Second-line treatment of endometrial cancer is an unmet medical need. Lurbinectedin showed promising antitumor activity in a phase I study in combination with doxorubicin in advanced endometrial cancer. This phase 2 Basket trial evaluated lurbinectedin 3.2 mg/m2 1-h intravenous infusion every 3 weeks in a cohort of 73 patients with pretreated endometrial cancer. The primary endpoint was overall response rate (ORR) according to RECIST v1.1. Secondary endpoints included duration of response (DoR), progression-free survival (PFS), overall survival (OS), safety and an exploratory translational study. Confirmed complete (CR) and partial response (PR) was reported in two and six patients, respectively (ORR = 11.3%; 95%CI, 5.0–21.0%). Median DoR was 9.2 months (95%CI, 3.4–18.0 months), median PFS was 2.6 months (95%CI, 1.4–4.0 months) and median OS was 9.3 months (95%CI, 6.1–12.8 months). Molecular subtypes showed differences in PFS rate at 6 months (p53abn 23.7% vs. “No Specific Molecular Profile” [NSMP] 42.9%) and median OS (p53abn 6.6 months vs. NSMP 16.1 months). The most common treatment-related adverse events (mostly grade 1/2) were fatigue (54.8% of patients), nausea (50.7%), vomiting (26.0%) decreased appetite (17.8%). and constipation, (19.2%). The most common grade 3/4 toxicity was neutropenia (43.8%; grade 4, 19.2%; febrile neutropenia, 4.1%). In conclusion, considering the exploratory aim of this trial and the hints of antitumor activity observed together with a predictable and manageable safety profile, further biomarker-based development of lurbinectedin is recommended in this indication in combination with other agents. Clinicaltrials.gov identifier: NCT02454972.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer is the sixth most common cause of cancer in females with 417,000 cases every year [1]. Patients who progress beyond first-line chemotherapy have a poor prognosis and novel therapy options are urgently needed. The Cancer Genome Atlas (TCGA) study of endometrial cancer identified four molecular subtypes [2]. Based on this, the Proactive Molecular Risk Classifier for Endometrial Cancer (ProMisE) Algorithm has been developed to assess endometrial cancer samples and classifies them in four molecular subgroups [3]. Several therapeutics are being explored using this biomarker analysis. The TCGA endometrial cancer data expanded the knowledge about the role of different immunotherapeutic approaches based on molecular subtypes. Immune checkpoint inhibitors demonstrated distinct antitumor activities as monotherapy or in combination [4]. In microsatellite unstable (microsatellite instability-high) endometrial cancer, immune checkpoint inhibitors showed promising activity in recurrent settings. On the other hand, single immune checkpoint inhibitors showed underwhelming efficacy in microsatellite stable endometrial cancer but this improved using a combination approach.
Lurbinectedin (ZEPZELCA™) is an oncogenic transcription inhibitor that binds guanine-rich DNA sequences at gene promoters, evicts oncogenic transcription function and inhibits mRNA synthesis through ubiquitination and degradation of RNA polymerase II [5,6,7]. In a Basket, multicenter, open-label, phase 2 study (ClinicalTrials.gov identifier: NCT02454972), nine cohorts of patients with different difficult-to-treat tumor types received lurbinectedin to establish the proof of concept of anticancer activity for potential further clinical development. Based on the results in the small cell cancer (SCLC) cohort [8], approval of lurbinectedin was obtained first in the US [9] and in several other countries later (Canada, Australia, Switzerland, Singapore, South Korea, Ecuador, Mexico, Arab Emirates or Qatar). More recently, results of other cohort of this Basket trial have shown antitumor activity in relapsed Ewing sarcoma [10].
This report focuses on the outcomes of the endometrial cancer cohort. In addition, retrospective biomarker analysis based on TCGA and PromiSe molecular subtypes was explored. This cohort was evaluated because promising antitumor activity was previously found in a phase I study for a combination of doxorubicin plus lurbinectedin in patients with advanced endometrial cancer [11]. The overall response rate (ORR) of 42.1% was higher than the 14–16% reported for doxorubicin alone [12, 13], then suggesting a synergistic effect. Furthermore, another trial evaluating lurbinectedin plus paclitaxel showed an ORR of 27% in a small cohort of 11 patients with pretreated endometrial cancer [14]. In this study we report the activity and safety analysis of lurbinectedin monotherapy in addition to a translational exploratory analysis of endometrial molecular subtypes showing better PFS and OS in the TP53 wild-type, low/absent p53 protein immunohistochemical (IHC) and No Specific Molecular Profile (NSMP) molecular subgroups.
Methods
The study protocol was approved by the Independent Local Ethics Committee of each participating center and was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulations for clinical trials. Signed informed consent was obtained from all patients prior to any study-specific procedure. Additionally, patients were invited to participate in a translational study designed to identify molecular predictors of response or resistance to lurbinectedin, through an independent informed consent. The trial is registered at https://www.clinicaltrials.gov as NCT02454972.
Patient selection
Seventy-three patients with endometrial cancer were treated at 19 investigational sites in Belgium (n = 3), France (n = 17), Germany (n = 2), Spain (n = 23), Switzerland (n = 2), the United Kingdom (n = 9), and the USA (n = 17). Eligibility criteria included patients ≥ 18 years old with pathologically proven diagnosis of endometrial carcinoma; pretreated with one prior adjuvant/advanced chemotherapy-containing line (including platinum or not); measurable disease as per the Response Criteria in Solid Tumors (RECIST) v.1.1 [15]; Eastern Cooperative Oncology Group performance status ≤ 2; and adequate major organ function. Patients were excluded if they had: previously received lurbinectedin or trabectedin; prior or concurrent malignant disease unless in complete remission for more than five years; known central nervous system involvement; concomitant unstable or serious medical condition, or impending need for radiotherapy.
Lurbinectedin treatment
All patients were treated with lurbinectedin 3.2 mg/m2 administered as a 1-h intravenous (i.v.) infusion every three weeks (q3wk). All patients received antiemetic prophylaxis. Primary granulocyte colony-stimulating factors (G-CSFs) prophylaxis was not allowed. Treatment continued until disease progression, unacceptable toxicity, treatment delay > three weeks; more than two dose reductions; or patient refusal.
Efficacy and safety assessments
The primary objective of this study was to assess the antitumor activity of lurbinectedin in terms of ORR, primary endpoint, assessed by the investigators. Radiological tumor evaluation was performed every six weeks (two cycles) until Cycle 6, and every nine weeks (three cycles) thereafter. Objective response was to be confirmed at least four weeks later. Secondary efficacy endpoints included disease control rate (ORR or stable disease), duration of response (DoR), progression-free survival (PFS), and OS.
Safety was evaluated in all patients who received at least one lurbinectedin infusion, complete or incomplete, by assessment of adverse events (AEs), clinical laboratory test results, physical examinations and vital signs. Laboratory tests were done weekly during Cycles 1 and 2, and on Day 1 of subsequent cycles. AEs were recorded and coded with the Medical Dictionary for Regulatory Activities (MedDRA), v.21.0. AEs and laboratory values were graded according to the National Cancer Institute-Common Toxicity Criteria for Adverse Events (NCI-CTCAE), v. 4.0. All patients were followed until recovery from any lurbinectedin-related AE.
Translational study
Fifty of 73 treated patients (68.5%) had available archived formalin-fixed paraffin-embedded tumor samples and consented to participate in an optional translational study. In order to characterize patients’ tumors, a next generation sequencing (NGS) custom gene panel was performed, targeting 151 genes involved in cancer pathogenesis and DNA-repair (see Supplemental Methods). Data of sufficient quality was obtained for 42 patients. Moreover, to classify patients into four endometrial cancer molecular subgroups, two additional techniques were performed: microsatellite instability (MSI) status by fluorescent polymerase chain reaction (PCR) and IHC p53 protein staining (see Supplemental Methods). Analytically valid results were obtained for each technique for 47 and 50 samples respectively. Molecular sub-classification was obtained through an hierarchical algorithm [16, 17]: first, patients with pathogenic mutation in POLE gene exonuclease domain (POLE+ subgroup); second, MSI positives (dMMR/MSI subgroup); third, high staining abnormal p53 IHC (> 20% stained cells) and/or carriers of deleterious class 5 TP53 mutations (p53 abnormal subgroup, p53 abn) (see Supplemental Methods) and, finally, the remaining were considered as “No Specific Molecular Profile” (NSMP subgroup).
Statistical methods
Up to 50 evaluable patients were to be recruited to test the null hypothesis that 10% or less patients get a response (p ≤ 0.10) versus the alternative hypothesis that 25% or more patients get a response (p ≥ 0.25). The variance of the standardized test was based on the null hypothesis. The type I error (alpha) associated with this one-sided test is 0.025 and the type II error (beta) is 0.144; hence, statistical power is ~86%. With these assumptions, if the number of patients who achieve a confirmed response is ≥ 10, then this would allow the rejection of the null hypothesis.
Initially, 15 patients were to be included in a first stage. If one confirmed response occurred in the first 15 evaluable patients, recruitment had to continue up to 25 evaluable patients. Two of the first 15 patients had confirmed partial response (PR) to lurbinectedin treatment and, therefore, recruitment continued. Due to the signs of activity also observed in combination with doxorubicin [11], paclitaxel [14] or irinotecan [18], a protocol amendment was implemented to include 50 evaluable patients but, finally, because of the fast recruitment until the 50 evaluable patients were evaluated, 73 patients were enrolled and treated.
Descriptive statistics were used. Non-continuous variables are described in frequency tables using counts and percentages. Continuous variables are described by median, minimum and maximum. Binomial exact estimates and its 95% confidence interval (CI) were calculated for the evaluation of the main endpoint (ORR). The Kaplan-Meier method was used to analyze DoR, PFS and OS. For the translational sub-study analysis, the correlation between mutational status and OS or PFS was evaluated by a Cox regression analysis and Kaplan-Meier curves represented. SAS and R software were used to generate statistical outputs.
Results
Patient characteristics
Seventy-three patients were recruited and treated with lurbinectedin between 30 October 2016 and 19 April 2017. Cut-off for final analysis of all cohorts in this Basket study was 16 November 2020. Most patients were white (61.6%), with ECOG PS 0–1 (91.8%), and with a median age of 64 years (range, 32–80 years; 49.3% were ≥ 65 years old) (Table 1). The most common histological types were endometrioid (61.6%) and serous (27.4%). The median number of metastatic sites involved at baseline was 2 (range, 1–7), with 45.2% of patients having ≥ 3 disease sites. Lymph nodes (61.6%), lung (46.6%), peritoneum (45.2%) and liver (31.5%) were the most common disease sites. Sixty-two patients (84.9%) had previously undergone surgery. Prior radiotherapy had been administered to 39 patients (53.4%). The patients had received a median of one prior line of chemotherapy for advanced disease (range, 0–4 lines). The most common prior agents were carboplatin (95.9%) and paclitaxel (95.9%). ORR to last prior line was 37.0%.
Lurbinectedin treatment
A total of 378 cycles were administered to the 73 treated patients. The median number of cycles per patient was 4 (range, 1–22 cycles), with 31.5% of patients having received ≥ 6 cycles. The median relative dose intensity was 97.7% (range, 64.9–102.9%). Twenty patients had treatment-related dose delays, being hematological toxicity the most common reason: grade 2–4 neutropenia in 13 patients and grade 3 anemia in two patients. Lurbinectedin dose was reduced due to treatment-related reasons in 6.9% of cycles in 20 patients, being hematological toxicity the most common cause: grade 2-4 neutropenia in eight patients and eight cycles; grade 3/4 febrile neutropenia in two patients and two cycles; and grade 3 leukopenia in one patient and one cycle. Of note, the protocol stated that in case of grade 4 neutropenia, lurbinectedin dose had to be reduced instead of continuing at the same dose with granulocyte colony-stimulating factor (G-CSF) prophylaxis.
Efficacy results
Seventy-one patients were evaluable for efficacy (Table 2). Two patients were not evaluable due to patient refusal prior to the first disease measurement, and death because of grade 5 septic shock considered unrelated to the study treatment in Cycle 1.
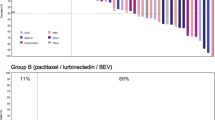
Confirmed complete response (CR) was reported in two patients (2.9%) and partial response (PR) in six patients (8.5%). Stable disease (SD) was observed in 29 patients (40.8%), with 17 of them (23.9%) reaching SD ≥ 4 months. Therefore, ORR was 11.3% (95%CI, 5.0–21.0%). Overall, 47.6% of patients had reduction in target lesions during the treatment period (Fig. 1A). Objective responses were observed in the two most common histological subtypes: endometrioid (five responses) and serous (two responses). The other response was reported in a patient with endometrial stromal sarcoma (epithelioid). The median of prior lines in responder patients was one (range, 1–4) (Supplemental Table 1).
A Waterfall plot showing maximum variation of target lesions size with lurbinectedin in patients with pretreated endometrial cancer. B Time to progression with last prior therapy (months) versus progression-free survival (months) with lurbinectedin in patients with endometrial cancer and clinical benefit (complete response, partial response or stable disease ≥ 4 months). Abbreviations: CR, complete response; NA, not available; PD, disease progression; PR, partial response; SD, stable disease; UK, unknown
Median DoR was 9.2 months (95%CI, 3.4–18.0 months). Clinical benefit rate (CR + PR + SD ≥ 4 months) and disease control rate (CR + PR + SD) were 35.2% (95%CI, 24.2–47.5%) and 52.1% (95%CI, 39.9–64.1%), respectively (Table 2). Median duration for clinical benefit rate and disease control rate was 7.1 months and 5.6 months, respectively. Time to progression with last prior therapy versus PFS with lurbinectedin is shown in Fig. 1B.
Median PFS was 2.6 months (95%CI, 1.4–4.0 months) and PFS rate at 6 months was 29.0% (95%CI, 18.2–39.8%). With a median follow-up of 28.9 months and a censoring rate of 19.8%, median OS was 9.3 months (95%CI, 6.1–12.8 months) (Table 2).
Thirty-five patients (47.9%) received further antitumor medical therapy, nine patients (12.3%) received further radiotherapy and two patients (2.7%) underwent further surgery after lurbinectedin. The most common agents subsequently received were paclitaxel (n = 14; 19.2%) and carboplatin (n = 13; 17.8%).
Translational study results
In the context of a retrospective translational study, an NGS panel was performed to identify molecular tumor biomarkers that might influence the clinical response to lurbinectedin. The mutational landscape observed was typical for an endometrial cancer cohort [2]: TP53 mutated (54.8% of tumors), PIK3CA (38.0%), PTEN (21.4%), KRAS (26.2%), and KMT2D and ARID1A (19.0% each) (Fig. 2A). The most remarkable results are shorter median PFS in patients with PIK3CA mutation positive tumors: 2.0 vs. 4.0 months in the wild-type group (p = 0.0059), and shorter median OS in patients with TP53 pathogenic mutation: 6.6 vs. 16.1 months in the wild-type group (p = 0.0020) (Supplemental Table 2 and Supplemental Fig. 1A-D).
A Oncoplot showing mutational profile on every patient sample, together with histology, tumor grade, p53 immunohistochemistry and molecular subtype classification. B Molecular classification algorithm for endometrial cancer patients included in this cohort. Abbreviations: IHC, immunohistochemistry; VOUS, variants of unknown significance; MSI/dMMR, “microsatellite instable/mismatch repair deficient” molecular subgroup; NSMP, “No Specific Molecular Profile” molecular subgroup; p53abn, “p53 abnormal” molecular subgroup including abnormal p53 IHC and nonsense/non-funtional TP53 mutants
In addition to the traditional classification, based on staging and histology, last guidelines recommend to incorporate tumor biomarkers to allow endometrial cancer subgroup classification in four molecular subtypes: p53 abnormal, dMMR/MSI+, POLE-mutated, and “no specific molecular profile” (NSMP) [16, 17]. To classify patient’s tumors, NGS characterization was complemented with evaluation of MSI status and p53 protein IHC staining (see Supplemental Methods). None of the samples was carrier of any described or likely pathogenic POLE variant; three samples (7.1%) were MSI positive, 23 samples (54.8%) showed p53/TP53 inactivation and 16 samples (38.1%) were classified as NSMP (Fig. 2B). Consistently with observations on TP53 pathogenic mutation, as under normal conditions wild-type p53 protein is rapidly degraded and inactive p53 accumulates [19], patients with high p53 IHC staining showed a numerically shorter mean PFS than p53 low/absent normal tumors (1.7 months vs. 2.7 months, p = 0.3309) and a shorter median OS (8.2 months vs. 12.8 months, p = 0.0345) (Supplemental Table 3 and Supplemental Fig. 1E-F).
Molecular subtypes showed differences in PFS, with a difference at 6 months of 19.2% between p53abn and NSMP molecular subgroups, 23.7% (95%CI, 5.8%-41.7%) and 42.9% (95%CI, 18.2%-67.5%) respectively (Supplemental Table 4). Overall survival was significantly shorter in p53 abnormal group with a median of 6.6 months (95%CI, 3.1–12.1) compared to the NSMP group, with a median of 16.1 months (95%CI, 5.3–26.6) (Supplemental Table 4 and Supplemental Fig. 1G-H). No significant differences were seen on MSI molecular subgroup (data not shown).
Safety results
All 73 treated patients were evaluable for safety (Table 3). The most common treatment-related adverse events were fatigue (54.8% of patients), gastrointestinal disorders (nausea, 50.7%, vomiting, 26.0%, and constipation, 19.2%), and metabolism and nutrition disorders (mainly decreased appetite, 17.8%). These adverse events were mostly grade 1/2. The most common treatment-related grade 3/4 AEs and laboratory abnormalities regardless of relationship were hematological disorders including anemia (27.4%), leukopenia (32.9%) and neutropenia (43.8%; grade 4, 19.2%; febrile neutropenia, 4.1%); fatigue (4.1%), nausea (2.7%), diarrhea (2.7%), and increased liver function tests, including increased transaminases (ALT, 4.2%; AST, 1.4%), alkaline phosphatase (5.6%) and GGT (19.2%). Eleven patients (15.1%) received G-CSFs secondary prophylaxis or therapeutic for neutropenia.
One patient died due to treatment-related grade 5 sepsis infection after two cycles (Table 3). This case was associated with severe neutropenia. During hospitalization, blood culture was positive for Klebsiella, Escherichia coli, Streptococcus viridans and Streptococcus and CT-scan showed disease progression that was later confirmed in the autopsy.
Most patients (n = 59; 80.8%) discontinued the study treatment due to disease progression. With respect to the other 14 patients, five (6.8%) died while on treatment (three due to disease progression, one due to grade 5 septic shock unrelated to treatment, and one due to treatment-related grade 5 sepsis, above explained); five (6.8%) refused to continue treatment; one (1.4%) discontinued lurbinectedin therapy due to a treatment-related adverse event: persistence of peripheral neuropathy (grade 2 was present at baseline and worsened to grade 3); and the other three patients discontinued lurbinectedin due to adverse events unrelated to the study treatment (n = 1) or because of investigator decision based on benefit-risk balance (n = 2).
Discussion
This cohort from a phase 2 exploratory Basket study included 73 patients with pretreated endometrial cancer who received therapy with single-agent lurbinectedin. ORR according to RECIST v.1.1 was 11.3% (95%CI, 5.0–21.0%). Responses were mostly observed in patients with endometrioid tumors and the median of prior lines was one. These results (eight objective responses) were lower than the threshold of ≥ 10 confirmed responses established in the statistical hypothesis for this endometrial carcinoma cohort. However, although the cohort did not meet the planned hypothesis, hints of antitumor activity were observed, with two patients achieving complete response and six patients with partial responses in a quite heterogeneous cohort that included patients with different number of prior lines administered (up to four previous lines), different histology subtypes (e.g., carcinosarcoma, endometrial stromal sarcoma), and not characterized molecularly at study entry according to current guidelines [20]. Of note, median duration of response was prolonged (9.2 months). This duration of response was similar to that found for physician’s choice following platinum-based therapy in patients with advanced endometrial cancer in a recent phase 3 study [21].
Studies in small cohorts of patients of lurbinectedin in combination with other drugs have shown to increase the single-agent activity in pretreated endometrial cancer. For instance, in combination with doxorubicin (ORR = 42% and median DoR = 7.5 months) [11], paclitaxel (ORR = 27% and median DoR = 6.1 months) [14], or irinotecan (ORR = 30%; median DoR not available) [18].
Retrospective tumor molecular and genomic profiling have shown different lurbinectedin response depending on the presence of mutations on particular genes, protein levels and specific molecular subtypes, with better PFS and OS in the TP53 wild-type, low/absent p53 protein IHC and NSMP molecular subgroup. These biomarkers/molecular classification might help to identify those patients who could get more benefit from lurbinectedin alone or in combination. However, as molecular subgroups are known to have a prognostic value [17] and no control arm was included in this trial, these results should be taken with caution and considered merely as hypothesis-generating. In any case, our results exemplified how molecular testing/classification should be incorporated in endometrial cancer clinical trials in the same extension as it is nowadays recommended to be included in the clinical management of endometrial cancer [16, 17].
Lurbinectedin administered at 3.2 mg/m2 as a 1-h i.v. q3wk infusion in patients with pretreated endometrial carcinoma demonstrates a predictable and manageable safety profile, with the main toxicity being reversible myelosuppression, fatigue and nausea/vomiting. Overall, the safety profile reported for lurbinectedin in this cohort of patients agrees with the results observed previously in patients with other solid tumors such as breast cancer [22], Ewing sarcoma [10] neuroendocrine tumors [23], ovarian cancer [24, 25], or SCLC [8].
In conclusion, the current efficacy results suggest that antitumor activity of lurbinectedin could be improved in patients with pretreated endometrial cancer when administered in combination with other agents and in populations with previous molecular classification. Immunotherapy added to chemotherapy has shown promising results in the first-line treatment of endometrial cancer [26,27,28,29,30]. Immunotherapy currently is placed in the second-line setting in advanced treatment of endometrial cancer, as single agent in deficient mismatch repair (dMMR), or in combination (e.g., pembrolizumab-lenvatinib) in proficient mismatch repair (pMMR). The evaluation of lurbinectedin combined with an immune checkpoint inhibitor in pMMR is warranted.
Data availability
Individual participant data are not publicly available since this requirement was not anticipated in the study protocol considering that this trial started patient enrolment in 2015. Clinical trial summary results were placed in the European Clinical Trials Database (EudraCT; https://eudract.ema.europa.eu; study 2014-003773-42) and ClinicalTrials.gov (Identifier: NCT02454972).
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497:67–73
Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, Boyd N, Pike J, Anglesio M, Kwon JS, Karnezis AN, Huntsman DG, Gilks CB, McAlpine JN (2017) Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer 123:802–813
Mahdi H, Chelariu-Raicu A, Slomovitz BM (2023) Immunotherapy in endometrial cancer. Int J Gynecol Cancer 33:351–357
Santamaria Nunez G, Robles CM, Giraudon C, Martinez-Leal JF, Compe E, Coin F, Aviles P, Galmarini CM, Egly JM (2016) Lurbinectedin specifically triggers the degradation of phosphorylated RNA polymerase II and the formation of DNA breaks in cancer cells. Mol Cancer Ther 15:1–14
Leal JF, Martinez-Diez M, Garcia-Hernandez V, Moneo V, Domingo A, Bueren-Calabuig JA, Negri A, Gago F, Guillen-Navarro MJ, Aviles P, Cuevas C, Garcia-Fernandez LF, Galmarini CM (2010) PM01183, a new DNA minor groove covalent binder with potent in vitro and in vivo anti-tumour activity. Br J Pharmacol 161:1099–1110
Harlow ML, Maloney N, Roland J, Guillen Navarro MJ, Easton MK, Kitchen-Goosen SM, Boguslawski EA, Madaj ZB, Johnson BK, Bowman MJ, D’Incalci M, Winn ME, Turner L, Hostetter G, Galmarini CM, Aviles PM, Grohar PJ (2016) Lurbinectedin inactivates the ewing sarcoma oncoprotein EWS-FLI1 by redistributing it within the nucleus. Cancer Res 76:6657–6668
Trigo J, Subbiah V, Besse B, Moreno V, López R, Sala MA, Peters S, Ponce S, Fernández C, Alfaro V, Gómez J, Kahatt C, Zeaiter A, Zaman K, Boni V, Arrondeau J, Martínez M, Delord J-P, Awada A, Kristeleit R, Olmedo ME, Wannesson L, Valdivia J, Rubio MJ, Anton A, Sarantopoulos J, Chawla SP, Mosquera-Martinez J, D’Arcangelo M, Santoro A, Villalobos VM, Sands J, Paz-Ares L (2020) Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol 21:645–654
Singh S, Jaigirdar AA, Mulkey F, Cheng J, Hamed SS, Li Y, Liu J, Zhao H, Goheer A, Helms WS, Wang X, Agarwal R, Pragani R, Korsah K, Tang S, Leighton J, Rahman A, Beaver JA, Pazdur R, Theoret MR, Singh H (2021) FDA approval summary: lurbinectedin for the treatment of metastatic small cell lung cancer. Clin Cancer Res 27:2378–2382
Subbiah V, Brana I, Longhi A, Boni V, Delord JP, Awada A, Boudou-Rouquette P, Sarantopoulos J, Shapiro GI, Elias A, Ratan R, Fernandez C, Kahatt C, Cullell-Young M, Siguero M, Zeaiter A, Chawla SP (2022) Antitumor activity of lurbinectedin, a selective inhibitor of oncogene transcription, in patients with relapsed ewing sarcoma: results of a basket phase II study. Clin Cancer Res 28:2762–2770
Kristeleit R, Moreno V, Boni V, Guerra EM, Kahatt C, Romero I, Calvo E, Baste N, Lopez-Vilarino JA, Siguero M, Alfaro V, Zeaiter A, Forster M (2021) Doxorubicin plus lurbinectedin in patients with advanced endometrial cancer: results from an expanded phase I study. Int J Gynecol Cancer 31:1428–1436
McMeekin S, Dizon D, Barter J, Scambia G, Manzyuk L, Lisyanskaya A, Oaknin A, Ringuette S, Mukhopadhyay P, Rosenberg J, Vergote I (2015) Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol Oncol 138:18–23
Miller DS, Scambia G, Bondarenko I, Westermann AM, Oaknin A, Oza AM, Lisyanskaya AS, Vergote I, Wenham RM, Temkin SM, Gabra H (2018) ZoptEC: Phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer (NCT01767155). J Clin Oncol 36:5503–5503
Forster MD, Moreno V, Boni V, Guerra E, Poveda A, Kristeleit R, Kahatt CM, Lardelli P, Vilarino JAL, Cuevas NM, Soto-Matos A, Calvo E (2017) Activity of lurbinectedin (PM01183) as single agent and in combination in patients with endometrial cancer. J Clin Oncol 35:5586–5586
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Oaknin A, Leon-Castillo A, Lorusso D (2020) Progress in the management of endometrial cancer (subtypes, immunotherapy, alterations in PIK3CA pathway): data and perspectives. Curr Opin Oncol 32:471–480
Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti A, Fotopoulou C, Gonzalez Martin A, Lax S, Lorusso D, Marth C, Morice P, Nout RA, O’Donnell D, Querleu D, Raspollini MR, Sehouli J, Sturdza A, Taylor A, Westermann A, Wimberger P, Colombo N, Planchamp F, Creutzberg CL (2021) ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 31:12–39
Aix SP, Cote GM, Gonzalez AF, Sepulveda JM, Aguilar EJ, Sanchez-Simon I, Flor MJ, Nuñez R, Gonzalez EM, Insa M, Siguero M, Cullell-Young M, Kahatt CM, Zeaiter AH, Paz-Ares LG (2020) Lurbinectedin (LUR) in combination with Irinotecan (IRI) in patients (pts) with advanced solid tumors: updated results from a phase Ib-II trial. J Clin Oncol 38:3514–3514
Kobel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG (2019) Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol 38(Suppl 1):S123–S131
National Comprehensive Cancer Network (2022) NCCN clinical practice guidelines in oncology (NCCN Guidelines): uterine neoplasms. Version 1.2022 – November 4, 2021. http://www.nccn.org/professionals/default.aspx. Accessed 22 March 2023
Makker V, Herraez A, Santin A, Colomba E, Miller D, Fujiwara K, Pignata S, Baron-Hay S, Ray-Coquard I, Shapira-Frommer R, Ushijima K, Sakata J, Yonemori K, Kim Y, Guerra E, Sanli U, McCormack M, Huang J, Smith A, Keefe S, Dutta L, Orlowski R, Lorusso D (2021) A multicenter, open-label, randomized, phase 3 study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patients with advanced endometrial cancer. Study 309/KEYNOTE-775 Society of Gynecologic Oncology 2021 Annual Meeting on Women’s Cancer. Abstract 11512
Cruz C, Llop-Guevara A, Garber JE, Arun BK, Perez Fidalgo JA, Lluch A, Telli ML, Fernandez C, Kahatt C, Galmarini CM, Soto-Matos A, Alfaro V, Perez de la Haza A, Domchek SM, Antolin S, Vahdat L, Tung NM, Lopez R, Arribas J, Vivancos A, Baselga J, Serra V, Balmana J, Isakoff SJ (2018) Multicenter phase II study of lurbinectedin in BRCA-mutated and unselected metastatic advanced breast cancer and biomarker assessment substudy. J Clin Oncol 36:3134–3143
Longo-Muñoz F, Castellano D, Alexandre J, Chawla SP, Fernández C, Kahatt C, Alfaro V, Siguero M, Zeaiter A, Moreno V, Sanz-García E, Awada A, Santaballa A, Subbiah V (2022) Lurbinectedin in patients with pretreated neuroendocrine tumours: results from a phase II basket study. Eur J Cancer 172:340–348
Poveda A, Del Campo JM, Ray-Coquard I, Alexandre J, Provansal M, Guerra Alia EM, Casado A, Gonzalez-Martin A, Fernandez C, Rodriguez I, Soto A, Kahatt C, Fernandez Teruel C, Galmarini CM, Perez de la Haza A, Bohan P, Berton-Rigaud D (2017) Phase II randomized study of PM01183 versus topotecan in patients with platinum-resistant/refractory advanced ovarian cancer. Ann Oncol 28:1280–1287
Gaillard S, Oaknin A, Ray-Coquard I, Vergote I, Scambia G, Colombo N, Fernandez C, Alfaro V, Kahatt C, Nieto A, Zeaiter A, Aracil M, Vidal L, Pardo-Burdalo B, Papai Z, Kristeleit R, O’Malley DM, Benjamin I, Pautier P, Lorusso D (2021) Lurbinectedin versus pegylated liposomal doxorubicin or topotecan in patients with platinum-resistant ovarian cancer: a multicenter, randomized, controlled, open-label phase 3 study (CORAIL). Gynecol Oncol 163:237–245
Mirza MR, Chase DM, Slomovitz BM, dePont CR, Novak Z, Black D, Gilbert L, Sharma S, Valabrega G, Landrum LM, Hanker LC, Stuckey A, Boere I, Gold MA, Auranen A, Pothuri B, Cibula D, McCourt C, Raspagliesi F, Shahin MS, Gill SE, Monk BJ, Buscema J, Herzog TJ, Copeland LJ, Tian M, He Z, Stevens S, Zografos E, Coleman RL, Powell MA, Investigators R (2023) Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med 388:2145–2158
Eskander RN, Sill MW, Beffa L, Moore RG, Hope JM, Musa FB, Mannel R, Shahin MS, Cantuaria GH, Girda E, Mathews C, Kavecansky J, Leath CA 3rd, Gien LT, Hinchcliff EM, Lele SB, Landrum LM, Backes F, O’Cearbhaill RE, Al Baghdadi T, Hill EK, Thaker PH, John VS, Welch S, Fader AN, Powell MA, Aghajanian C (2023) Pembrolizumab plus chemotherapy in advanced endometrial cancer. N Engl J Med 388:2159–2170
O’Malley DM, Bariani GM, Cassier PA, Marabelle A, Hansen AR, De Jesus AA, Miller WH Jr, Safra T, Italiano A, Mileshkin L, Xu L, Jin F, Norwood K, Maio M (2022) Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol 40:752–761
Oaknin A, Gilbert L, Tinker AV, Brown J, Mathews C, Press J, Sabatier R, O’Malley DM, Samouelian V, Boni V, Duska L, Ghamande S, Ghatage P, Kristeleit R, Leath C III, Guo W, Im E, Zildjian S, Han X, Duan T, Veneris J, Pothuri B (2022) Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer 10:e003777
Makker V, Colombo N, Herraez AC, Monk BJ, Mackay H, Santin AD, Miller DS, Moore RG, Baron-Hay S, Ray-Coquard I, Ushijima K, Yonemori K, Kim YM, Guerra Alia EM, Sanli UA, Bird S, Orlowski R, McKenzie J, Okpara C, Barresi G, Lorusso D (2023) Lenvatinib plus pembrolizumab in previously treated advanced endometrial cancer: updated efficacy and safety from the randomized phase III study 309/KEYNOTE-775. J Clin Oncol 41:2904–2910
Acknowledgements
We gratefully acknowledge the patients, their families and the investigator teams. Vivek Subbiah is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. Vivek Subbiah acknowledges support of The Jacquelyn A. Brady Fund.
Funding
The study was funded by Pharma Mar S.A, including partial funding by grants from the Centro para el Desarrollo Tecnológico Industrial (CDTI) during the conduct of the study (grant number IDI-20150006). VS is supported by a US National Institutes of Health (NIH) grant (no. R01CA242845 and R01CA273168); MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (no. RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (no. 1U01 CA180964), NCATS (Center for Clinical and Translational Sciences) Grant (no. UL1 TR000371), and the MD Anderson Cancer Center Support Grant (no. P30 CA016672).
Author information
Authors and Affiliations
Contributions
Rebecca Kristeleit: Investigation, Resources, Writing – review & editing. Alexandra Leary: Investigation, Resources, Writing – review & editing. Jean Pierre Delord: Investigation, Resources, Writing – review & editing. Victor Moreno: Investigation, Resources, Writing – review & editing. Ana Oaknin: Investigation, Resources, Writing – review & editing. Daniel Castellano: Investigation, Resources, Writing – review & editing. Geoffrey I. Shapiro: Investigation, Resources, Writing – review & editing. Cristian Fernández: Conceptualization, Methodology, Writing – Original Draft, Writing – review & editing, Supervision. Carmen Kahatt: Conceptualization, Methodology, Writing – review & editing, Supervision. Vicente Alfaro: Methodology, Writing – Original Draft, Writing – review & editing. Mariano Siguero: Methodology, Formal analysis, Writing – review & editing. Ali Zeaiter: Methodology, Writing – review & editing, Supervision. Ahmad Awada: Investigation, Resources, Writing – review & editing. Anna Santaballa: Investigation, Resources, Writing – review & editing. Khalil Zaman: Investigation, Resources, Writing – review & editing. Jalid Sehouli: Investigation, Resources, Writing – review & editing. Vivek Subbiah: Conceptualization, Investigation, Resources, Writing – Original Draft, Writing – review & editing.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committees, and with the Declaration of Helsinki or comparable ethical standards.
Informed consent
Written informed consent was obtained from all patients included in the study.
Competing interests
Alexandra Leary has grants or contracts from Agenus, Astra Zeneca, BMS, GSK, Iovance, MSD, OSE Immuno, Roche as principal investigator in clinical trials and from ARCAGY-GYENCO, AZ, Sanofi for translational research; has payment as invited speaker from Astra Zeneca, Clovis, GSK, Kephren publishing, Medscape; payment for consultancy from GLG and Orion, and payment for writing engagement from Onko+; has participation in Advisory Boards from Ability Pharma, Apmonia, Astra Zeneca, Blueprint, Clovis, GSK, Merck Serono, MSD, Tesaro and Zentaris; has participation in a steering committee from MSD; was IDMC member or chair for Clovis and Pfizer; and participated in an Academic Research Project for LX Repair and Owkin. Ana Oaknin has grants or contracts from Abbvie Deutschland Gmbh & Co Hg, Ability Pharmaceuticals, Advaxis, Agenus, Aprea Therapeutics AB, Astrazeneca AB, Beigene USA, Inc., Belgian Gynaecological Oncology Group (BGOG), Bristol‐Myers Squibb International Corporation (BMSM Clovis Oncology, Corcept Therapeutics, Eisai, F. Hoffmann‐La Roche, Grupo Español de Investigación en Cáncer de Ovario (GEICO), Immunogen, Iovance Biotherapeutics, Lilly, Medimmune, Merck Healthcare, Merck Sharp & dohme, Millennium Pharmaceuticals, Mundipharma Research, Novartis Farmacéutica, Regeneron Pharmaceuticals, Seagen, Seattle Genetics, Sutro Biopharma, Tesaro, University Health Network, and Werastem; has payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from ESMO, Edizioni Minerva Medica SpA and Doctaforum Servicios S.L.; has support for attending meetings and/or travel from AstraZeneca; Clovis Oncology; PharmaMar, and Roche; and participation on a Data Safety Monitoring Board or Advisory Board from Agenus, AstraZeneca, Clovis Oncology, Inc., Corcept Therapeutics, Deciphera Pharmaceutical, Eisai Europe Limited, EMD Serono, Inc., F. Hoffmann‐La Roche, GlaxoSmithKline, Immunogen, KL Logistics, Medison Pharma, Merck Sharp & Dohme de España, Mersana Therapeutics, Novocure GmbH, Pharma Mar, prIME Oncology, ROCHE FARMA, Sattucklabs, and Sutro Biopharma, Inc., as well as participation to non‐remunerated activities and non‐remunerated leadership roles for GCIC and GEICO. Daniel Castellano have payment for Advisory Board or Expert Opinion from Pfizer, Roche, BMS, MSD, Astellas, Astra Zeneca, Novartis, Gilead, Ipsen, Pierre Fabre, Sanofi, Eisai, Janssen and GSK. Geoffrey. I. Shappiro has payment to Dana-Farber Cancer Institute from PharmaMar for conduct of the clinical trial; has sponsored research agreement with payment to DFCI from Merck KGaA/EMD-Serono, Merck & Co. and Lilly; has funding for investigator-initiated clinical trials to DFCI from Pfizer; consulting fees from XinThera, Inc.; has patent issued to Cyclacel Pharmaceuticals and Geoffrey I. Shapiro for dosage regimen for sapacitabine and seliciclib, and pending to Liam Cornell and Geoffrey I. Shapiro for compositions and methods for predicting response and resistance to CDK4/6 inhibition; and has participation on advisory boards for Pfizer, Eli Lilly, Merck KGaA/EMD-Serono, Bicycle Therapeutics, Fusion Pharmaceuticals, Cybrexa Therapeutics, Bayer, Boehringer Ingelheim, ImmunoMet, Asana, Artios, Atrin, Concarlo Holdings, Syros, Zentalks, CytomX Therapeutics, Blueprint Medicines, Kymera Therapeutics, and Janssen. Cristian Fernández, Carmen Kahatt, Vicente Alfaro, Mariano Siguero, Daniel Rueda and Ali Zeaiter have personal fees for salary as full time employee from PharmaMar S.A. Cristian Fernández, Carmen Kahatt, Vicente Alfaro, Daniel Rueda and Ali Zeaiter are stock ownership of PharmaMar S.A. Ahmad Awada has advisory role with Amgen, AstraZeneca, Bayer, Daiichi, EISAI, Genomic Health, Hengrui, Innate, Ipsen, Leo Pharma, Lilly, Merck, MSD, Novartis, Pfizer and Seattle Genetics; has speaker fees with Amgen, AstraZeneca, Bayer, Daiichi, EISAI, Genomic Health, Ipsen, Leo Pharma, Lilly, Merck, MSD, Novartis, Pfizer and Seattle Genetics; and has research grants to his Institute by BSM and Roche. Vivek Subbiah received grants from PharmaMar, Eli Lilly/LOXO Oncology, Blueprint Medicines Corporation, Turning PointTherapeutics and Boston Pharmaceuticals; and grants fromHelsinn Pharmaceuticals during the conduct of the study; in addition, Vivek Subbiah received a grant and advisory board/consultant position with Eli Lilly/Loxo Oncology during the conduct of the study; research grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, AbbVie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, NCI-CTEP, University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, Novartis, PharmaMar and Medimmune; an advisory board/consultant position with Helsinn, Incyte, QED Pharma, Daiichi-Sankyo, Signant Health, Novartis, Relay therapeutics, Roche and Medimmune; travel funds from PharmaMar, Incyte, ASCO, ESMO; and other support from Medscape, all outside the submitted work. The remaining authors made no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kristeleit, R., Leary, A., Delord, J.P. et al. Lurbinectedin in patients with pretreated endometrial cancer: results from a phase 2 basket clinical trial and exploratory translational study. Invest New Drugs 41, 677–687 (2023). https://doi.org/10.1007/s10637-023-01383-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01383-2