Abstract
Malignant tumors represent an important cause of mortality within the global population. Tumor angiogenesis, recognized as one of the key hallmarks of malignant tumors, is crucial for supplying essential nutrients and oxygen for tumor growth. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) are key drivers of tumor angiogenesis. Targeted therapeutic interventions not only effectively inhibit tumor growth by specifically blocking tumor angiogenesis but have also made breakthroughs in the treatment of malignant tumors. Fruquintinib, an anti-angiogenic small molecule drug developed independently in China, functions as a potent tyrosine kinase inhibitor with high selectivity. It effectively curtails tumor growth by binding to and inhibiting VEGFR-1, VEGFR-2, and VEGFR-3. Additionally, fruquintinib offers several advantages including minimal off-target toxicity, robust resistance profiles, and commendable efficacy. This agent can be used alone or in combination with other treatments. It has shown high effectiveness and survival benefits across various malignant tumors such as colorectal cancer, gastric cancer, non-small cell lung cancer, breast cancer, and other malignant tumors. Therefore, this article conducts a systematic review encompassing the mechanism of action, pharmacokinetics, clinical efficacy, and safety profile of fruquintinib. Through this review, we aimed to offer a reference for the clinical application and subsequent development of fruquintinib.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a leading cause of mortality worldwide. As of 2022, there were nearly 20 million new cancer cases and 9.7 million cancer deaths globally [1]. In China, approximately 4,824,700 new cancer cases were reported in 2022, with an age-standardized incidence rate of 208.58/100,000 [2]. Cancer is both a global health challenge and a major disease affecting social development.
In clinical practice, combined treatments, including chemotherapy and radiotherapy, are commonly employed for advanced malignant tumors. However, after multiple lines of treatment, the efficacy of the combined treatment will gradually diminish. Therefore, it is particularly vital to find new drugs with fewer adverse effects and better efficacy. As research into anti-angiogenesis targeted therapies deepens and our understanding of the vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling pathways expands, drugs that inhibit angiogenesis have provided new intervention strategies for treating malignant tumors. These drugs are increasingly recognized for their efficacy and patient tolerability.
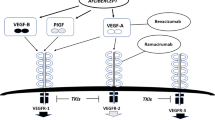
Tumor angiogenesis is crucial for tumor growth as it provides necessary nutrients and oxygen, promotes abnormal growth, and creates pathways for metastasis [3]. Studies have shown that VEGF is one of the key drivers of tumor angiogenesis, and its receptor signaling pathway has a substantially pivotal role in regulating tumor angiogenesis [4, 5]. Also, a previous study confirmed that VEGF is up-regulated in a variety of benign and malignant tumors, including infantile hemangioma, melanoma, breast cancer, glioblastoma multiforme, lung cancer, gastrointestinal cancer, renal cancer, head and neck cancer, and ovarian cancer. Additionally, there is a positive correlation between VEGFR-2 mutations and tumor progression [6]. VEGF plays a central role in tumor growth and metastasis, primarily by regulating angiogenesis and vascular permeability through two tyrosine residues in VEGF-R2. VEGF primarily acts on endothelial cells, with VEGF-R2 being the main signaling receptor whose activation promotes the mitosis and permeability of vascular endothelial cells [7]. In a tumor environment, activation of the VEGF/VEGFR signaling axis leads to increased vascular density, immune escape, and invasiveness. In some cases, it can even promote the metastasis of tumors [8]. Therefore, the study of molecular regulation of VEGF, along with the fundamental research targeting VEGF directly or indirectly, is a crucial method for innovatively and specifically blocking tumor-induced neovascularization. Additionally, this approach is also crucial to suppressing tumor growth.
Fruquintinib is a highly selective tumor angiogenesis inhibitor independently developed in China. It is distinguished from other approved small-molecule VEGFR inhibitors by its high efficiency, low toxicity, and suitability for combination therapy. As reported by the FRESCO (Fruquintinib Efficacy and Safety in 3 + Line Colorectal Cancer Patients) China phase III study, fruquintinib significantly benefits the survival of patients with metastatic colorectal cancer (mCRC). In 2018, an innovative small molecule drug independently developed by Hutchison MediPharma received the first global approval in China. This small molecule drug can be used for the treatment of patients with mCRC who have undergone at least two standard anticancer therapies [9, 10]. On November 8, 2023, fruquintinib was approved for the treatment of adult patients with mCRC in the United States [11]. The literature on fruquintinib treatment for malignant tumors up to May 2024 was sourced from databases including Pub Med, VIP, Wangfang, and CNKI. This review provided a summary of the structure, mechanism of action, pharmacokinetics, clinical efficacy, and adverse effects of fruquintinib. The objective of this review was to offer references for the clinical use and future studies of fruquintinib.
Mechanism of action of fruquintinib
The earlier generation of small molecule VEGFR inhibitors, including sunitinib, regorafenib, sorafenib, and pazopanib, requires inhibition of multiple targets. As a result, the drug exposure of these inhibitors at the maximum tolerated dose is limited, resulting in poor kinase inhibitor selectivity. Such a limitation also leads to a suboptimal inhibitor effect on any given target, particularly VEGFR, and/or a short duration of action. Fruquintinib, a novel and highly selective oral tyrosine kinase inhibitor (TKI), exhibits strong affinity for VEGFR-1, VEGFR-2, and VEGFR-3. Such a characteristic minimizes off-target toxicity and provides high drug exposure at the mean idle time, thereby increasing the duration of effective inhibition of VEGFR targets [5]. The chemical name of fruquintinib is 6-[(6,7-dimethoxy-4-quinazolinyl)oxy]-N, 2-dimethyl-3-benzofurancarboxamide, with the molecular formula C21H19N3O5. The chemical structure is displayed in Fig. 1 [12].
At the molecular level, kinase activity assays and [32p-ATP] binding assays demonstrated that fruquintinib exhibited potent inhibition of VEGFR-1, VEGFR-2, and VEGFR-3. The half maximal inhibitory concentration (IC50) values were 33 nmol/L, 35 nmol/L, and 0.5 nmol/L, respectively. Furthermore, fruquintinib showed weak inhibition of the RET proto-oncogene, fibroblast growth factor receptor 1, or tyrosine kinase receptor. Besides, fruquintinib exhibited no significant inhibition of other kinase activities (IC50 > 1,000 nmol/L) [12]. Because of its narrow target toxicity distribution, fruquintinib offered flexibility and suitability for combination therapy.
At the cellular level, fruquintinib inhibited VEGF/VEGFR family kinases and their signaling pathways in human umbilical vein endothelial cells and human lymphatic endothelial cells, with low IC50 values. Additionally, in a model of chick embryo chorioallantoie membrane, fruquintinib blocked the function of human umbilical vein endothelial cells, showing an anti-angiogenic effect [5].
At the tissue level, oral administration of fruquintinib significantly suppressed VEGF-induced phosphorylation of VEGFR-2 in mouse lung tissue [13]. The scope and duration of the inhibition were closely correlated with drug exposure [5].
At the animal level, fruquintinib administered orally inhibited VEGFR-2 phosphorylation and tumor angiogenesis, thereby suppressing tumorigenesis [14]. In a patient-derived tumor xenograft model, fruquintinib suppressed tumor growth through anti-angiogenesis and maintained a good dose-response relationship [5].
The results of the above studies suggested that fruquintinib could evidently inhibit the VEGFR kinase activity in different animal models and cell models, which further suppressed angiogenesis and tumorigenesis. In summary, fruquintinib reverses the immunosuppressive microenvironment of tumors, principally by inhibiting tumor angiogenesis, normalizing tumor vasculature, and promoting the infiltration of effector immune T cells in the tumor microenvironment [15, 16]. Therefore, fruquintinib stands out as a promising antitumor agent in the clinical treatment of various solid tumors, particularly mCRC, offering patients enhanced survival benefits. The primary mechanisms of action and biological effects of fruquintinib in treating malignant tumors were detailed in Table 1.
Pharmacokinetics
Absorption
Early clinical trials on the pharmacokinetics of fruquintinib in humans were conducted in both China and the United States. The outcomes of studies revealed that the maximum (peak) plasma concentration (Cmax) and the area under the concentration-time curve at 24 h (AUC0–24) after administration were proportional to the dosage in the range of 1–6 mg (0.2–1.2 times the recommended dose) [17, 18]. In healthy Chinese male volunteers, a single oral dose of 5 mg of fruquintinib capsule was absorbed well and rapidly. The median time to reach peak concentration was 3 h (1.5–24 h). The mean Cmax was 155 ng/mL, and the mean AUC0–∞ was 5,700 h.ng/mL [14, 17]. In Chinese patients with advanced solid tumors, the oral administration of fruquintinib capsules (5 mg/dose) resulted in a mean Cmax of 195 ng/mL, a median time to peak drug concentration of 2 h (0.5–2 h), and an average AUC0–72 of 5495 h.ng/mL [19]. A phase 1/1b open-label, dose-escalation study in patients with advanced solid tumors in the United States manifested that the 3 mg and 5 mg dose cohorts of fruquintinib had comparable pharmacokinetic profiles to those established in the Chinese study [18].
Distribution
The distribution characteristics of fruquintinib in the healthy population and in patients with advanced solid tumors indicated that fruquintinib and humans had a plasma protein binding ratio of approximately 80% [20]. The exposure level of fruquintinib in plasma was high, whereas its tissue distribution level was relatively low. Preclinical pharmacokinetic studies depicted that fruquintinib had a moderately high distribution in the gastrointestinal tract, kidneys, and adipose tissues, while its distribution in the brain and bone marrow was minimal [21]. Furthermore, another study discovered that, in healthy Chinese male volunteers administered radiolabeled fruquintinib, the predominant entity circulating in the plasma was the unchanged parent compound [17].
Metabolism
Fruquintinib is primarily metabolized in the liver via cytochrome P450 (CYP) 3 A, with secondary metabolism occurring through CYP2C8, CYP2C9, and CYP2C19 [12]. Within 96 h post-administration, fruquintinib predominantly exists as the parent compound in human plasma, accounting for 72.48% of the total plasma drug amount. M379-3, the main circulating metabolite, constitutes 17.31% of the total plasma drug amount, while other circulating metabolites comprise less than 5% [14, 17].
Excretion
Fruquintinib and its metabolites are excreted through urine and feces, with the major part recovered in urine as metabolites (60%) and a small amount of the parent compound excreted in urine (0.5%) or feces (5%) [22]. In Chinese patients with solid tumors, oral fruquintinib (5 mg/d) was excreted after metabolic reactions such as demethylation, hydroxylation, and glucuronidation, with a low in vivo clearance and a mean terminal elimination half-life of 42 h [14, 19, 23]. This was in line with the elimination half-life of fruquintinib reported in phase 1/1b studies of advanced solid tumors conducted in the USA [20]. In another phase I study in healthy Chinese male volunteers, 14 days after a single oral dose of 5 mg radiolabeled fruquintinib, recovery procedures were applied to the radiolabeled drug; 60.31% of radiopharmaceuticals were recovered in the urine and 29.80% in the feces [17, 20]. Besides, less than 6% of radiolabeled fruquintinib was excreted in the form of the prototype drug in the urine and feces [24].
Clinical studies
Metastatic colorectal cancer
Overview
In recent years, the incidence of colorectal cancer worldwide has presented a rising trend year by year. Nearly 20% of patients with colorectal cancer are diagnosed with metastasis at the first visit, and their 5-year survival is only 14%. Therefore, more effective treatments are urgently needed for a portion of patients with mCRC who have failed at least three lines of standard treatment (who failed standard 3 + line) [25,26,27]. Fruquintinib, a highly selective TKI, may offer a new therapeutic strategy for patients with mCRC driven by genetic mutations, potentially minimizing the adverse cytotoxicity and resistance to systemic chemotherapy [28].
Phase III clinical trials
In the FRESCO trial (a randomized, double-blind, placebo-controlled, and multi-center clinical trial conducted in China) [23], 416 patients (mean age: 54.6 years) were randomized to the fruquintinib group (278 patients) or the placebo group (138 patients). The primary study endpoint of the trial was overall survival (OS), and the secondary study endpoints included progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR). The results of the FRESCO trial illustrated that the median OS in the fruquintinib group (9.3 months) was better than that in the placebo group (6.6 months), with a significant prolongation of the median PFS by 1.9 months. Moreover, the ORR and the DCR were apparently higher compared with those in the placebo group. This phase III clinical study preliminary confirmed that fruquintinib could remarkably improve the OS and the PFS in patients with colorectal cancer, demonstrating safe and effective therapeutic effects.
In 2018, fruquintinib received its first global approval in China for the treatment of patients with mCRC who had failed previous second-line and third-line therapies [9]. Subsequently, an international, randomized, double-blind, and placebo-controlled phase III study (FRESCO-2) was conducted in 124 hospitals and cancer centers in multiple countries worldwide, enrolling a total of 934 patients with mCRC [29]. The results of the FRESCO trial demonstrated that the median OS (7.4 months) in the fruquintinib group was evidently better than that in the placebo group (4.8 months) (p < 0.001). Additionally, the median duration of remission (10.7 months) and DCR (56%), respectively, were higher in the fruquintinib group than in the placebo group. These data indicated that fruquintinib was effective and well-tolerated in patients with mCRC who had failed at least three lines of standard therapy.
The results of these two phase III studies displayed that fruquintinib had significant efficacy in the treatment of mCRC. Fruquintinib, compared to regorafenib, presented no significant difference in OS. However, it showed better trends in PFS, the overall incidence of adverse reactions, and cost-effectiveness [30,31,32]. A direct comparative clinical trial of the efficacy and safety of these two drugs revealed [33] that fruquintinib was superior to regorafenib in terms of the median PFS, median OS, and ORR (Table 2).
Combination therapy with programmed death-1 inhibitors
Treatment options for patients with mCRC are limited. Approximately 95% of patients with CRC have proficient mismatch repair/microsatellite stable (pMMR/MSS) tumors that show little response to programmed death-1 (PD-1) antibody therapy [34]. In Japan, regorafenib in combination with nivolumab has shown promising anticancer activity in patients with refractory mCRC, providing a new therapeutic option [35].
Studies have shown that PD-1 promotes tumor angiogenesis by binding to the immunosuppressive molecule PD-ligand 1 (PD-L1). VEGF/VEGFR promotes tumor angiogenesis by inducing a variety of intracellular pathways to regulate cell division, survival, budding, and endothelial cell migration. Both PD-1/PD-L1 and VEGF/VEGFR are equally involved in cancer immune evasion [36, 37]. Anti-angiogenic therapy can activate the immune responses of antitumor CD8 T cells and enhance the infiltration of these cells. This mechanism may be achieved through the activation of endothelial cells and vascular normalization, coupled with the inhibition of regulatory T cell infiltration [16, 38]. Fruquintinib, when administered at lower doses, has the ability to shift the inherently immunosuppressive tumor microenvironment to an immune-supportive state. Furthermore, it can also enhance the response to immunotherapy in both colon cancer model mice and clinical patients with colorectal cancer, thereby improving the efficacy of anti-PD-1 therapy [4, 39]. In a preclinical study, the combination of fruquintinib with PD-1 inhibitors presented stronger tumor growth suppression in the models that represented MSS and microsatellite instability (MSI) CRC compared to either agent alone [4]. Gou et al. [40] demonstrated that, compared to fruquintinib monotherapy (11.1% vs. 4.9%), the ORR was obviously higher when fruquintinib was combined with PD-1 inhibitors for the treatment of patients with refractory non-microsatellite instability-high/pMMR mCRC. Wang et al. [16] disclosed in a murine syngeneic model of CT26 cells that the combination of fruquintinib and PD-1 inhibitors could optimize the antitumor microenvironment by reducing regulatory T cells and enhancing T lymphocyte function, thereby showing remarkable synergistic antitumor effects. Li et al. [4] discovered in an allograft transplantation tumor model established using colon cancer cells of mice CT26 and MC38 that the combination of fruquintinib and sintilimab greatly inhibited the growth of CRC by altering the immune microenvironment of tumors.
A retrospective single-center analysis conducted by Yang et al. [41] evaluated the outcomes of patients with MSS mCRC who were treated with fruquintinib in combination with anti-PD-1 antibodies (sintilimab and toripalimab) after the failure of standard therapies. The analysis outcomes revealed that the combination of fruquintinib and anti-PD-1 antibodies improved the OS and PFS of patients with refractory MSS mCRC in China and exhibited a tolerable toxicity profile. Li et al. [42] denoted that fruquintinib in combination with sintilimab provided the higher DCR and the longer median PFS and was better tolerated compared with a trifluridine-tipiracil hydrochloride mixture. Ma et al. [43] conducted a single-arm, single-center, prospective, phase II clinical study on the third-line treatment of refractory advanced mCRC using sintilimab combined with toripalimab. This study demonstrated that the ORR of the combination was 21.05%, which was superior to that of patients treated with fruquintinib alone (ORR = 4.7%). This result supported the conclusion that fruquintinib in combination with an anti-PD-1 monoclonal antibody had better efficacy than fruquintinib alone for the third-line treatment of patients with advanced colorectal cancer with MSS in China. Another retrospective study verified that fruquintinib in combination with a PD-1 inhibitor significantly improved patients’ PFS time compared with regorafenib in combination with a PD-1 inhibitor in the treatment of advanced mCRC with MSS/pMMR [44].
However, all current studies on the combination of anti-angiogenic agents and PD-1 inhibitors are small-sample phase I/II prospective or retrospective studies with a single-arm design. The conclusions vary somewhat, and there is a lack of double-blind, randomized controlled phase III studies. The LEAP-017 study is the only ongoing phase III study (NCT04776148) [41]. More well-structured, prospective, and extensive studies are needed in the future to confirm and validate the efficacy of fruquintinib in combination with PD-1 inhibitors.
Combination with other comprehensive therapies
PD1/PD-L1 inhibitors have been shown to be effective in patients with mCRC with microsatellite instability-high or mismatch repair-deficient genes [45, 46]. However, immune checkpoint inhibitors (ICIs) have limited efficacy in patients with advanced colon cancer with microsatellite instability-low or pMMR genes [47]. Studies have shown that systemic antitumor immune responses induced by radiotherapy through an “abscopal effect” can reverse the immunosuppressive tumor microenvironment and promote antitumor immunity [48,49,50]. In addition, radiotherapy also affects the tumor vascular system, where a single high-dose irradiation induces endothelial cell apoptosis and senescence, causing vascular regression and collapse, leading to tissue hypoxia. Conversely, fractionated low-dose irradiation increases the expression of growth factors by inducing angiogenesis (e.g., VEGF) [48, 51]. This provides an opportunity for intervention with anti-angiogenic drugs, potentially enhancing immunotherapy sensitivity in patients with MSS.
According to recent studies, patients with mCRC who have a limited number of metastases may benefit from a combination of radical local treatment and systemic therapy. This combined approach has the potential to achieve long-term tumor control. Wang et al. [48] conducted the RIFLE study (a Phase II trial of stereotactic ablative radiotherapy combined with fruquintinib and tislelizumab in mCRC), which provided a new therapeutic strategy for patients and improved their prognosis. Moreover, the objective of this trial was to enhance the current understanding of the combination of radiotherapy, targeted therapy, and immunotherapy. In addition, the trial is also intended to optimize the clinical application of systemic treatment for mCRC. Combining ICI with an anti-VEGFR-TKI and local radiation interventions may be an effective strategy for heavily pretreated patients with mCRC exhibiting a MSS phenotype. In addition, Wang et al. [52] reported a case report of a patient with mCRC treated with fruquintinib who exhibited microsatellite instability as well as a KRAS exon 2 p. G12D mutation. This patient underwent local radiotherapy and then continued to receive fruquintinib, which resulted in sustained partial remission, and his PFS exceeded 30 months. Such outcomes suggested that fruquintinib may be an effective treatment for specific populations following local radiotherapy and that in-depth studies by expanding the sample size are needed.
Although TKIs and ICIs have achieved certain successes in the treatment of MSS and CRC, the efficacy of their combination is still limited. Studies have suggested that modulating the composition of the gut microbiota can enhance the efficacy of anti-PD-1 immunotherapy. Zhao et al. [53] conducted the first prospective study evaluating the antitumor activity and safety of fecal microbiota transplantation in combination with tislelizumab and fruquintinib as a third-line or above treatment for patients with MSS mCRC. They observed a significant improvement in the survival benefit (9.6 months of median PFS and 13.7 months of median OS), and ORR and DCR were 20% and 95%, respectively. This suggested that the combination of the three treatments has good antitumor activity with controllable toxicity.
The above results suggest that local radiotherapy, acting as an immunomodulator, can further enhance disease control on the basis of ICI. Furthermore, fecal microbiota transplantation, based on the gut microbiome, can also serve as a promising adjuvant approach to enhance antitumor immunity and promote response to ICI. More treatment options and survival benefits for cancer patients can be provided through the study of different treatment regimen combinations and the conduct of more prospective and extensive trials.
Adverse reactions and safety
Li et al. [29] indicated that there were a range of adverse reactions associated with treatment with fruquintinib. To be specific, nearly 63% of the fruquintinib group had grade 3 or higher adverse reactions. Common adverse reactions included hypertension, fatigue, anorexia, diarrhea, hypothyroidism, and lethargy. Additionally, the most frequently observed serious adverse reactions included hypertension, hand-foot skin reactions, and proteinuria. Given the high incidence of hypertension induced by fruquintinib, close attention should be paid to patients during the first two weeks of treatment. Clinical pharmacists should provide pharmacotherapy recommendations to clinicians through pharmaceutical care. Also, supportive treatments and dosage adjustments should be implemented to prevent severe adverse reactions.
In a previous research, a patient with advanced colon cancer experienced elevated blood pressure during targeted therapy with fruquintinib. The blood pressure decreased after suspending fruquintinib and administering antihypertensive therapy. Subsequently, treatment with fruquintinib was continued in combination with levamlodipine and irbesartan-hydrochlorothiazide to maintain stable blood pressure [54]. In addition, some rare adverse effects were observed in the above study, including symptoms such as hyperuricaemia, chest pain, hemorrhoids, changes in the ST segment in the electrocardiogram, and insomnia [55]. Recently, Wang et al. [56] reported a case of posterior reversible encephalopathy syndrome associated with fruquintinib for the first time. This suggests that the administration of fruquintinib may increase the risk of posterior reversible encephalopathy syndrome.
Although treatment with fruquintinib leads to some adverse reactions, such as hypertension, hand-foot skin reactions, and proteinuria, most of these effects are target-related. They can be predicted, controlled, and reversed in clinical settings through pharmacological monitoring. Therefore, fruquintinib generally demonstrates a high safety profile [57].
Gastric cancer
Overview
As one of the most common cancers worldwide, gastric cancer ranks fifth in incidence among malignant tumors, with an estimated annual occurrence of over one million new cases. Moreover, gastric cancer holds the fifth position in cancer-related mortality rates [1, 58]. Currently, the first-line treatment for advanced unresectable or metastatic gastric cancer primarily consists of platinum and fluorouracil two-agent chemotherapy, often combined with targeted agents [59]. Nevertheless, second-line treatment options for advanced gastric cancer after the failure of first-line treatment are still very limited. Despite the recommendation of paclitaxel combined with ramucirumab as the preferred regimen, the search for more effective and personalized treatment regimens for gastric cancer remains a priority for future research [60].
Clinical studies
Fruquintinib has been reported to enhance antitumor activity and exhibit good tolerability when combined with chemotherapeutic agents in a patient-derived xenograft model [5]. Zhang et al. [61] initially conducted a phase II study (NCT02415023) of fruquintinib in combination with paclitaxel for second-line treatment of advanced gastric cancer. They enrolled 34 patients and determined the recommended phase II dose. In addition, they observed that 7 patients in the 4 mg dose group achieved partial remission of the tumor response, with an ORR of 25.9%; 40.7% of patients achieved stable disease status, with a DCR of 66.7%; and 22 patients (81.5%) achieved tumor shrinkage. Such results further confirmed the antitumor activity of fruquintinib in combination with chemotherapy. A further phase III clinical trial (NCT03223376) was also conducted for the combination of fruquintinib and paclitaxel in the treatment of gastric cancer [9].
To fill the gap in the neoadjuvant treatment of gastric cancer with fruquintinib, Wu et al. [62] conducted a phase II, multicenter, single-arm, open-label clinical trial (NCT5122091). Through such a trial, they expected to evaluate the efficacy and safety of fruquintinib in combination with SOX (S-1 and oxaliplatin) in patients with locally advanced gastric cancer.
Adverse reactions
During the treatment of advanced gastric cancer with fruquintinib + paclitaxel, patients experienced a range of adverse reactions, primarily grade 3 and above. These included leukopenia, neutropenia, hypertension, hand-foot syndrome, and hemorrhage. Among the 28 evaluable patients, the common all-grade adverse reactions were mainly leukopenia, neutropenia, alopecia, and anemia [63]. However, the sample size of the clinical studies at present is small, and most of the enrolled patients have good organ function. The real situation of the adverse reactions of fruquintinib treatment for advanced gastric cancer needs to be further explored, and the sample size needs to be enlarged for more in-depth studies.
Advanced non-small cell lung cancer
Overview
Lung cancer is the leading cause of death from malignant tumors in the world, with more than 1.6 million deaths annually. Approximately 85% of these cases are non-small cell lung cancer (NSCLC), principally comprising lung adenocarcinoma and lung squamous cell carcinoma [63]. Recently, the development of new drugs and therapies has seen the application of fruquintinib in patients with NSCLC, with the aim of improving their prognosis.
Clinical studies
In a randomized, double-blind, placebo-controlled, multicenter phase II clinical study involving patients with advanced squamous NSCLC, patients in the fruquintinib group showed a longer-term survival benefit. The study included 91 evaluable patients and demonstrated that the median PFS in the fruquintinib group was 3.8 months (risk ratio for blinded independent central review was 0.34; 95% confidence interval: 0.20–0.57). The 3-month and 6-month survival rates of patients in the fruquintinib group (90.2% and 67.2%, respectively) were significantly higher than those in the placebo group (73.3% and 58.8%, respectively). Additionally, the ORR and the DCR (13.1% and 60.7%, respectively) were also significantly higher than those in the placebo group (0% and 13.3%, respectively) [64]. Lu et al. [6] conducted a phase III trial on advanced lung squamous NSCLC, enrolling 527 patients with advanced/metastatic lung squamous NSCLC. The enrolled patients were divided into two groups: the fruquintinib group (n = 354) and the placebo group (n = 173). They received either 5 mg of oral fruquintinib or placebo once daily. The median OS was 8.9 and 10.4 months, while the median PFS was 3.7 and 1.0 months for the fruquintinib and placebo groups, respectively. The ORR (13.8% vs. 0.6%) and DCR (66.7% vs. 24.9%) were significantly higher in the fruquintinib group than in the placebo group. All of these studies suggest that fruquintinib shows longer-term survival benefits in the treatment of advanced NSCLC.
Another experimental study exhibited that the combination therapy of fruquintinib and gefitinib also achieved favorable efficacy and safety in advanced NSCLC with epidermal growth factor receptor mutations [65]. The study has progressed to a phase II trial, and the results demonstrated that the ORR and DCR of fruquintinib in combination with gefitinib were 73.5% and 98.0%, respectively. Additionally, the median PFS of the combined drugs was significantly improved by approximately 30% compared with gefitinib monotherapy (14.72 months vs. 10.4 months). The study preliminarily verified that the efficacy of fruquintinib combined with gefitinib in the treatment of advanced NSCLC was superior to that of gefitinib monotherapy.
Adverse reactions
In terms of safety analysis, the most common grade ≥ 3 adverse reaction of fruquintinib for lung squamous NSCLC was hand-foot syndromes, followed by hypertension, proteinuria, and hemoptysis [66]. In the combination of fruquintinib and gefitinib, all patients (including the 4 mg fruquintinib group and the 5 mg fruquintinib group) experienced at least one adverse reaction of special concern, the most common of which were hepatotoxicity, thyroid dysfunction, and proteinuria [65]. A few patients experienced adverse reactions such as headache, alopecia, nasal bleeding, hyperthyroidism, anal fissure, dizziness, and femoral vein thrombosis [55]. Overall, most of the adverse reactions caused by fruquintinib treatment were within manageable limits, and the overall safety profile was favorable.
Other tumors
Two cases of patients with metastatic pancreatic cancer after failure of chemotherapy with fruquintinib treatment were recently reported, which showed that the PFS of these two patients was prolonged to 10 months [67]. In the treatment of Hodgkin lymphoma, a patient treated with fruquintinib + raltitrexed + S-1 also achieved a good survival benefit, suggesting the possibility of a combination regimen [68]. Clinical trials of fruquintinib for the treatment of a variety of other advanced malignancies are also progressing steadily, providing new ideas for the treatment of various malignancies. For instance, significant progress has been achieved in retrospective studies on advanced bone and soft tissue sarcomas. Additionally, a phase Ib/II trial (NCT04577963) focusing on breast cancer is currently in the enrollment phase [20]. Future clinical studies of fruquintinib in the treatment of other advanced malignancies still require continued attention.
Summary and outlook
Low drug selectivity, accompanied by significant side effects and limited anticancer efficacy, is the main reason why most VEGFR tyrosine kinase small molecule inhibitors are not suitable for patients with advanced malignancies. Fruquintinib, a potent selective inhibitor of VEGFR-1, VEGFR-2, and VEGFR-3, provides a new therapeutic option for patients with chemotherapy-refractory mCRC. Fruquintinib has demonstrated an excellent pharmacokinetic profile, tolerable safety profile, and antitumor activity in a wide range of tumor types. The clinical trials of fruquintinib, which cover multiple countries, offer good guidance for cancer treatment worldwide (Fig. 2). Due to the specificity of fruquintinib, its off-target effects are minimized, allowing the use of sufficient doses to achieve the desired VEGFR inhibition without significant toxicity. The most common serious adverse reactions to fruquintinib primarily include hypertension, hand-foot syndromes, and proteinuria, but these can be mitigated with close monitoring and intervention.
As fruquintinib is a novel small molecule VEGFR inhibitor, research on it has been relatively short, and many issues still need to be addressed. Firstly, except for mCRC, the efficacy of fruquintinib in the clinical application of other solid tumors is not apparent, and the dosage of the drug is not consistent in the treatment of other tumors. To continue in-depth research, it is necessary to expand the sample size. A large sample size will achieve better drug efficacy assessments and dosage specifications, which is crucial for supporting the approval of fruquintinib for broader use in treating malignant tumors. Secondly, the current clinical studies on fruquintinib as a monotherapy for mCRC are relatively mature and complete. However, the studies on fruquintinib combined with PD-1 inhibitors for treating mCRC are mainly focused on single-arm, prospective, or retrospective phase I/II studies with small sample sizes. Additionally, there is a lack of double-blind, randomized controlled phase III studies. Finally, there are limited reports on the use of fruquintinib in combination with surgery, radiotherapy, chemotherapy, and other combination therapies. Further exploration through prospective and extensive trials involving various treatment combinations is necessary.
In conclusion, fruquintinib shows great promise in the clinical treatment of malignant tumors. Further studies are needed to investigate the role of fruquintinib in early-stage treatment, its integration with standard chemotherapy, and its combination with ICI. Such research endeavors are expected to offer earlier and more effective survival advantages for patients with malignant tumors.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Bray F et al (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 74(3):229–263
Zheng RS et al (2024) [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi 46(3):221–231
Qin S et al (2019) Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol 12(1):27
Li Q et al (2022) Fruquintinib enhances the Antitumor Immune responses of Anti-programmed Death Receptor-1 in Colorectal Cancer. Front Oncol 12:841977
Sun Q et al (2014) Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol Ther 15(12):1635–1645
Mabeta P, Steenkamp V (2022) The VEGF/VEGFR Axis Revisited: implications for Cancer Therapy. Int J Mol Sci, 23(24)
Apte RS, Chen DS, Ferrara N (2019) VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 176(6):1248–1264
Jinnin M et al (2008) Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med 14(11):1236–1246
Shirley M (2018) Fruquintinib: First Global approval. Drugs 78(16):1757–1761
Zhang Y C.X., Fruquintinib Plans Overseas expansion: Hutchison MediPharma attempts FDA approval again, C. Bussiness, Editor
F H Creating a Hutchison Landmark on the Global Map of Pharmaceutical Innovation, in Chian Pharmaceutical News
Patell K et al (2024) Metabolism, toxicity and management of fruquintinib: a novel drug for metastatic colorectal cancer. Expert Opin Drug Metab Toxicol 20(4):197–205
Liu X et al (2020) Fruquintinib inhibits VEGF/VEGFR2 axis of choroidal endothelial cells and M1-type macrophages to protect against mouse laser-induced choroidal neovascularization. Cell Death Dis 11(11):1016
Gu Y et al (2014) Preclinical pharmacokinetics and disposition of a novel selective VEGFR inhibitor fruquintinib (HMPL-013) and the prediction of its human pharmacokinetics. Cancer Chemother Pharmacol 74(1):95–115
Peng Z et al (2020) Cost-effectiveness analysis of fruquintinib for metastatic colorectal cancer third-line treatment in China. BMC Cancer 20(1):990
Wang Y et al (2020) Combination of Fruquintinib and Anti-PD-1 for the treatment of Colorectal Cancer. J Immunol 205(10):2905–2915
Zhou S et al (2017) A phase I study to investigate the metabolism, excretion, and pharmacokinetics of [(14)C]fruquintinib, a novel oral selective VEGFR inhibitor, in healthy Chinese male volunteers. Cancer Chemother Pharmacol 80(3):563–573
Wang-Gillam A et al (2023) Phase 1/1b open-label, dose-escalation study of fruquintinib in patients with advanced solid tumors in the United States. Invest New Drugs 41(6):851–860
Cao J et al (2016) A phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1, -2, and– 3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer Chemother Pharmacol 78(2):259–269
Sun LY, Huang MY (2021) Research Progress of Fruquintinib on Advanced Colorectal Cancer. Cancer Res Prev Treat 48(12):1135–1142
Chen Z, Jiang L (2019) The clinical application of fruquintinib on colorectal cancer. Expert Rev Clin Pharmacol 12(8):713–721
Fang WT (2019) New drug for metastatic colorectal cancer treatment: Fruquintinib. Chin J New Drugs Clin Remedies 38(09):522–526
Li J et al (2018) Effect of Fruquintinib vs Placebo on overall survival in patients with previously treated metastatic colorectal Cancer: the FRESCO Randomized Clinical Trial. JAMA 319(24):2486–2496
XR Z (2019) Efficacy of Fruquintinib applied to patients with advanced Solid Tumors failed in standard therapy, A.M. University, Editor
Shin AE, Giancotti FG, Rustgi AK (2023) Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci 44(4):222–236
Qi J et al (2023) National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health 8(12):e943–e955
Biller LH, Schrag D (2021) Diagnosis and treatment of metastatic colorectal Cancer: a review. JAMA 325(7):669–685
Stucchi E et al (2024) Fruquintinib as new treatment option in metastatic colorectal cancer patients: is there an optimal sequence? Expert Opin Pharmacother 25(4):371–382
Dasari A et al (2023) Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet 402(10395):41–53
Jing Z, Rui Z, Binglan Z (2019) A comparison of regorafenib and fruquintinib for metastatic colorectal cancer: a systematic review and network meta-analysis. J Cancer Res Clin Oncol 145(9):2313–2323
Guan X et al (2021) Cost-effectiveness analysis of fruquintinib versus regorafenib as the third-line therapy for metastatic colorectal cancer in China. J Med Econ 24(1):339–344
Zhu JB, Lv WJ (2022) Cost-utility analysis of Fruquintinib Versus Regorafenib as third-line treatment for metastatic colorectal Cancer. Chin J Pharmacoepidemiology 31(03):173–177
Deng YY et al (2023) Comparison of the efficacy and safety of fruquintinib and regorafenib in the treatment of metastatic colorectal cancer: a real-world study. Front Oncol 13:1097911
He L et al (2023) Significant response from fruquintinib plus anti-PD-1 immunotherapy for microsatellite stable metastatic colorectal cancer with liver and lung metastasis in the third line: case report. J Gastrointest Oncol 14(6):2617–2626
Fukuoka S et al (2020) Regorafenib plus Nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). 38(18):2053–2061
Alam MR, Rahman MM, Li Z (2024) The link between intracellular calcium signaling and exosomal PD-L1 in cancer progression and immunotherapy. Genes Dis 11(1):321–334
Georganaki M, van Hooren L, Dimberg A (2018) Vascular targeting to increase the efficiency of Immune Checkpoint Blockade in Cancer. Front Immunol 9:3081
Shigeta K et al (2020) Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer, 8(2)
Fukumura D et al (2018) Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 15(5):325–340
Gou M et al (2022) Fruquintinib in Combination with PD-1 inhibitors in patients with Refractory Non-MSI-H/pMMR metastatic colorectal Cancer: a real-world study in China. Front Oncol 12:851756
Yang X et al (2023) Efficacy, safety, and predictors of fruquintinib plus anti-programmed death receptor-1 (PD-1) antibody in refractory microsatellite stable metastatic colorectal cancer in a real-world setting: a retrospective cohort study. J Gastrointest Oncol 14(6):2425–2435
Li L et al (2023) Fruquintinib in combination with sintilimab or TAS-102 as third-line or above treatment in patients with metastatic colorectal cancer: a real-world study. Transl Cancer Res 12(11):3034–3044
Ma S et al (2023) Efficacy and safety of toripalimab with fruquintinib in the third-line treatment of refractory advanced metastatic colorectal cancer: results of a single-arm, single-center, prospective, phase II clinical study. J Gastrointest Oncol 14(2):1052–1063
Sun L et al (2021) Efficacy and safety of Fruquintinib Plus PD-1 inhibitors Versus Regorafenib Plus PD-1 inhibitors in refractory microsatellite stable metastatic colorectal Cancer. Front Oncol 11:754881
Andre T et al (2020) Pembrolizumab in microsatellite-instability-high Advanced Colorectal Cancer. N Engl J Med 383(23):2207–2218
Overman MJ et al (2018) Durable clinical benefit with Nivolumab Plus Ipilimumab in DNA mismatch Repair-Deficient/Microsatellite instability-high metastatic colorectal Cancer. J Clin Oncol 36(8):773–779
Le DT et al (2015) PD-1 blockade in tumors with Mismatch-Repair Deficiency. N Engl J Med 372(26):2509–2520
Wang K et al (2023) RIFLE: a phase II trial of stereotactic ablative radiotherapy combined with fruquintinib and tislelizumab in metastatic colorectal cancer. Gastroenterol Rep (Oxf) 11:goad063
Brooks ED, Chang JY (2019) Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol 16(2):123–135
Liu Y et al (2018) Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol 11(1):104
Goedegebuure RSA et al (2018) Combining Radiotherapy with anti-angiogenic therapy and immunotherapy; a therapeutic Triad for Cancer? Front Immunol 9:3107
Wang R et al (2023) Case report: long-term sustained remission in a case of metastatic colon cancer with high microsatellite instability and KRAS exon 2 p.G12D mutation treated with fruquintinib after local radiotherapy: a case report and literature review. Front Pharmacol 14:1207369
Zhao W et al (2023) Fecal microbiota transplantation plus tislelizumab and fruquintinib in refractory microsatellite stable metastatic colorectal cancer: an open-label, single-arm, phase II trial (RENMIN-215). EClinicalMedicine 66:102315
Huang C, Qi LD (2021) Clinical pharmacist participating in the Pharmaceutical Care of High Blood pressure Induced by Fruquintinib. Chin J Pharmacoepidemiology 30(11):760–762
Lin J, Wu WN (2021) Correlation between adverse reactions and efficacy of Fruquintinib. Herald Med 40(07):943–947
Wang L, Zeng Z, Wu Z (2023) Case report: PRES associated with fruquintinib in a patient with metastatic colon cancer. Neurol Sci 44(11):4111–4114
N W The study on Fruquintinib in China was published in the Journal of the American Medical Association. Cancer Frontier, 2018(03): p. 22–23
Smyth EC et al (2020) Gastric cancer. Lancet 396(10251):635–648
Boileve J, Touchefeu Y, Matysiak-Budnik T (2023) Clinical management of gastric Cancer Treatment regimens. Curr Top Microbiol Immunol 444:279–304
Wang FH et al (2024) The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun (Lond) 44(1):127–172
Zhang Y et al (2023) A phase Ib/II study of fruquintinib in combination with paclitaxel as the second-line therapy for advanced gastric cancer. Cancer Commun (Lond) 43(1):150–153
Wu L et al (2024) Fruquintinib plus oxaliplatin combined with S-1 (SOX) as neoadjuvant therapy for locally advanced gastric cancer (GC) or gastro-oesophageal junction adenocarcinoma (GEJ): a multicentre, phase II, single-arm, open-label clinical trial (FRUTINEOGA) protocol. BMJ Open 14(2):e075696
Herbst RS, Morgensztern D, Boshoff C (2018) The biology and management of non-small cell lung cancer. Nature 553(7689):446–454
Lu S et al (2018) Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II study of Fruquintinib after two prior chemotherapy regimens in Chinese patients with Advanced Nonsquamous non–small-cell Lung Cancer. J Clin Oncol 36(12):1207–1217
Lu S et al (2021) Fruquintinib with gefitinib as first-line therapy in patients carrying EGFR mutations with advanced non-small cell lung cancer: a single-arm, phase II study. Transl Lung Cancer Res 10(2):839–854
Lu S et al (2020) A phase III, randomized, double-blind, placebo-controlled, multicenter study of fruquintinib in Chinese patients with advanced nonsquamous non-small-cell lung cancer - the FALUCA study. Lung Cancer 146:252–262
Wu D et al (2024) Extended survival with metastatic pancreatic cancer under fruquintinib treatment after failed chemotherapy: two case reports. World J Clin Cases 12(7):1296–1304
Wan Y, Luo D (2023) Using a combination of fruquintinib, raltitrexed, and S-1 as a third-line treatment for metastatic colorectal cancer with co-existence of Hodgkin lymphoma: a case report. J Gastrointest Oncol 14(1):450–457
Huang YW et al (2013) VEGF-c expression in an in vivo model of orthotopic endometrial cancer and retroperitoneal lymph node metastasis. Reprod Biol Endocrinol 11:49
Mei YB et al (2019) Validated UPLC-MS/MS method for quantification of fruquintinib in rat plasma and its application to pharmacokinetic study. Drug Des Devel Ther 13:2865–2871
Acknowledgements
Not applicable.
Funding
This research was supported by Special Project for Research on Traditional Chinese Medicine in Henan Province (2024ZY2100 & 2022ZY2046 & 2023ZY2110); National Traditional Chinese Medicine Expert Inheritance Studio Construction Project (Guozhong Pharmaceutical Renjiao Fa [2022] No. 75); Henan Chief Science Popularization Expert Wang Xiangqi Studio (HNKP2024176).
Author information
Authors and Affiliations
Contributions
Xin Zhang and Li-jia Ma conceived and designed the study. Xin Zhang and Li-jia Ma conducted the study. Chen-gang Zhou contributed to data acquisition. Chen-gang Zhou analyzed the data. Chen-gang Zhou interpreted the data. Xin Zhang and Li-jia Ma edited the manuscript draft. Xin Zhang and Li-jia Ma reviewed and edited the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, S., Wang, W., Li, J. et al. Clinical research progress of fruquintinib in the treatment of malignant tumors. Invest New Drugs (2024). https://doi.org/10.1007/s10637-024-01476-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10637-024-01476-6