis altivelis (hatching date, daily age, body size, date of upstream migration, spawning period, and number of drifting larvae) before and after the tsunami were investigated in two rivers (the Sakari River and the Unosumai River) located on the Sanriku coast (Fig. 1). The Sakari River flows into Ofunato Bay and is the largest river among the three rivers flowing into the bay. A water break that was installed at the entrance to the bay was completely destroyed by the tsunami. This changed the bay environment, such as water currents, after the tsunami. The Unosumai River flows into Otsuchi Bay with two adjacent rivers. A sandbar developed around the river mouth and formed a brackish water embayment with a small opening and a surf zone before the tsunami. However, the sandbar was completely eroded by the tsunami, and the brackish water area and surf zones disappeared.
Maps of Japan and the study area showing the sampling sites in the two rivers on the Sanriku coast of Iwate Prefecture, northern Japan. Squares in Ofunato Bay and Otsuchi Bay indicate the water-break installed at the bay entrance and the sandbar that developed around the river mouth, respectively, both of which were disappeared after the tsunami
Fish collection
About 3 months after the tsunami, Plecoglossus altivelis altivelis that migrated up the Sakari (7 June 2011) and Unosumai (11, 20 June 2011) rivers (upstream migrants) were collected with cast nets within 2–4 km upstream from the river mouth (Fig.1). The P. altivelis altivelis had hatched during the previous year of upstream migration; thus, the migrants collected in 2011 experienced the tsunami during their oceanic period. P. altivelis altivelis migrants had also been collected by the same method in both rivers before the tsunami (the Sakari River: 7 May and 18 June 2009, 7 June 2010, and the Unosumai River: 23 June 2010). Those fish represented the local population because upstream migrations of P. altivelis altivelis usually stop before the middle to late of June on the Sanriku coast (Hata et al. unpublished data). Among the upstream migrants collected in 2009 and 2010, the stocked fish that were artificially produced at hatcheries were morphologically distinguished by observing the arrays of lateral line organs under the lower jaw and counting the number of scales above lateral line. The stocked fish have irregular numbers of lateral line organs (usually 0–5) under the lower jaw with asymmetrical alignment and fewer scales (15–16) above the lateral line. In contrast, the wild fish have four pairs of symmetrical aligned lateral line organs under the lower jaw and more scales (18–21) (Aizawa and Nakagawa 2008). The fish distinguished as stocked were excluded from analyses. All fish collected in 2011 were considered wild because artificially produced P. altivelis altivelis were not stocked in any of the Sanriku coast rivers in 2011 because of severe damage to the riverine environment and hatcheries caused by the earthquake and tsunami. The wild fish were measured for standard length (SL) and body weight (BW) to the nearest 0.1 mm and 0.1 g, respectively in a fresh state and were stored in 99 % ethanol. Then the otoliths were extracted for otolith microstructure and microchemistry analyses.
Plecoglossus altivelis altivelis drifting larvae were collected from the Sakari and the Unosumai rivers between early September and late December at 2-weeks intervals from 2008 to 2011 (2008 data available only for the Sakari River). The collection sites in both rivers were located at the lowest reach of freshwater region about 1.8 km upstream from the river mouth (Fig. 1). A conical plankton net (mouth diameter: 56 cm, mesh size: 0.33 mm) was set on the bottom at the center of flow for 3–5 min every hour during 17:00–24:00 to collect drifting larvae. We occasionally collected for only 20 s when there were large numbers of drifting larvae. The collected samples were preserved immediately in 99 % ethanol after collection. Filtering volume during all collections was estimated using a flow meter attached to the net mouth. The number of P. altivelis altivelis larvae collected was counted and the density (number of larvae m−3 h−1) was estimated using the filtered volume for each collection. Total flow rate (m3 h −1) was determined for each collection day by summing the flow rates in 1 m subsections across the river and was calculated based on water depth and current velocity measured with an electro-magnetic current meter (AEM1-D, JFE Advantech, Tokyo, Japan). Flow rate was also calculated at the time of collection in a 1 m subsection where the net was set, and total flow rate at the time was estimated proportionally. Then, the number of drifting larvae per second was calculated using larval density and estimated total flow rate, assuming that larval density was constant among all subsections of the river. The cumulative number of larvae drifting during 17:00–24:00 was determined and divided by 0.85 to obtain the total number of drifting larvae in a day, because 85 % of the larvae drifted down the river between 17:00 and 24:00 each day (Hata et al. unpublished data). Finally, the total number of drifting larvae during one spawning season was estimated assuming a linear correlation between collection days. The date that larvae drifting down the river was considered the hatching date in both rivers, as larvae drifted 1–3 days after hatching (Hata et al. unpublished data).
Otolith analyses
An otolith increment analysis was performed to determine daily age and hatching date of the upstream migrants. The sagittal otolith from each fish was embedded in epoxy resin (Epofix, Struers; Ballerup, Denmark), mounted on a glass slide, and the sagittal section was ground to expose the core using a grinding machine equipped with 70-μm and 13-μm diamond cup-wheels (Diskoplan-TS; Struers). The ground surface was polished with an active oxide polishing suspension (OP-S suspension, Struers) on a polishing wheel with a semi-automatic specimen mover (MD-Chem and RotoPol-35 equipped with PdM-Force-20; Struers). The daily age of each fish was determined by counting the daily rings under a binocular microscope connected to a digital camera and a computer system with image analysis software (Jiseki ARP/W ver. 5.20; Ratoc System Engineering, Tokyo, Japan). Only the increments outside of the hatch check, which is a marked increment formed at hatching, were counted. The hatch check was observed at about 14-μm from the core in otolith of P. altivelis altivelis from the Sanriku area (Hata et al. unpublished data). The hatching date of each fish was back-calculated from daily age and date of capture.
The otolith Sr:Ca ratio was determined to estimate daily age, body size, and date at first freshwater entry. Each otolith surface was cleaned with Milli-Q water, air dried, and coated with Pt-Pd in an ion-sputter (E-1010; Hitachi, Tokyo, Japan). Sr and Ca concentrations were measured in all aged specimens along the line of the increment analysis using a wave length dispersive X-ray electron microprobe (JXA-8900R or JXA-8230; JEOL, Tokyo, Japan). The accelerating voltage and beam current were 15 kV and 12 nA, respectively. The electron beam was focused on a 4-μm diameter point, with measurements spaced at 4-μm intervals, and counting time was 4 s. Strontianite (SrTiO3) and calcite (CaCO3) were used as standards. The otolith Sr:Ca ratio was determined as a weight percent ratio. Datum of each analytical point is presented as a moving average of three consecutive points, which is equivalent to five to seven days. All daily rings were assigned to the nearest analytical point, and the daily Sr:Ca ratio was determined.
The analytical point indicating first freshwater entry was defined as the first point where the Sr:Ca ratio decreased to less than 2.4, because mean otolith Sr:Ca ratios of P. altivelis altivelis reared in freshwater for two months are 1.40–2.34 (Hata et al. unpublished data). Fish in which a drop in the Sr:Ca ratio was not observed were considered not to have completely shifted their habitat from marine to freshwater, and were excluded from all comparisons. The nearest daily ring assigned to the analytical point indicating first freshwater entry was regarded as precipitated on the day of freshwater entry, and the otolith radius, daily age, and date were considered first freshwater entry. When the lines used for the electron probe micro-analysis (EPMA) and increment analysis did not correspond, the distance from the core to the analytical point indicating freshwater entry was proportionally corrected on the incremental analysis line.
SL at first freshwater entry was further estimated assuming the allometric relationship between SL and otolith radius (OR), which is denoted as: SL = aORb, where a and b are constants. The biological intercept (Campana 1990) was incorporated into the model to determine the constants separately and the following simultaneous equations were resolved: SL0 = aOR0 b and SLC = aORC b, where SL0 and OR0 are biological intercepts, and SLC and ORC are SL and OR at capture, respectively. SL of larvae artificially hatched at 15 °C (6.3 mm, Hata et al. unpublished data) was used for the SL0. OR0 was the radius of hatch check of each individual. Then, the SL at freshwater entry was individually back-calculated using the formula and otolith radius at freshwater entry determined by EPMA.
The otolith Sr:Ca ratios were further analyzed to estimate the salinity conditions experienced by the upstream migrants around the time the tsunami occurred. The Sr:Ca ratios of the rings assigned to 11 March were compared between upstream migrants in 2010 and 2011 for the Sakari and Unosumai rivers. The characteristics of the upstream migrants surviving the tsunami were verified based on the assumption that the oceanic migratory patterns were similar among years.
Water temperature
Water temperature in the Sakari River from June 2008 to May 2011 was provided by the Center for Public Works of the Ofunato Regional Development Bureau, Iwate prefecture. Water temperature was recorded monthly between December and April and bimonthly between June and October. Water temperature in the Unosumai River was measured using a data-logger (Hobo water temp pro; Climatec Inc., Tokyo, Japan) at one-hour intervals in the freshwater region about 4 km upstream from the river mouth from June 2010 to May 2011 (Fig.1).
Daily seawater temperature data in Ofunato Bay and Otsuchi Bay were provided at the Iwate Fisheries Technology Center website (www.suigi.pref.iwate.jp) and by the International Coastal Research Center of the Atmosphere and Ocean Research Institute, the University of Tokyo, respectively. As the sensors installed in Otsuchi Bay were lost during the tsunami, Otsuchi Bay temperature data after 11 March 2011 were not available.
Water temperatures when the 2011 upstream migrants inhabited the ocean (1 September 2010 to 11 March 2011) were 7.0–23.2 °C (mean ± standard deviation [SD], 14.3 ± 5.53 °C) in Ofunato Bay and 7.1–23.3 °C (14.3 ± 5.40 °C) in Otsuchi Bay with a downward trend (Fig.2). During that period, mean seawater temperatures during September–November were about 2 °C higher than those in previous years. Seawater temperatures in Ofunato Bay during April and May 2011 corresponding to the upstream migration period were 6.2–11.5 °C and were significantly higher than those recorded during the previous two years (Steel-Dwass test, p < 0.01 for 2009 and p < 0.05 for 2010) (Fig.2).
Seawater temperatures in Ofunato Bay (a) and Otsuchi Bay (b), and river water temperatures in the Sakari River (c) and the Unosumai River (d) during June 2008 to February or May 2011. Seawater temperature was not measured after March 2011 in Otsuchi Bay because of the loss of a temperature sensor in the 2011 tsunami. Blue line, red line and green line indicate temperatures in June 2008–May 2009, June 2009–May 2010 and June 2010–May 2011, respectively
Results
Changes in the hatching date of upstream migrants
The 2011 upstream migrants were composed completely of fish that hatched after October (later-hatched fish) in both rivers, whereas fish that hatched in September (early-hatched fish) constituted the majority of upstream migrants before the tsunami (Fig. 3). The 2011 hatching dates of the upstream migrants in the Sakari River ranged from 3 October to 18 November, and the mean hatching date was significantly later than those of the 2009 and 2010 upstream migrants (1 September to 17 October, mean: 26 September; 8 September to 20 October, mean: 28 September, respectively) (Steel-Dwass test, p < 0.01) (Table 1). The later-hatched fish constituted a minor portion of the 2009 and 2010 upstream migrants. In contrast, fish that hatched in September, which constituted 54 % of the 2009 upstream migrants and 52 % of the 2010 upstream migrants, were not detected. Similar results were found for upstream migrants in the Unosumai River. The hatching dates of the 2011 upstream migrants in the Unosumai River ranged from 6 October to 4 November (Table 1). The later-hatched fish solely constituted the 2011 upstream migrants, although they occupied a minor portion of the 2010 migrants (38 %). In contrast, fish that hatched in September, which constituted 62 % of the 2010 upstream migrants, were not detected. As a consequence, the mean hatching date in 2011 (16 October) was significantly later than that in 2010 (29 September; Welch’s t-test, p < 0.01).
Distribution of numbers of larvae drifting down the rivers every 10 days during the reproductive season (white bar), and the hatching periods of upstream migrants (black bar) in the 2008 (a), 2009 (b), and 2010 (c) hatching groups for the Sakari River and in the 2009 (d) and 2010 (e) hatching groups for the Unosumai River. Hatched larvae drifted down river just after hatching, and the date of larval collection was almost the same as each hatch date. The 2008, 2009, and 2010 hatching groups were pre-tsunami year groups, and the 2010 hatching group was a post-tsunami year group
Discrepancies between the hatching date composition of upstream migrants and drifting larvae were found within the same cohort in 2011 in both rivers (Fig. 3). In the Sakari River, the total number of drifting larvae was estimated to be 432.4 x 106 during the 2010 spawning period, with a peak in mid-October (Table 2). The peak was included in those of 2008 and 2009 which were early-mid October and late October, respectively. Despite the considerable number of larvae that hatched in September (6.6 %) of the 2010 spawning season, they did not appear in the 2011 migrants. This result contrasted to the cohort before the tsunami, in which the hatching date of drifting larvae and upstream migrants were conversely related; early-hatched fish were a minor portion of drifting larvae but were a high proportion of upstream migrants (Fig. 3). The total number of drifting larvae in the Unosumai River was estimated to be 257.2 x 106 during the 2010 spawning period, with a peak in late September (Table 2), and 23.3 % of the larvae drifted down river in September, but none of the larvae that hatched in September were found in the upstream migrant sample (Fig. 3).
Changes in the timing of the upstream migration
The timing of upstream migration also changed after the tsunami. The first freshwater entry of upstream migrants into the Sakari River occurred 182–244 days (210.1 ± 17.4 days) after hatching (Fig. 4) and at a SL of 64.4–93.6 mm (76.2 ± 7.2 mm) (Table 1). This daily age at first freshwater entry was significantly different from the upstream migrants in the years before the tsunami (203–260 days [231.2 ± 13.5 days] in 2009; 206–254 days [229.4 ± 12.7 days] in 2010) (Steel-Dwass test, p < 0.01). In addition, SL at first freshwater entry in 2011 was significantly smaller than that in the upstream migrants before the tsunami (64.2–107.6 mm [80.8 ± 10.0 mm] in 2009 and 62.1–95.1 mm [84.9 ± 8.0 mm] in 2010) (Steel-Dwass test, p < 0.01). A similar tendency was found in the Unosumai River. Daily age at first freshwater entry was significantly younger in the 2011 upstream migrants than in those before the tsunami (189–245 days [221.4 ± 17.6 days] in 2011and 228–259 days [242.1 ± 11.3 days] in 2010) (Welch’s t-test, p < 0.01). However, SL at first freshwater entry was not significantly different (82.7–121.4 mm [96.5 ± 10.6 mm] in 2011and 84.3–110.7 mm [100.5 ± 8.2 mm] in 2010) (Table 1, Fig. 4).
In contrast, the dates at first freshwater entry were not significantly different among years before and after the tsunami, despite the significant differences in fish age and size at freshwater entry. The upstream migrations stopped in late May to early June in the Sakari River and in mid-June in the Unosumai River, whereas the migration commencement dates varied slightly.
Salinity environment that the 2011 tsunami survivors experienced
Despite the expected huge disturbances by the tsunami, no instantaneous change in Sr:Ca ratios were observed in the 2011 upstream migrants (Fig. 5b). The Sr:Ca ratios of the 11 March 2011 upstream migrants were 4.40–9.93 (7.35 ± 1.19) in the Sakari River and 5.51–7.85 (6.88 ± 0.73) in the Unosumai River (Fig. 6a, b). These Sr:Ca ratios were significantly higher than those for the corresponding date in the previous year (11 March 2010) for the 2010 upstream migrants (3.56–8.42 [5.82 ± 1.18] in the Sakari River and 3.58–8.01 [(5.08 ± 1.09) in the Unosumai River) (Welch’s t-test, p < 0.01) (Figs. 5a, b and 6a, b). These results indicate that juveniles distributed under relatively higher salinity conditions around the time of the tsunami survived and migrated into the rivers as a major component of upstream migrants. In other words, the juveniles that had moved into brackish area were selectively sacrificed by the tsunami.
Spawning period and number of drifting larvae
Drifting larvae were collected throughout the 2011 sampling period (early October to early November) in both rivers, although the total numbers of drifting larvae and the peak periods were not comparable with the years before the tsunami, because of the lack of sampling in September and December. We estimated that most larvae drifted down the rivers in mid- October. A total of 34.7 x 106 larvae drifted down the Unosumai River (Table 2). This corresponded to 31 % and 40 % of the drifting larvae during the peak drifting periods in 2008 and 2010, but was larger than the number in 2009, probably because of a severe typhoon disturbance on the spawning grounds. Similar results were obtained in the Sakari River.
Discussion
Our results show typical upstream migration of Plecoglossus altivelis altivelis despite the huge tsunami associated with the 2011 Great East Japan Earthquake. The upstream migrants were certainly fish that survived the tsunami rather than fish recruited from other regions. Distribution of P. altivelis altivelis are restricted to areas within 2.5 km offshore (Tsukamoto et al. 1989; Tago 2002; Azuma 2004) or at most 20 km off the coast (Azuma et al. 2002) during the oceanic period. This distributional pattern probably prevented long-distance recruitment from a region not exposed to the tsunami. Therefore, it is conceivable that the tsunami did not extinguish the local P. altivelis altivelis population, despite the marked decrease in fish abundance after the tsunami (Masuda 2013; Nakayama et al. 2013) and severe destruction of the coastal environment and structure around the study area (Mori et al. 2011; Tanaka et al. 2012).
However, life history traits of the 2011 upstream migrants that experienced the tsunami were distinct from those in 2010; they were composed of later-hatched fish that migrated upstream at a significantly younger and smaller size than migrants before the tsunami. This substantial change was the result of a difference in hatching date composition. The hatching date discrepancies observed within the same cohort (i.e. between drifting larvae in 2010 and upstream migrants in 2011) and between before and after the tsunami clearly suggest that early-hatched fish suffered selective high mortality during the tsunami. In contrast, early-hatched fish in the Sanriku region before the tsunami typically showed lower mortality than that of later-hatched fish and represented the majority of upstream migrants, as shown in Fig. 3. These comparisons suggest that the direct effect of the tsunami on the P. altivelis altivelis population was selective mortality of early-hatched fish.
This selective mortality observed in 2011 was probably the result of physical disturbances caused by the tsunami, rather than the unsuitable environment before the tsunami. Seawater temperature potentially affects survival of P. altivelis altivelis. Previous studies have reported that the hatching date composition of P. altivelis altivelis can be altered during their oceanic period by unusually high water temperature causing differential mortality based on hatching date (Takahashi et al. 1999, 2003). In the 2010 P. altivelis altivelis cohort, which was disturbed by the tsunami, seawater temperature potentially affected the selective mortality during their oceanic period because lethal seawater temperatures for P. altivelis altivelis larvae (>20 °C; Ito et al. 1967, 1968, 1971; Takahashi et al. 2003) were observed in Ofunato Bay and Otsuchi Bay during September–November 2010. However, considerable numbers of P. altivelis altivelis larvae and juveniles hatched during late September to late November were collected from the surf zone, sandy beach, and offshore area in Ofnato Bay and Otsuchi Bay during October 2010 –February 2011 (Hata et al. unpublished data). And the fish collected before December was majorly composed of early hatched one. The high seawater temperature persisted through the 2010 P. altivelis altivelis spawning period, its effect on the mortality may have been similar among different hatching groups.
Considering the P. altivelis altivelis migratory pattern, differences in habitat depended on the hatching date resulting in selective mortality within the 2010 cohort. Generally, P. altivelis altivelis that hatch earlier in the spawning season migrate earlier between habitats and then migrate into the river at a younger age than later-hatched larvae (Tsukamoto 1988; Tsukamoto et al. 1989; Takahashi et al. 2002). This migratory pattern can be applied to P. altivelis altivelis population in the Sanriku region (Hata et al. unpublished data). The otolith Sr:Ca ratio of the 2011 upstream migrants indicated that they inhabited more saline water than the upstream migrants before the tsunami around the date of the tsunami. This suggests that fish surviving the tsunami had not yet moved to brackish water or the estuary from offshore or the surf zone. Therefore, although the distributions of P. altivelis altivelis in the bays were not estimated completely, it is assumed that the early-hatched fish assembled around the estuary when the tsunami occurred and decreased dramatically.
The otolith analysis further revealed the subsequent effects of selective mortality by the tsunami. The 2011 upstream migrants migrated into the rivers at a younger age and thus had a shorter oceanic growth period than those of the 2010 migrants in both rivers. The upstream P. altivelis altivelis migration period has been suggested to be regulated by seawater temperature, as the fish begin to migrate upstream when seawater temperature increases to 10 °C (Nakamura and Kasuya 2004). Additionally, termination of the upstream migration is probably regulated by seawater temperature, because the upstream migration of P. altivelis ryukyuensis, a subspecies of P. altivelis altivelis, continues until seawater temperature reaches 24 °C (Kishino and Shinomiya 2003). Although later-hatched fish tend to migrate into the river later in the season (Tsukamoto 1988), the later-hatched young juveniles were forced to migrate into the river by the increasing water temperature, resulting in a similar upstream migration period as that observed before the tsunami.
Concurrently, P. altivelis altivelis migrated into the Sakari River in 2011 at a smaller size than those in 2010. This decrease in body size was probably caused by the shortened oceanic period described above, rather than a decrease in the growth ratio. However, no change in body size at the time of the upstream migration was observed in 2011 in the Unosumai River, despite that the fish migrated at a younger age. We hypothesized that unknown ecological changes after the tsunami, such as changes in nutrients or primarily production, favored growth of juveniles. Such a difference in the response to the tsunami was slight between the two studied rivers, suggesting that restoration of the P. altivelis altivelis populations will differ between the rivers.
The environmental disturbance caused by a disaster such as a tsunami can initiate an extinction vortex of a local population through various effects, including reduced population size, destruction of habitat, and declines in genetic diversity (Gilpin and Soulé 1986). The observed ecological changes in P. altivelis altivelis experiencing the tsunami could trigger a decrease in the size of the P. altivelis altivelis population in the Sanriku region. Considering the physical disturbance with co-seismic subsidence (Simons et al. 2011; Tanaka et al. 2012) and the drastic changes in coastal morphology in the study area, the tsunami certainly destroyed preferred habitat of P. altivelis altivelis larvae and juveniles along the Sanriku coastal sea. The disappearance of early-hatched fish will delay the spawning period because early-hatched fish tend to mature and spawn earlier in the spawning season (Otake et al. 2005). This expected delay also suggests that reduced spawning activity will occur and that ecological changes caused by the tsunami will be inherited by descendants.
Another concern is a decline in genetic diversity. Artificially produced P. altivelis altivelis seedlings are frequently stocked in the rivers throughout Japan, including the Sanriku region, to enhance the resource. The decrease in wild fish could enhance hybridization between hatchery-reared and wild fish (Kaewsangk et al. 2000; Otake et al. 2002) and displace the wild genotype. Assuming these substantial effects, the impact of the tsunami revealed in the present study could potentially shrink the local P. altivelis altivelis population. Continuous studies are required to reveal the temporal ecological changes after the tsunami in relation to population dynamics, such as growth, spawning activity, and genetic diversity.
Our results clearly show the immediate impacts of the huge tsunami on the populations of the amphidromous fish, P. altivelis altivelis. Diverse diadromous fish species, such as Rhinogobius spp., Japanese fluvial sculpin Cottus pollux (Gunther, 1873), chum salmon Oncorhynchus keta (Walbaum, 1792) and masu salmon Oncorhynchus masou (Brevoort, 1856), are distributed in the Sanriku region and are important components of local ecosystems and fisheries. The tsunami also likely impacted those species through direct disturbance and habitat destruction. The extent of damage to other fish and benthic animals caused by the tsunami and their restoration vary depending on the species (Masuda 2013; Nakayama et al. 2013; Seike et al. 2013; Watanabe et al. 2014). Therefore, comprehensive studies about how the 2011 tsunami impacted coastal ecosystems will contribute to a full understanding of the vulnerability of local ecosystems to disturbances and the ecological processes forming local community structure. Furthermore, these studies will help establish the basis for precise ecological forecasting in response to future anthropogenic disturbances, natural disasters and climate change.
References
Aizawa Y, Nakagawa K (2008) Examination of bio-production and suitable stock size of ayu, Plecoglossus altivelis altivelis in Hayakawa River. Bull Kanagawa Pref Technol Center for Fish 3:79–85 (in Japanese)
Azuma K (2004) The distributional ecology of the ayu in the marine life period. Bull Mar Sci Fish Kochi Univ 23:59–112
Azuma K, Hiraga H, Horiki N, Taniguchi N (2002) Distribution of ayu, Plecoglossus altivelis larvae in surf zones in the central area of Wakayama prefecture, Japan. Suisanzoshoku 50:9–15 (in Japanese)
Azuma K, Takahashi I, Fujita S, Kinoshita I (2003) Recruitment and movement of larval ayu occurring in the surf zone of a sanding beach facing Tosa Bay. Fish Sci 69:355–360
Campana SE (1990) How reliable are growth back-calculations based on otoliths? Can J Fish Aquat Sci 47:2219–2227
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297
Cochard R, Ranamukhaarachchi SL, Shivakoti GP, Shipin OV, Edwards PJ, Seeland KT (2008) The 2004 tsunami in Aceh and southern Thailand: a review on coastal ecosystems, wave hazards and vulnerability. Perspect Plant Ecol Evol Syst 10:3–40
Gilpin ME, Soulé ME (1986) Minimum viable populations: processes of species extinction. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer, Sunderland, MA, pp. 19–34
Hosoya K (2002) Plecoglossidae in: Nakabo T (ed.) fishes of Japan with pictorial keys to the species, English edn. Tokai University Press, Tokyo, p. 297 (in Japanese)
Ito T, Iwai T, Furuichi T (1967) Studies on the artificial production of ayu fish seeding XXXIII the early spawning experiment and the perfect culture of ayu fish. Res Rep Kisosansen Surv Team 4:359–501 (in Japanese)
Ito T, Iwai T, Furuichi T (1968) Studies on the artificial production of ayu seeding, LXI the influence of water temperature on the early survival of yolk-sac fry of ayu fish. Res Rep Kisosansen Surv Team 5:571–584 (in Japanese)
Ito T, Iwai T, Furuichi T (1971) Studies on the artificial production of ayu seeding, LXXV influence of salinity and temperature of rearing water upon the early survival and growth of yolk-sac fry. Art Propagation Ayu Fish 1:165–185 (in Japanese)
Jaramillo E, Dugan JE, Hubbard DM, Melnick D, Manzano M, Duarte C, Campos C, Sanchez R (2012) Ecological implications of extreme events: footprints of the 2010 earthquake along the Chilean coast. PLoS One 7:e35348
Kaewsangk K, Hayashizaki KI, Asahida T, Ida H (2000) An evaluation of the contribution of stocks in the supplementation of ayu Plecoglossus altivelis in the Tohoku area, using allozyme markers. Fish Sci 66:915–923
Kishino T, Shinomiya A (2003) Upstream migration of Ryukyu-ayu Plecoglossus altivelis ryukyuensis in the Yakugachi River, Amami-Oshima Island, Japan. Nippon Suisan Gakkaishi 69:624–631 (in Japanese with English abstract)
Lomovasky BJ, Firstater FN, Salazar AG, Mendo J, Iribarne OO (2011) Macro benthic community assemblage before and after the 2007 tsunami and earthquake at Paracas Bay, Peru. J Sea Res 65:205–212
Masuda R (2012) Underwater visual census of fish assemblages in Moune Bay, Kesennuma. Aquabiology 34:562–568 (in Japanese with English abstract)
Masuda R (2013) Underwater visual census of fish and invertebrate assemblages in Moune, Kesennuma after the disturbance by tsunami. Aquabiolody 35:587–591 (in Japanese with English abstract)
McAdoo BG, Ah-Leong JS, Bell L, Ifopo P, Ward J, Lovell E, Skelton P (2011) Coral reefs as buffers during the 2009 South Pacific tsunami, Upolu Island, Samoa. Earth-Sci Rev 107:147–155
Miura O, Sasaki Y, Chiba S (2012) Destruction of populations of Batillaria attramentaria (Caenogastropoda: Batillariidae) by tsunami waves of the 2011 Tohoku earthquake. J Mollus Stud 78:377–380
Mori N, Takahashi T, Yasuda T, Yanagisawa H (2011) Survey of 2011 Tohoku earthquake tsunami inundation and run-up. Geophys Res Lett 38:L00G14
Nakamura T, Kasuya K (2004) Forecasting of the first ascending day and the number of ascending schools of amphidromous ayu Plecoglossus altivelis altivelis in the Naka River, Tochigi prefecture, Central Japan. Nippon Suisan Gakkaishi 70:288–296 (in Japanese with English abstract)
Nakayama K, Kumagai Y, Tanaka M (2013) Recovery process of demersal fish assemblage of Moune Bay, Kesennuma, after earthquake disaster. Aquabiology 35:582–586 (in Japanese with English abstract)
Otake T, Uchida K (1998) Application of otolith microchemistry for distinguishing between amphidromous and non-amphidromous stocked ayu, Plecoglossus altivelis. Fish Sci 64:517–521
Otake T, Yamada C, Uchida K (2002) Contribution of stocked ayu (Plecoglossus altivelis altivelis) to reproduction in the Nagara River, Japan. Fish Sci 68:948–950
Otake T, Tanaka H, Hashimaru D, Mizutani Y, Masuda M, Mizoguchi Y, Ye H, Kondo Y, Takasaki Y, Sugahara H, Yamada C (2005) Early life history of amphidromous ayu, Plecoglossus altivelis altivelis: the relationship between their early life history in coastal waters and their spawning. The 7th Indo-Pacific Fish conference Abstract book, p 180
Ozawa S, Nishimura T, Suito H, Kobayashi T, Tobita M, Imakiire T (2011) Coseismic and postseismic slip of the 2011 magnitude-9 Tohoku-Oki earthquake. Nature 475:373–376
Secor DH, Rooker JR (2000) Is otolith strontium a useful scalar of life cycles in estuarine fishes? Fish Res 46:359–371
Secor DH, Henderson-Arzapalo A, Piccoli PM (1995) Can otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes? J Exp Mar Biol Ecol 192:15–33
Seike K, Shirai K, Kogure Y (2013) Disturbance of shallow marine soft-bottom environments and megabenthos assemblages by a huge tsunami induced by the 2011 M90 Tohoku-Oki earthquake. PLoS One 8:e65417
Simons M, Minson SE, Sladen A, Ortega F, Jiang J, Owen SE, Meng L, Ampuero JP, Wei S, Chu R, Helmberger DV, Kanamori H, Hetland E, Moore AW, Webb FH (2011) The 2011 magnitude 9.0 Tohoku-Oki earthquake: mosaicking the megathrust from seconds to centuries. Science 332:1421–1425
Tago Y (2002) Larval distribution of ayu Plecoglossus altivelis in the surface layer of estuary regions in Toyama bay. Nippon Suisan Gakkaishi 68:61–71 (in Japanese with English abstract)
Takahashi I, Azuma K, Hiraga H, Fujita S (1999) Different mortality in larval stage of ayu Plecoglossus altivelis by birth dates in the Shimanto estuary and adjacent coastal waters. Fish Sci 65:206–210
Takahashi I, Azuma K, Fujita S, Kinoshita I (2002) Habitat shift of ayu Plecoglossus altivelis altivelis in early stages from waters adjacent to the bank to the center of flow in the Shimanto estuary. Fis Sci 68:554–559
Takahashi I, Azuma K, Fujita S, Kinoshita I, Hiraga H (2003) Annual changes in the hatching period of the dominant cohort of larval and juvenile ayu Plecoglossus altivelis altivelis in the Shimanto estuary and adjacent coastal waters during 1986–2001. Fish Sci 69:438–444
Tanaka H, Tinh NX, Umeda M, Hirao R, Pradjoko E, Mano A, Udo K (2012) Coastal and estuarine morphology changes induced by the 2011 great East Japan earthquake tsunami. Coast Eng J 54:1250010
Tsukamoto K (1988) Migratory mechanisms and behavioral characteristics in ayu. In: Ueno T, Okiyama M (eds) Ichthyology currents. Asakura Publishing, Tokyo, pp. 100–133
Tsukamoto K, Kajihara T (1987) Age-determination of ayu with otolith. Nippon Suisan Gakkaishi 53:1985–1997
Tsukamoto K, Mochizuki K, Otake T, Yamazaki Y (1989) Distribution, migration and growth of ayu larvae at the mouth of river Kumano. Fish Engineer 50:47–57
Urabe J, Suzuki T, Nishita T, Makino W (2013) Immediate ecological impacts of the 2011 Tohoku earthquake tsunami on intertidal flat communities. PLoS One 8:e62779
Watanabe K, Yaegashi S, Tomozawa H, Koshimura S, Omura T (2014) Effects on river macroinvertebrate communities of tsunami propagation after the 2011 great East Japan earthquake. Freshw Biol 59:1474–1483
Whanpetch N, Nakaoka M, Mukai H, Suzuki T, Nojima S, Kawai T, Aryuthaka C (2010) Temporal changes in benthic communities of seagrass beds impacted by a tsunami in the Andaman Sea, Thailand. Estuar Coast Shelf Sci 87:246–252
Acknowledgments
We thank to K. Kawasaki and Y. Sato of the Fisherman’s Cooperative Associations of the Unosumai River and Sakari River, respectively, and Y. Amano of the Atmosphere and Ocean Research Institute (AORI), the University of Tokyo, for their help with field sampling before and after the 2011 tsunami disaster. We also thank to M. Otsuki and K. Suzuki of AORI for their help with otolith microchemistry. This study was supported, in part, by JSPS KAKENHI Grant Numbers 21380122 and 24380105 to T. Otake and the Tohoku Ecosystem-Associated Marine Science (TEAMS) project. This study followed the animal experimental use guidelines of The University of Tokyo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hata, M., Kawakami, T. & Otake, T. Immediate impact of the tsunami associated with the 2011 Great East Japan Earthquake on the Plecoglossus altivelis altivelis population from the Sanriku coast of northern Japan. Environ Biol Fish 99, 527–538 (2016). https://doi.org/10.1007/s10641-016-0495-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-016-0495-8