Abstract
Background
Anlotinib plus chemotherapy as first-line treatment for extensive-stage small-cell lung cancer (ES-SCLC) achieves good efficacy, but there is still room for improvement. This clinical study examined the effectiveness of anlotinib plus etoposide for maintenance therapy in ES-SCLC.
Methods
The current single-arm, prospective phase II study was performed at Jiangsu Cancer Hospital (March 2019 to March 2022). After successful primary etoposide-based therapy, anlotinib was administered at 12 mg/day on days 1 to 14 of 21-day cycles until disease progression or consent withdrawal. All patients also received etoposide at 50 mg/day on days 1 to 14 of 21-day cycles for a maximum of six cycles. Progression-free survival (PFS) constituted the primary study endpoint. Secondary endpoints were overall survival (OS), objective remission rate (ORR), disease control rate (DCR), and safety. In addition, adverse events (AEs) were assessed.
Results
Twenty-eight patients were treated. Median PFS and OS were 8.02 (95%CI 5.36–10.67) and 11.04 (95%CI 10.37–11.68) months, respectively. Totally 9 and 18 participants showed a partial response and stable disease, respectively; ORR and DCR were 32.14% and 96.43%, respectively. The commonest all-grade AEs were fatigue (n = 11, 39.28%), hypertension (n = 11, 39.28%), loss of appetite (n = 9, 32.14%), oral mucositis (n = 7, 25.00%) and proteinuria (n = 6, 21.40%). Grade 3–4 AEs included fatigue (n = 4, 14.28%), hypertension (n = 2, 7.14%), hand and foot syndrome (n = 2, 7.14%), oral mucositis (n = 1, 3.57%), hemoptysis (n = 1, 3.57%), proteinuria (n = 1, 3.57%), gingival bleeding (n = 1, 3.57%), and serum creatinine elevation (n = 1, 3.57%).
Conclusion
Maintenance anlotinib plus etoposide achieves promising PFS and OS in clinical ES-SCLC.
Registration number
ChiCTR1800019421.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small-cell lung cancer (SCLC) SCLC features fast doubling time, elevated growth fraction, and extensive metastases in early disease phases, generally showing widespread hematogenous metastases [1]. SCLC cases comprise approximately 15% of all lung cancers [2], and the age-adjusted incidence of SCLC is 6.23 per 100,000 persons in the United States of America [1, 3]. Extensive-stage (ES)-SCLC includes stage IV (any T, any N, and M1a/b) or T3-T4 excluded from the limited-stage disease per the 8th edition American Joint Committee on Cancer (AJCC) [1].
The best SCLC management is based on surgery, chemotherapy, targeted therapy, and radiotherapy [1, 4], but 5-year overall survival (OS) is only 6.3%, including 27.3% for localized disease, 15.6% for regional disease, and 2.8% for metastatic disease [5]. The recommended primary therapies for ES-SCLC encompass carboplatin/cisplatin, etoposide, and atezolizumab/durvalumab, followed by atezolizumab/durvalumab maintenance therapy [1]. Maintenance therapy after the initial first-line treatment is a recognized treatment approach in clinical ES-SCLC [1], but chemotherapy alone is not as effective as maintenance therapy and could have toxicity issues [6]. Although immunotherapy is an effective maintenance therapy for ES-SCLC [7,8,9,10], its high cost prevents access for many patients. Hence, novel treatment strategies are required for improving prognosis in ES-SCLC.
The vascular endothelial growth factor (VEGF) receptor (VEGFR) shows high expression in SCLC [11], justifying the use of anti-VEGF/VEGFR antibodies in SCLC. Many trials examined the efficacy of antiangiogenic drug maintenance therapy for ES-SCLC, but the reported outcomes were unsatisfactory, with low objective response rates (ORRs) (no or small difference vs. control arm), poor progression-free survival (PFS) (4.7–9.1 months), poor OS (8.9–12.1 months), and toxicity issues [1, 12,13,14]. Anlotinib represents a recently developed tyrosine kinase inhibitor (TKI) targeting multiple receptor tyrosine kinases, including VEGFR1-4, platelet-derived growth factor receptor (PDGFR)α/β, fibroblast growth factor receptor (FGFR)1–4, and c-kit [15]. Anlotinib prevents angiogenesis, decreases tumor cell proliferation, and improves the immune tumor microenvironment [15,16,17]. Anlotinib is an oral drug (more convenient to use than intravenous drugs), whose tolerance profile is favorable [15]. The first-line therapy for ES-SCLC using anlotinib plus chemotherapy achieves good efficacy, with ORRs of 86–90% and median PFS of 6.0-10.3 months [18,19,20], and there is still room for improvement.
Therefore, the current work aimed to explore the effectiveness of anlotinib plus etoposide for maintenance therapy in clinical ES-SCLC.
Materials and methods
Study design
The current single-arm, prospective phase II trial was performed at the Oncology Department of Jiangsu Cancer Hospital between March 2019 and March 2022. The study followed the Declaration of Helsinki (2000), and had approval from the Ethics Committee of Jiangsu Cancer Hospital. Each patient provided signed informed consent. The current trial was registered at the Chinese clinical trial registry (www.chictr.org.cn, ChiCTR1800019421).
Participants
Inclusion criteria were: (1) 18 to 75 years old; (2) proven with ES-SCLC by histopathological examination; (3) treatment with standard first-line Etoposide-platinum solely chemotherapy, without progression; (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; (5) one or more computed tomography (CT) measurable lesions; (6) expected survival of at least 3 months; (7) major organ function indicators meeting the following criteria 7 days before the start of treatment: (a) hemoglobin (Hb) ≥ 90 g/L, (b) absolute neutrophil count (ANC) ≥ 1.5 × 109/L, (c) platelets (PLT) ≥ 80 × 109/L, (d) total bilirubin (TBIL) ≤ 1.5 fold the upper limit of normal range (ULN), (e) alanine (ALT) and aspartate (AST) aminotransferase levels ≤ 2.5×ULNs (ALT and AST ≤ 5×ULNs in patients with liver metastasis), (f) serum creatinine (Cr) ≤ 1.5×ULN or creatinine clearance (CCr) ≥ 60 ml/min, and (g) Doppler ultrasound evaluation showing left ventricular ejection fraction (LVEF) ≥ 50%; 7) contraceptive measures in patients of child-bearing age (female patients and female companions of male patients).
Exclusion criteria were: (1) presence of tumor types other than SCLC and mixed-SCLC; (2) a history of severe allergy or allergic constitution; (3) pregnant or breastfeeding women; (4) participation in other clinical trials; (5) pleural effusion or ascites that induced respiratory syndrome (CTCAE grade ≥ 2 dyspnea); (6) symptomatic brain metastases or symptoms controlled for < 2 months; (7) severe and/or uncontrolled diseases such as (a) suboptimal blood pressure control (systolic [SBP] and diastolic [DPB] blood pressure ≥ 150 and ≥ 100 mmHg, respectively), (b) grade ≥ 1 myocardial ischemia or infarction, arrhythmia (QTc ≥ 480 ms), or NYHA grade ≥ 2 congestive heart failure, (c) active or uncontrolled severe infection (CTCAE grade ≥ 2), (d) liver cirrhosis, decompensated liver disease, active hepatitis, or chronic hepatitis that required antiviral treatment, (e) renal failure that required dialysis, (f) a history of immunodeficiency diseases, i.e., HIV or other acquired or congenital immunodeficiency disease, or organ transplantation history, (g) suboptimal control of diabetes (fasting blood glucose (FBG) above 10 mmol/L), (h) urine routine examination showed urine protein ≥++ and 24 h urine protein above 1.0 g, or (i) neurological disease, such as epilepsy, dementia, severe depression, or mania; 8) major surgery, open biopsy, or substantial traumatic injuries within 28 days before inclusion; 9) imaging examination showing tumor invasion of the tissues surrounding vital blood vessels, or a tumor highly possibly invading vital blood vessels; 10) any grade of bleeding constitution or bleeding history, with any grade ≥ 3 bleeding or hemorrhagic events, or nonunion trauma, ulcer, or bone fracture; 11) atrial/venous embolism events within the past 6 months, including cerebrovascular events (e.g., transient ischemic attacks), deep venous thrombosis, or pulmonary embolism; 12) previous psychotropic drug abuse and incapacity of quitting, or with mental diseases; or 13) dysphagia or diagnosed drug absorption disorder.
Intervention
All patients underwent baseline imaging assessment after enrollment. The participants started treatment within 3 weeks (21 calendar days) from screening. All participants were administered anlotinib plus etoposide. Specifically, all participants were treated with oral anlotinib 12 mg q.d. on days 1–14 of 21-day cycles. Three weeks (21 d) were considered as one cycle. Anlotinib was continually administered until disease progression, consent withdrawal, or intolerable toxicity. The participants were also treated with oral etoposide 50 mg on days 1 to 14 of 21-day cycles. Etoposide treatment lasted for six cycles maximum.
During treatment, imaging examinations were performed every 2 cycles to assess clinical efficacy according to RECIST 1.1 criteria, including complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). Safety were evaluated every 3 weeks (21 ± 7 days) until disease progression, consent withdrawal, loss to follow-up, or intolerable toxicity. In case of disease progression, the participants were included in the survival follow-up phase, in which follow-up was performed every 56 ± 7 days until death, loss to follow-up, or consent withdrawal. Anti-tumor therapy after disease progression was decided by the investigators. Follow-up was recommended, and patient data were recorded.
Medication was discontinued in case of disease progression. In case of grade 3 or 4 adverse effects, the oral dose of anlotinib was lowered to the next dose level. In patients using a starting dose of 12 mg/day, 10 mg/day and then 8 mg/day were subsequently used. If the initial dose was 10 mg/day, it was lowered to 8 mg/day. No dose lowering was allowed after 8 mg/d; in such cases requiring dose adjustment, treatment discontinuation was applied. When the initial dose was 8 mg/day, treatment discontinuation was directly applied if dose adjustment was necessary.
Endpoints
The primary endpoint was PFS, which was the time elapsed from the start of therapy to first disease progression as judged by the investigators or per imaging findings or death. Secondary endpoints encompassed OS, ORR, disease control rate (DCR), and safety. OS represented the time elapsed from the start of therapy to death. Objective Response Rate (ORR) was defined as (CR + PR) / (CR + PR + SD + PD) × 100%, and Disease Control Rate (DCR) was defined as (CR + PR + SD) / (CR + PR + SD + PD) × 100%. Treatment-emergent adverse events (TEAEs) were evaluated on the basis of the Common Terminology Criteria for Adverse Events (CTCAE) 4.0.
Sample size
Previous data revealed a PFS of 2 months in ES-SCLC without maintenance therapy following standard first-line etoposide chemotherapy [21]. The anticipated PFS of patients after anlotinib plus etoposide was 5.5 months. With α = 0.05 and β = 0.2 (power = 80%), 24 participants were required in the present trial. Considering a possible loss to follow-up of 10%, 27 participants were required.
Statistical analysis
The intent-to-treat (ITT) set encompassed all participants administered the treatment. The per-protocol analysis (PP) set included all participants with high compliance with the study protocol and strictly completed the trial processes per the study protocol. All participants in the PP set completed the drug therapy throughout the study according to the protocol.
Data were analyzed with SPSS 22.0 (SPSS, USA). Normally and skewedly distributed continuous variables were presented as mean ± standard deviation and median (range), respectively. Categorical variables were presented as n (%). Kaplan-Meier curve analysis was utilized to estimate PFS and OS.
Results
Characteristics of participants
From March 2019 to March 2022, eligibility screening was conducted for 32 advanced SCLC cases with no progression through first-line therapy (Fig. 1). Cases were excluded for laboratory test failure (n = 3) or protocol violation (n = 1). Finally, 28 patients were included. Table 1 summarizes baseline patient data. Mean age was 66 (38–75) years, and 96.42% were male. Brain metastases were found in 6 (21.43%) participants. All 6 patients with brain metastases were detected before the initiation of etoposide-platinum chemotherapy. The first-line primary therapies were etoposide + cisplatin in 12 (42.86%) participants and etoposide + carboplatin in 16 (57.14%).
Efficacy
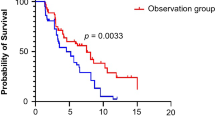
Median PFS was 8.02 (95%CI 5.36–10.67) months (Table 2; Fig. 2A). Median OS was 11.14 (95%CI 7.56–14.72) months (Table 2; Fig. 2B). In the ITT, PR was observed in 9 participants and SD in 18; ORR and DCR were 32.14% and 96.43%, respectively. Based on the PP set, PR was observed in 9 participants and SD in 11; ORR and DCR were 42.86% and 95.24%, respectively (Table 2 and Supplementary Figure S1). In the brain metastasis subgroup, median PFS was 8.02 (95%CI 4.96–11.08) months, and OS was 10.68 (95%CI 6.82–14.55) months (Fig. 3A). In the non-brain metastasis subgroup, median PFS and OS were 6.93 (95%CI 3.24–10.62) and 11.14 (95%CI 6.96–15.32) months, respectively (Fig. 3B).
Safety
Almost all participants (96.43%) experienced any-grade TEAEs. Any-grade TEAEs leading to anlotinib dose modification were observed in 35.71% of patients. TEAEs leading to anlotinib dose interruption were observed in 14.29% of cases. TEAEs leading to etoposide dose modification were observed in 7.14% of participants (Table 3). The commonest any-grade TEAE was proteinuria (57.14%), followed by anorexia (42.86%), neutropenia (35.71%), asthenia (32.14%), thrombocytopenia (28.57%), hypertension (28.57%), anemia (25.00%), and hand-foot syndrome (25.00%) (Table 3).
Grade 3–4 TRAEs were observed in 42.85% of participants. Grade 3–4 TEAEs leading to anlotinib dose modification were observed in 28.57% of cases. TEAEs leading to anlotinib dose interruption were observed in 7.14% of patients. TEAEs leading to etoposide dose modification were observed in 7.14% of participants (Table 3). The commonest grade 3 TRAEs included hand-foot syndrome (14.28%), neutropenia (10.71%), and asthenia (7.14%). In this study, there were no Grade 5 TEAEs, and there were no treatment-related deaths.
Discussion
This work examined the effectiveness of anlotinib combined with etoposide for maintenance treatment of ES-SCLC cases. The above findings suggest that maintenance treatment with anlotinib and etoposide after primary treatment with etoposide + platinum was effective and safe in clinical ES-SCLC.
Patient survival in ES-SCLC after treatment with etoposide and platinum is poor. Indeed, in a study by Paz-Ares et al. [8] up to six cycles of first-line therapy with platinum and etoposide yielded a median OS of 10.3 months. Roviello and colleagues [21] reported that standard first-line etoposide chemotherapy without maintenance yielded a PFS of 2 months in ES-SCLC. Adding etoposide maintenance following platinum and etoposide first-line therapy does not fare much better. In a phase II trial of four cycles of platinum with etoposide with subsequent etoposide maintenance, median PFS and OS were 9 and 14 months, respectively [22]. Zhang and collaborators [23] compared six cycles of platinum and etoposide followed or not by oral etoposide maintenance therapy and reported that etoposide maintenance improved PFS compared with no maintenance (8.9 vs. 5.9 months), while OS times were similar in both groups (15.0 vs. 14.3 months). Additionally, a meta-analysis by Zhou and colleagues [6] of SCLC supported a lack of efficacy for maintenance chemotherapy in ESCLC cases without statistically significant effects on OS (HR = 0.87, 95%CI 0.71–1.06) or PFS (HR = 0.87, 95%CI 0.62–1.22) compared with the control arm.
Although VEGF is expressed in SCLC, the anti-VEGF antibody bevacizumab yielded conflicting or disappointing results, with ORRs of 58.0-91.9 and median PFS of 4.7–7.8 months [13, 24,25,26], without differences compared with the control arm [13]. Hence, bevacizumab maintenance therapy is not recommended for SCLC [1]. Classical TKIs (e.g., sorafenib, sunitinib, and pazopanib) are not recommended in SCLC [1] because of a lack of efficacy or high toxicity [27,28,29]. Sorafenib showed ORRs of 2–11% and median OS times of 5.3–6.7 months in ES-SCLC cases previously administered a maximum of one line of platinum-based therapy [27]. Sunitinib showed a median PFS of 3.7 months, with 19% cases with grade 3 fatigue [28]. In patients responding to primary etoposide plus platinum, adding pazopanib led to maintenance of 3.8 months vs. 1.8 months for placebo [29]. Apatinib might improve PFS (7.8 vs. 4.9 months) and OS (12.1 vs. 8.2 months) in ES-SCLC cases [30]. Another trial of apatinib revealed an ORR of 50% and a median PFS of 3.7 months [31].
Third-line anlotinib monotherapy showed promising efficacy in ES-SCLC cases with/without brain metastasis in the ALTER1202 trial [32, 33]. Following the demonstration of anlotinib monotherapy as a third-line treatment option for SCLC, anlotinib was examined as a first-line therapy in combination with other anticancer drugs. Previous studies of first-line platinum plus etoposide plus anlotinib followed by anlotinib ± etoposide maintenance have reported PFS times of 6.0-10.8 months and OS times of 14.0-17.1 months [18,19,20]. Still, these three studies used anlotinib in the initial treatment in combination with etoposide and platinum. Exposing patients too early to anlotinib might increase the risk of anlotinib resistance since SCLC has a high rate of treatment resistance due to rapid cell proliferation [34]. Instead, in the present study, anlotinib was administered with etoposide as maintenance treatment in cases already responding to platinum with etoposide. By doing so, exposure to anlotinib is shorter, and treatment costs are lower. This also allows observing the effect of anlotinib without interference from platinum. In this work applying first-line treatment with platinum and etoposide followed by etoposide and anlotinib maintenance, median PFS and OS were 8.0 and 11.1 months, respectively, which are apparently higher than reported for etoposide maintenance [22, 23] but corroborated a previous clinical study of SCLC cases administered etoposide plus platinum followed by anlotinib maintenance (median PFS and OS of 7.7 and 11.0 months, respectively) [35]. Those numbers appeared to be closer to immunotherapy maintenance in ES-SCLC cases, with ORRs of 60–74% and median PFS times of 5.2–13.0 months [7,8,9,10]. Unfortunately, immunotherapy is expensive, which cannot be afforded by many patients. Hence, anlotinib combined with etoposide for maintenance therapy could be an effective and more affordable option for immunotherapy. Still, comparisons among trials must be taken with caution because of differences in eligibility criteria and clinical characteristics of the study populations. Future trials should be designed to directly compare anlotinib vs. immunotherapy for maintenance treatment in ES-SCLC cases.
In this work, 96.43% of the examined participants experienced any-grade TEAEs, with grade 3–4 TEAEs in 42.85% of cases. Those numbers are lower than reported for ES-SCLC cases administered other TKIs as maintenance treatment in phase II trials. Indeed, maintenance treatment with sorafenib resulted in 23% treatment discontinuation due to AEs [27]. A study of sunitinib maintenance reported a dose reduction in 48% of participants and grade 3–4 AEs in 54% of cases [28]. Pazopanib was also associated with high rates of toxicities, with treatment interruption in 52% of the examined participants [29]. Maintenance with immunotherapy also has safety issues, with any-grade AEs observed in 100% of participants, grade 3–4 AEs in 45–68%, and grade 5 AEs in 5% [7, 8, 10]. Therefore, anlotinib has a safety profile that is at least not worse, and maybe even better, than other TKIs and immunotherapy tested for maintenance therapy in ES-SCLC. In this study, the commonest any-grade TEAEs included proteinuria, anorexia, neutropenia, asthenia, thrombocytopenia, hypertension, anemia, and hand-foot syndrome, without new safety signals [18,19,20, 36, 37]. These results suggest the tolerability of anlotinib + etoposide for maintenance treatment in ES-SCLC.
This study had multiple limitations. First, this was a phase II trial without a control group. In addition, a single center design was used with small sample size. The recent advent of immunotherapy with platinum and etoposide as primary therapy [1] might require examining anlotinib in future studies.
In conclusion, anlotinib and etoposide is promising and safe in ES-SCLC after primary therapy with etoposide + platinum.
Data Availability
The data generated or analyzed during this study are included in this published article.
References
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) (2022) Small cell Lung Cancer. Version 2.2023. National Comprehensive Cancer Network, Fort Washington
Rudin CM, Brambilla E, Faivre-Finn C et al (2021) Small-cell lung cancer. Nat Rev Dis Primers 7:3. https://doi.org/10.1038/s41572-020-00235-0
Siegel RL, Miller KD, Wagle NS et al (2023) Cancer statistics, 2023. CA Cancer J Clin 73:17–48. https://doi.org/10.3322/caac.21763
Rivera MP, Mehta AC, Wahidi MM (2013) Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: american college of chest Physicians evidence-based clinical practice guidelines. Chest 143:e142S–e165S. https://doi.org/10.1378/chest.12-2353
Siegel RL, Miller KD, Fuchs HE et al (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Zhou H, Zeng C, Wei Y et al (2013) Duration of chemotherapy for small cell lung cancer: a meta-analysis. PLoS ONE 8:e73805. https://doi.org/10.1371/journal.pone.0073805
Liu SV, Reck M, Mansfield AS et al (2021) Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung Cancer treated with atezolizumab, carboplatin, and Etoposide (IMpower133). J Clin Oncol 39:619–630. https://doi.org/10.1200/JCO.20.01055
Paz-Ares L, Dvorkin M, Chen Y et al (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394:1929–1939. https://doi.org/10.1016/S0140-6736(19)32222-6
Horn L, Mansfield AS, Szczesna A et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell Lung Cancer. N Engl J Med 379:2220–2229. https://doi.org/10.1056/NEJMoa1809064
Goldman JW, Dvorkin M, Chen Y et al (2021) Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 22:51–65. https://doi.org/10.1016/S1470-2045(20)30539-8
Tanno S, Ohsaki Y, Nakanishi K et al (2004) Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer 46:11–19. https://doi.org/10.1016/j.lungcan.2004.03.006
Tiseo M, Boni L, Ambrosio F et al (2017) Italian, Multicenter, Phase III, Randomized Study of Cisplatin Plus Etoposide with or without Bevacizumab as First-Line treatment in extensive-disease small-cell Lung Cancer: the GOIRC-AIFA FARM6PMFJM Trial. J Clin Oncol 35:1281–1287. https://doi.org/10.1200/JCO.2016.69.4844
Pujol JL, Lavole A, Quoix E et al (2015) Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trialdagger. Ann Oncol 26:908–914. https://doi.org/10.1093/annonc/mdv065
Montanino A, Manzo A, Carillio G et al (2021) Angiogenesis inhibitors in small cell Lung Cancer. Front Oncol 11:655316. https://doi.org/10.3389/fonc.2021.655316
Shen G, Zheng F, Ren D et al (2018) Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol 11:120. https://doi.org/10.1186/s13045-018-0664-7
Liu S, Qin T, Liu Z et al (2020) Anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis 11:309. https://doi.org/10.1038/s41419-020-2511-3
Yang Y, Li L, Jiang Z et al (2020) Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother 69:2523–2532. https://doi.org/10.1007/s00262-020-02641-5
Liu C, Liao J, Wu X et al (2022) A phase II study of anlotinib combined with etoposide and platinum-based regimens in the first-line treatment of extensive-stage small cell lung cancer. Thorac Cancer 13:1463–1470. https://doi.org/10.1111/1759-7714.14414
Kong T, Chen L, Zhao X et al (2022) Anlotinib plus etoposide and cisplatin/carboplatin as first-line therapy for extensive-stage small cell lung cancer (ES-SCLC): a single-arm, phase II study. Invest New Drugs 40:1095–1105. https://doi.org/10.1007/s10637-022-01279-7
Deng P, Hu C, Chen C et al (2022) Anlotinib plus platinum-etoposide as a first-line treatment for extensive-stage small cell lung cancer: a single-arm trial. Cancer Med 11:3563–3571. https://doi.org/10.1002/cam4.4736
Roviello G, Zanotti L, Cappelletti MR et al (2016) No advantage in Survival with targeted therapies as maintenance in patients with Limited and extensive-stage small cell Lung Cancer: A literature-based Meta-analysis of Randomized trials. Clin Lung Cancer 17:334–340. https://doi.org/10.1016/j.cllc.2016.05.008
Li L, Li Q, Xu Y et al (2013) Phase II study of oral etoposide maintenance for patients with extensive stage small cell lung cancer who have responded to the induction on an EP regimen. Thorac Cancer 4:234–240. https://doi.org/10.1111/1759-7714.12019
Zhang C, Duan J, He Z et al (2021) The benefits of etoposide capsules as maintenance therapy for patients with extensive-stage small cell lung cancer: a prospective two-stage, two-center study. J Thorac Dis 13:343–352. https://doi.org/10.21037/jtd-21-106
Petrioli R, Roviello G, Laera L et al (2015) Cisplatin, Etoposide, and Bevacizumab Regimen followed by oral etoposide and bevacizumab maintenance treatment in patients with extensive-stage small cell Lung Cancer: a Single-Institution experience. Clin Lung Cancer 16:e229–234. https://doi.org/10.1016/j.cllc.2015.05.005
Spigel DR, Townley PM, Waterhouse DM et al (2011) Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 29:2215–2222. https://doi.org/10.1200/JCO.2010.29.3423
Horn L, Dahlberg SE, Sandler AB et al (2009) Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 27:6006–6011. https://doi.org/10.1200/JCO.2009.23.7545
Gitlitz BJ, Moon J, Glisson BS et al (2010) Sorafenib in platinum-treated patients with extensive stage small cell lung cancer: a Southwest Oncology Group (SWOG 0435) phase II trial. J Thorac Oncol 5:1835–1840. https://doi.org/10.1097/JTO.0b013e3181f0bd78
Schneider BJ (2015) Maintenance sunitinib for extensive-stage small cell lung cancer: a new standard, an option or a step in the right direction? Transl Lung Cancer Res 4:635–638. https://doi.org/10.3978/j.issn.2218-6751.2015.06.03
Sun JM, Lee KH, Kim BS et al (2018) Pazopanib maintenance after first-line etoposide and platinum chemotherapy in patients with extensive disease small-cell lung cancer: a multicentre, randomised, placebo-controlled phase II study (KCSG-LU12-07). Br J Cancer 118:648–653. https://doi.org/10.1038/bjc.2017.465
Luo H, Zhang L, Yang B et al (2020) A randomized phase 2 trial of apatinib vs observation as maintenance treatment following first-line induction chemotherapy in extensive- stage small cell lung cancer. Invest New Drugs 38:148–159. https://doi.org/10.1007/s10637-019-00828-x
Teng F, Xing P, Yang K et al (2022) Apatinib as maintenance therapy following standard first-line chemotherapy in extensive disease small cell lung cancer: a phase II single-arm trial. Thorac Cancer 13:557–562. https://doi.org/10.1111/1759-7714.14298
Cheng Y, Wang Q, Li K et al (2022) Anlotinib for patients with small cell lung cancer and baseline liver metastases: a post hoc analysis of the ALTER 1202 trial. Cancer Med 11:1081–1087. https://doi.org/10.1002/cam4.4507
Liu Y, Cheng Y, Li K et al (2021) Effect of prior thoracic radiotherapy on prognosis in relapsed small cell lung cancer patients treated with anlotinib: a subgroup analysis of the ALTER 1202 trial. Transl Lung Cancer Res 10:3793–3806. https://doi.org/10.21037/tlcr-21-632
Yuan J, Cheng F, Xiao G et al (2022) Efficacy and safety of Anlotinib in the treatment of small cell Lung Cancer: A Real-World Observation Study. Front Oncol 12:917089. https://doi.org/10.3389/fonc.2022.917089
Wang N, Zhao L, Zhang D et al (2023) Efficacy and safety of anlotinib as maintenance therapy after induction chemotherapy in extensive-stage small-cell lung cancer. Anticancer Drugs 34:558–562. https://doi.org/10.1097/CAD.0000000000001488
Wu D, Nie J, Hu W et al (2020) A phase II study of anlotinib in 45 patients with relapsed small cell lung cancer. Int J Cancer 147:3453–3460. https://doi.org/10.1002/ijc.33161
Song PF, Xu N, Li Q (2020) Efficacy and safety of Anlotinib for Elderly patients with previously treated extensive-stage SCLC and the Prognostic significance of common adverse reactions. Cancer Manag Res 12:11133–11143. https://doi.org/10.2147/CMAR.S275624
Acknowledgements
We are grateful to all participants involved in the present trial as well as to the clinicians who supported this research.
Funding
The current project was funded by the National Natural Science Foundation of China (Grant No. 82272863), the Beijing Medical and Health Foundation Project (Grant No. YWJKJJHKYJJ-F2030E), and the Huilan Public Interest Project (Grant No. HL-HS-2020102) (to Bo Shen) and the China International Medical Foundation Project (Grant NO. Z-2014-06-2103) and the Project of Jiangsu Cancer Hospital (Grant No. ZJ202110) (to Yuan Wu).
Author information
Authors and Affiliations
Contributions
Conceptualization: BS and YW. Data curation: YW, XFZ, WQZ, QW, ZXH, LFW, and WJZ. Formal analysis: YW, TZ, HZS, KHY, LS, BZP, and RHG. Funding acquisition: BS and YW. Investigation: YW, XFZ, and BS. Methodology: YW, XFZ, FJF, and BS. Project administration: GRZ, FJF, and BS. Resources: all authors. Software: BS. Supervision: FJ, FJF, and BS. Validation: YW, XFZ, WQZ, QW, ZXH, FJF, and BS. Visualization: YW, XFZ, FJF, and BS. Writing-original draft: YW, XFZ, and BS. Writing-review & editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This trial complied with the Declaration of Helsinki (2000) of the World Medical Association. It had approval from the Ethics Committee of the Jiangsu Cancer Hospital. Signed informed consent was provided by each patient. The study was registered at the Chinese clinical trial registry (www.chictr.org.cn, ChiCTR1800019421).
Consent to participate
Signed informed consent was provided by each patient.
Consent to publish
N/A.
Animal Studies
N/A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, Y., Zhou, X., Zhao, W. et al. Therapeutic effectiveness of anlotinib combined with etoposide in extensive-stage small-cell lung cancer: a single-arm, phase II trial. Invest New Drugs 41, 825–833 (2023). https://doi.org/10.1007/s10637-023-01398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01398-9