Summary
We conducted two indirect comparisons to estimate the efficacy of zanubrutinib versus orelabrutinib in Chinese patients with relapsed or refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) or R/R mantle cell lymphoma (MCL). An unanchored matching-adjusted indirect comparison (MAIC) was performed in R/R CLL/SLL patients. Individual patient data from zanubrutinib trial (BGB-3111-205) were adjusted to match the aggregated data from the orelabrutinib trial (ICP-CL-00103). A naïve comparison was performed in R/R MCL for the different response assessment methodology and efficacy analysis set between the zanubrutinib (BGB-3111-206) and orelabrutinib (ICP-CL-00102) trials. Efficacy outcomes included ORR and PFS. In R/R CLL/SLL patients, after matching, IRC-assessed ORR was comparable (86.6% vs. 92.5%; risk difference, -5.9% [95% CI: -15.8%-3.8%]); IRC-assessed PFS was similar with a favorable trend in zanubrutinib over orelabrutinib (HR, 0.74 [95% CI: 0.37-1.47]) and the 18-month PFS rate was numerically higher in zanubrutinib (82.9% vs. 78.7%). In R/R MCL patients, naïve comparison showed investigator-assessed ORR was similar (83.7% vs. 87.9%; risk difference, -4.2% [95% CI: -14.8%-6.0%]), and CR rate was significantly higher in zanubrutinib over orelabrutinib (77.9% vs. 42.9%; risk difference, 35.0% [95% CI: 14.5%, 53.7%]). Investigator-assessed PFS was similar with a favorable trend (HR, 0.77 [95% CI: 0.45-1.32]) in zanubrutinib over orelabrutinib and the 12-month PFS rate was numerically higher in zanubrutinib (77.5% vs. 70.8%). MAIC result showed zanubrutinib demonstrated favorable PFS over orelabrutinib for R/R CLL/SLL patients. The naïve comparison showed zanubrutinib had favorable PFS and higher CR rate than orelabrutinib for R/R MCL patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The B-cell receptor (BCR) signal pathway is a critical contributor to the survival and proliferation of malignant B cells [1, 2]. Inhibition of the BCR signal has been shown as a promising therapeutic method for B-cell malignancies [3]. Bruton’s tyrosine kinase (BTK), an essential member of the BCR signal pathway, plays an important role in the B-cell development involved in B-cell proliferation, maturation, differentiation, apoptosis, and migration [4]. Ibrutinib, a first-in-class BTK inhibitor, was first approved in the US in 2013 for the treatment of relapsed or refractory (R/R) mantle cell lymphoma (MCL) and has been a standard of care for naïve and R/R chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), R/R MCL, and R/R Waldenström macroglobulinemia (WM). However, the off-target kinase inhibition of ibrutinib, which was thought to cause diarrhea, bleeding, and atrial fibrillation [5], may limit its use as a treatment option. Highly selective, next-generation BTK inhibitors with fewer off-target effects are needed and being developed. Currently, several next-generation BTK inhibitors have been approved and launched, including acalabrutinib, zanubrutinib, and orelabrutinib. Zanubrutinib and orelabrutinib have been launched in China for the treatment of R/R CLL/SLL and R/R MCL.
Zanubrutinib, a novel next-generation BTK inhibitor, has greater selectivity for BTK and less off-target activity versus ibrutinib [6, 7]. Zanubrutinib was first approved in the US in 2019 as a breakthrough therapy for patients with MCL who received at least one prior therapy. It has received approval from China’s National Medical Products Administration (NMPA) for the treatment of R/R MCL, R/R CLL/SLL, and R/R WM. Two phase 3 head-to-head studies [8, 9] have demonstrated that zanubrutinib had less off-target toxicities (particularly cardiovascular toxicity) than ibrutinib in the treatment of WM and R/R CLL/SLL, and superior ORR and PFS over ibrutinib for R/R CLL/SLL [9, 10].
Orelabrutinib is also a novel next-generation BTK inhibitor with high selectivity for BTK [11] that has been only approved in China for the treatment of R/R CLL/SLL and R/R MCL.
BTK resynthesis was faster in patients with CLL than in healthy volunteers; therefore, it is hypothesized that complete and sustained BTK occupancy may improve efficacy outcomes [12]. Zanubrutinib has exposure coverage above its enzymatic half-maximal inhibitory concentration (IC50) during the entire dose interval for both twice daily and once daily dosing schedules. The ratio of Ctrough (trough concentrations) /IC50 is 7 and 2 resulting in significantly higher concentrations than the IC50 during the entire 24-hr dosing period for both dosing schedules [13]. While the corresponding ratios of Ctrough/IC50 for orelabrutinib were estimated to be lower than 1 [14].
Because zanubrutinib and orelabrutinib are both approved and are widely used in China as treatment options for R/R CLL/SLL and R/R MCL, a further evaluation to understand the difference between these two novel BTKis is helpful for planning future research. In addition, with the absence of head-to-head studies, an indirect treatment comparison is an appropriate approach to evaluate the comparative efficacy of zanubrutinib and orelabrutinib based on different clinical trials. Currently, orelabrutinib only has available data on the Chinese patient population, thus the studies of zanubrutinib on the Chinese population were used in the indirect treatment comparison.
In the present study, we conducted two separate indirect comparisons to assess the efficacy of the two NMPA-approved next-generation BTK inhibitors zanubrutinib and orelabrutinib in the treatment of R/R CLL/SLL or R/R MCL patient based on Chinese populations.
Methods
An unanchored matching-adjusted indirect comparison (MAIC) [15] was performed in patients with R/R CLL/SLL, and a naïve comparison was performed in patients with R/R MCL due to the different response assessment methodology and efficacy analysis set between the zanubrutinib and orelabrutinib trials. Individual patient data (IPD) from zanubrutinib studies (BGB-3111-205, BGB-3111-206) and aggregated data reported in publications from orelabrutinib studies (ICP-CL-00103, ICP-CL-00102) were used. Data were available from several cutoff dates of each included study. The data cutoff of the orelabrutinib study with the longest follow-up time of the primary endpoint was selected. The data cutoff of the zanubrutinib study with a comparable median follow-up time was selected for the comparisons.
Data sources
For R/R CLL/SLL
The data source of the efficacy of zanubrutinib in patients with R/R CLL/SLL was IPD, which is from the BGB-3111-205 study (NCT03206918). BGB-3111-205 [16, 17] is a single-arm, multicenter, phase II study of zanubrutinib in Chinese patients with R/R CLL/SLL. The primary endpoint was the overall response rate (ORR) assessed by an independent review committee (IRC). The longest data cutoff date of BGB-3111-205 used in this study was 1 December 2020, with a median follow-up time of 34 months [17], which was similar to the orelabrutinib study of ICP-CL-00103 study.
The literature review identified two publications [18, 19] of the ICP-CL-00103 study (NCT03493217) as the aggregated data source of orelabrutinib in patients with R/R CLL/SLL. The data cutoff date of the primary data source [18] of the ICP-CL-00103 study was 16 January 2021, which the IRC-assessed ORR was based on the longest follow-up (median follow-up: 25.6 months). The data cutoff date of the secondary data source of the ICP-CL-00103 study [19] was 10 August 2021, which the investigator-assessed ORR was based on the longest follow-up (median follow-up: 33.1 months). The ICP-CL-00103 study is an open-label, multicenter, single-arm phase II study of orelabrutinib in patients with R/R CLL/SLL. The primary endpoint was IRC-assessed ORR.
The eligibility criteria were similar between the BGB-3111-205 and ICP-CL-00103 studies, as shown in Supplementary Table 1.
For R/R MCL
The data source of zanubrutinib was IPD from the BGB-3111-206 study (NCT03206970). BGB-3111-206 [20, 21] is a single-arm, open-label, phase II study of zanubrutinib in patients with R/R MCL. The primary endpoint was ORR assessed by an IRC. The data cutoff date of BGB-3111-206 used in this study was 15 February 2019 with a median follow-up time of 18.4 months [20], to achieve a similar median follow-up time as the ICP-CL-00102 study.
The literature review identified one publication [22] (data cutoff: 10 April 2020; median follow-up: 16.4 months) of the ICP-CL-00102 study (NCT03494179) as the aggregated data source of orelabrutinib. ICP-CL-00102 is a multicenter, open-label, phase II study of orelabrutinib in patients with R/R MCL. The primary endpoint was ORR assessed by an IRC.
The eligibility criteria were similar between the BGB-3111-206 and ICP-CL-00102 studies, as shown in Supplementary Table 2.
Efficacy outcomes and assessment
Efficacy outcomes included IRC-assessed and investigator-assessed ORR and PFS.
PFS
The definitions of PFS were similar across all four studies. PFS was defined as the time from the first dose of treatment to progression or death.
ORR
For R/R CLL/SLL
The definitions of ORR were similar between the BGB-3111-205 and ICP-CL-00103 studies. For patients with R/R CLL/SLL, responses for patients with CLL included partial response (PR), PR with lymphocytosis, nodular PR, complete response (CR), or CR with incomplete hematologic recovery, and for patients with SLL, either PR or CR.
The response assessment methodology and efficacy analyses set were also similar between the two studies. The response assessment was according to the International Workshop on CLL guidelines [23] or the Lugano classification for SLL [24]. The efficacy analyses set included patients who received at least one dose of treatment.
For R/R MCL
The definitions of efficacy outcomes were similar between the BGB-3111-206 and ICP-CL-00102 studies. ORR was defined as either a PR or CR. However, the primary endpoint of IRC-assessed ORR was not reported in the ICP-CL-00102 study [22]; only investigator-assessed ORR was available. Moreover, the response assessment methodology and efficacy analyses set were different between the BGB-3111-206 and ICP-CL-00102 studies. Response evaluation was a PET-based assessment according to Lugano criteria [24] in the BGB-3111-206 study, while was a CT-based assessment according to Lugano criteria [24] in the ICP-CL-00102 study (CR was evaluated according to PET in only 28 out of 106 patients). The efficacy analysis set included all patients who received at least one dose of zanubrutinib, while the efficacy analysis set of the ICP-CL-00102 study excluded patients who were not evaluable due to protocol violation, adverse event (AE), or dropped out before the first assessment. The naïve comparison results will be conservative for zanubrutinib since the orelabrutinib trial excluded patients from the denominator in the process of estimating response rates; while those excluded unevaluable patients were included and counted as non-responders in the zanubrutinib trial.
Study comparisons
For R/R CLL/SLL
The study design, eligibility criteria, efficacy endpoints, and baseline characteristics showed sufficient similarities between the BGB-3111-205 and ICP-CL-00103 studies. The imbalances in the patient population between the two studies necessitated an MAIC to reduce bias when indirectly comparing zanubrutinib to orelabrutinib.
For R/R MCL
The study design and eligibility criteria were similar between the BGB-3111-206 and ICP-CL-00103 studies. However, the response assessment methodology and efficacy analysis set were different between the BGB-3111-206 and ICP-CL-00102 studies. Therefore, a naïve comparison was conducted to indirectly and descriptively compare the efficacy of zanubrutinib versus orelabrutinib in patients with R/R MCL.
Statistical analysis methods
For R/R CLL/SLL
An unanchored MAIC was conducted in patients with R/R CLL/SLL. MAIC is a propensity-score-weighting-based method to generate comparative effectiveness evidence when IPD is available in one study and aggregate data in another [15]. In this analysis, the unanchored MAIC adjusts the mean of effect modifiers and prognostic factors in BGB-3111-205 to match those reported characteristics for ICP-CL-00103.
The first step when implementing a MAIC is to align the patient population of the trials to be compared. We excluded one SLL patient with Ann-Arbor stage Phase 1 and five CLL patients with Binet stage A from BGB-3111-205 to match the population of ICP-CL-00103. Patients across two trials were matched on available potential effect modifiers and prognostic variables, including age category, sex, ECOG performance status, bulky disease, IGHV unmutated, cytogenetic mutation (Del[17p] or TP53 mutation, Del[11q], Trisomy 12), and the number of prior lines of treatment. The baseline characteristics to be matched in MAIC were selected based on the preliminary feasibility assessment and discussions with clinical experts. The weight of individual patients was calculated by the method of moments, in line with published guidance from the National Institute for Health and Care Excellence Decision Support Unit [25]. The weighted efficacy outcomes of BGB-3111-205 were compared with those reported in ICP-CL-00103. The survival outcomes of ICP-CL-00103 were estimated from the pseudo IPD generated from the digitized Kaplan-Meier curve and the at-risk table of ICP-CL-00103 [26]. We notice that the digitization process may introduce bias. Therefore, the reconstructed pseudo IPD was visually inspected by the plots of the estimated Kaplan-Meier curve versus read-in, the estimated numbers of patients at risk versus reported, and the estimated survival probabilities minus read-in survival probabilities over time by the R package IPDfromKM [26]. All validation plots showed the pseudo IPD was numerically accurate and thus the numerical error was almost negligible. The hazard ratio with a 95% confidence interval of the survival outcomes between two trials was estimated by a (weighted) Cox model. Risk differences with a 95% confidence interval of all binary outcomes between two trials were estimated by the Miettinen-Nurminen method.
For R/R MCL
A naïve indirect comparison was performed in patients with R/R MCL due to the different response assessment methodology and efficacy analysis set between BGB-3111-206 and ICP-CL-00102. As only investigator-assessed outcomes were reported in the publication of the orelabrutinib trial, the investigator-assessed outcomes were used and compared for R/R MCL patients. The difference of important baseline characteristics and efficacy outcomes were estimated for descriptive purpose. The survival outcomes of ICP-CL-00102 were estimated by the same method as that for ICP-CL-00103. The hazard ratio and risk differences with a 95% confidence interval were estimated by the same methods as those for R/R CLL/SLL.
All analyses were performed using R version 4.0.2. The results reported in this paper are post hoc analysis result.
Results
Baseline characteristics
For R/R CLL/SLL
Before matching, the baseline characteristics were highly comparable between orelabrutinib and zanubrutinib (Table 1). Only the proportion of patients with IGHV unmutated was a little higher in the zanubrutinib group than in the orelabrutinib group (56% vs. 41%). After matching, the baseline characteristics were balanced between the two populations, with an effective sample size of 66 in the zanubrutinib group.
For R/R MCL
The baseline characteristics were comparable between orelabrutinib and zanubrutinib (Table 2). Patients in the zanubrutinib group had a higher proportion of ≥2 prior lines of treatment (71% vs. 55%) and a lower proportion of ECOG PS ≥1 (30% vs. 54%) compared with those in the orelabrutinib group. As the response assessment methodology and efficacy analysis set were different between the BGB-3111-206 and ICP-CL-00102 studies, a naïve comparison was conducted to assess the efficacy between zanubrutinib and orelabrutinib.
Efficacy outcomes
Response rates
For R/R CLL/SLL
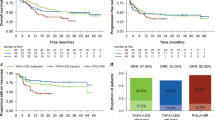
Based on the IRC assessment, after matching, ORR was comparable between zanubrutinib and orelabrutinib (86.6% vs. 92.5%; risk difference, -5.9% [95% CI: -15.8%, 3.8%]; Table 3). CR rate was significantly different between zanubrutinib versus orelabrutinib (5.7% vs. 16.3%; risk difference, -10.5% [95% CI: -20.9%, -1.1%]).
Based on the investigator’s assessment, after matching, ORR was also comparable between zanubrutinib versus orelabrutinib (90.1% vs. 93.8%; risk difference, -3.7% [95% CI: -12.8%, 5.2%]). CR rate was significantly lower in zanubrutinib compared with orelabrutinib (11.0% vs. 26.3%; risk difference, -15.2% [95% CI, -27.2%, -3.4%]).
For R/R MCL
The naïve comparison showed that ORR assessed by the investigator was similar between zanubrutinib and orelabrutinib (83.7% vs. 87.9%; risk difference, -4.2 [95% CI: -14.8%, 6.0%]). CR rate was significantly higher in zanubrutinib compared with orelabrutinib (77.9% vs. 42.9%; risk difference, 35.0% [95% CI: 14.5%, 53.7%]; Table 4).
Survival outcomes
For R/R CLL/SLL
As the survival outcome assessed by IRC, with the median follow-up time of 34 months in zanubrutinib and 25.6 months in orelabrutinib, PFS was not reached (NR) in both groups but was more favorable in zanubrutinib compared with orelabrutinib with a hazard ratio (HR) of 0.74 (95% CI, 0.37-1.47) after matching (Table 3; Fig. 1). The unadjusted comparison had the consistent result that PFS was favorable in zanubrutinib compared with in orelabrutinib (median PFS: NR vs. NR; HR, 0.69 [95% CI: 0.36-1.31]). The 18-month PFS rate was numerically higher in zanubrutinib than in orelabrutinib (82.9% vs. 78.7%) after matching.
The survival outcomes assessed by the investigator were also analyzed. With the median follow-up time of 34 months in zanubrutinib and 33.1 months in orelabrutinib, zanubrutinib had favorable PFS compared with orelabrutinib (median PFS: NR vs. NR; HR, 0.84 [95% CI: 0.47-1.53]) after matching. The 30-month PFS rate was also numerically higher in zanubrutinib than in orelabrutinib (73.6% vs. 69.7%) after matching.
For R/R MCL
With the median follow-up time of 18.4 months in zanubrutinib and 16.4 months in orelabrutinib, the PFS assessed by the investigator was favorable in zanubrutinib compared with orelabrutinib (median PFS: NR vs. NR; HR, 0.77 [95% CI: 0.45-1.32]; Table 4; Fig. 2). The 12-month PFS rate was also numerically higher in zanubrutinib than in orelabrutinib (77.5% vs. 70.8%).
Discussion
CLL/SLL are slow-growing types of indolent non-Hodgkin Lymphoma. The ultimate treatment goal of CLL/SLL is to achieve a longer overall survival (OS), while minimizing toxicities and improving the quality of life. In the absence of a survival benefit, achieving a long PFS is a reasonable goal of therapy [27]. For patients with CLL/SLL, continuous treatment with a single-agent BTK inhibitor significantly improved PFS and OS versus standard chemotherapy and/or chemoimmunotherapy [28,29,30,31]. Moreover, the interim analysis of a head-to-head study (ALPINE) showed an improved PFS in patients with R/R CLL/SLL treated with zanubrutinib compared with ibrutinib [9], and the final analysis showed that zanubrutinib is superiority to ibrutinib in PFS (HR: 0.65 [95% CI, 0.49-0.86]; P = 0.0024) in patients with R/R CLL/CLL [10]. There is no head-to-head study comparing any two next-generation BTK inhibitors. Shadman et al found that zanubrutinib showed higher selectivity than ibrutinib and acalabrutinib, and comparable selectivity to orelabrutinib. In addition, zanubrutinib had a lower IC50 value (0.71 vs. 15 nM) against BTK compared with orelabrutinib [14], which might contribute to the favorable efficacy of zanubrutinib over orelabrutinib. In this study, with the median follow-up of 34 months in zanubrutinib and 25.6 months in orelabrutinib, ORR was comparable, and median PFS was not reached but demonstrated a favorable trend on PFS for zanubrutinib over orelabrutinib in R/R CLL/SLL patients (IRC and investigator-assessed PFS HR of 0.74 and 0.69, respectively). No significant difference in PFS was observed, which might be due to the limited sample size and the relatively short follow-up time. Further updated analyses with a long follow-up time may help to consolidate these results. What we have found in this study was supported by a network meta-analysis [32], which aimed to estimate the efficacy of zanubrutinib versus standard of treatment for R/R CLL. Results suggested a remarkable improvement in PFS for zanubrutinib over acalabrutinib (HR, 0.52 [95% CI: 0.30-0.90]) and a trend favoring zanubrutinib over acalabrutinib in OS (HR, 0.75 [95% CI: 0.35-1.59]). These findings indicate that PFS on zanubrutinib might be superior to other next-generation BTK inhibitors for patients with R/R CLL/SLL.
R/R MCL has historically poor long-term survival compared with other B-cell malignancies [33]. In addition to survival outcomes, the treatment strategies for R/R MCL tend to focus on the relief of patients’ symptoms and their response. Ibrutinib was approved by the FDA for R/R MCL based on results of a phase 2 trial with a long-term ORR of 78% and CR of 27% [34]. The FDA approved zanubrutinib for R/R MCL when it showed an ORR of 83.7% and CR of 77.9% [35]. There is no study on the comparison of different next-generation BTK inhibitors. In this study, we conducted a naïve indirect treatment comparison due to the different response evaluation methodology between zanubrutinib and orelabrutinib studies (a PET-based assessment in the zanubrutinib study while a CT-based assessment in the orelabrutinib study). With the short median follow-up time of 18.4 months in zanubrutinib and 16.4 months in orelabrutinib, zanubrutinib has shown a favorable PFS trend over orelabrutinib with the HR of 0.77. CR rate was also significantly higher in zanubrutinib than in orelabrutinib.
We did not analyze the AEs in this study considering the potential differences in AE collection, reporting, follow-up time between studies, and limited sample size.
With the growing number of approved next-generation BTK inhibitors, it is important to understand the potential differences between them. The findings in the present study may provide useful information for future study.
However, there are several limitations in this study. In the R/R CLL/SLL analysis, because both included trials are single-arm trials, the comparison was conducted through an unanchored MAIC analysis, which relies on the assumption that all prognostic factors and effect modifiers are identified and included in population matching. Despite all available prognostic factors and effect modifiers having been included to reduce the bias, this assumption is still strong and can never be verified. Potential violation of this assumption leads to bias. In the R/R MCL analysis, the response assessment methodology and efficacy analysis set were different between the two included trials. Therefore, only a naïve comparison was conducted, and all the results are descriptive. However, the results of both analyses, even though they are likely biased, generated signals and hypotheses for further research.
Conclusions
In conclusion, MAIC results showed that zanubrutinib demonstrated a comparable ORR but more favorable PFS compared with orelabrutinib for R/R CLL/SLL patients, and the naïve comparison showed that zanubrutinib had a higher CR rate and favorable PFS over orelabrutinib for R/R MCL patients.
Data availability
The datasets used are available from the corresponding author on reasonable request.
References
Bernard S, Danglade D, Gardano L, Laguillier C, Lazarian G, Roger C, Thieblemont C, Marzec J, Gribben J, Cymbalista F, Varin-Blank N, Ledoux D, Baran-Marszak F (2015) Inhibitors of BCR signalling interrupt the survival signal mediated by the micro-environment in mantle cell lymphoma. Int J Cancer 136:2761–2774. https://doi.org/10.1002/ijc.29326
Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G (2011) B-cell receptor signaling in chronic lymphocytic leukemia. Blood J Am Soc Hematol 118:4313–4320. https://doi.org/10.1182/blood-2011-06-338855
Niemann CU, Wiestner A (2013) B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol 23:410–421. https://doi.org/10.1016/j.semcancer.2013.09.001
Niiro H, Clark EA (2002) Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol 2:945–956. https://doi.org/10.1038/nri955
Coutre SE, Byrd JC, Hillmen P, Barrientos JC, Barr PM, Devereux S, Robak T, Kipps TJ, Schuh A, Moreno C, Furman RR, Burger JA (2019) Long-term safety of single-agent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. 3:1799–1807. https://doi.org/10.1182/bloodadvances.2018028761
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA, Buggy JJ (2010) The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 107:13075–13080. https://doi.org/10.1073/pnas.1004594107
Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, Harrup R, Johnston PB, Marlton P, Munoz J, Seymour JF, Simpson D, Tedeschi A, Elstrom R, Yu Y, Tang Z, Han L, Huang J, Novotny W, Wang L, Roberts AW (2019) Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 134:851–859. https://doi.org/10.1182/blood.2019001160
Tam CS, Opat S, D’Sa S, Jurczak W, Lee HP, Cull G, Owen RG, Marlton P, Wahlin BE, Sanz RG, McCarthy H, Mulligan S, Tedeschi A, Castillo JJ, Czyz J, Fernández de Larrea C, Belada D, Libby E, Matous JV, Motta M, Siddiqi T, Tani M, Trneny M, Minnema MC, Buske C, Leblond V, Trotman J, Chan WY, Schneider J, Ro S, Cohen A, Huang J, Dimopoulos M (2020) A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood 136:2038–2050. https://doi.org/10.1182/blood.2020006844
Hillmen P, Eichhorst B, Brown JR, Lamanna N, O’Brien SM, Tam CS, Qiu L, Kazmierczak M, Zhou K, Šimkovič M, Mayer J, Gillespie-Twardy A, Shadman M, Ferrajoli A, Ganly PS, Weinkove R, Grosicki S, Mital A, Robak T, Österborg A, Yimer HA, Salmi T, Ji M, Yecies J, Idoine A, Wu K, Huang J, Jurczak W (2022) Zanubrutinib Versus Ibrutinib in Relapsed/Refractory Chronic lymphocytic leukemia and small lymphocytic lymphoma: interim analysis of a Randomized Phase III Trial. J Clin Oncol JCO 22.00510
Brown JR, Eichhorst B, Hillmen P, Jurczak W, Kaźmierczak M, Lamanna N, O’Brien SM, Tam CS, Qiu L, Zhou K, Simkovic M, Mayer J, Gillespie-Twardy A, Ferrajoli A, Ganly PS, Weinkove R, Grosicki S, Mital A, Robak T, Osterborg A, Yimer HA, Salmi T, Wang MD, Fu L, Li J, Wu K, Cohen A, Shadman M (2023) Zanubrutinib or Ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 388:319–332. https://doi.org/10.1056/NEJMoa2211582
Zhang B, Zhao R, Liang R, Gao Y, Liu R, Chen X, Wang Z, Yu L, Shakib Z, Cui J (2020) Orelabrutinib, a potent and selective Bruton’s tyrosine kinase inhibitor with superior safety profile and excellent PK/PD properties. Cancer Res 80:132
Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, van Hoek M, de Zwart E, Mittag D, Demont D, Verkaik S, Krantz F, Pearson PG, Ulrich R, Kaptein A (2017) Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther 363:240–252. https://doi.org/10.1124/jpet.117.242909
Tam CS, Ou YC, Trotman J, Opat S (2021) Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev Clin Pharmacol 14:1329–1344. https://doi.org/10.1080/17512433.2021.1978288
Shadman M, Flinn IW, Levy MY, Porter R, Burke JM, Cultrera JL, Misleh J, Zafar SF, Freeman B, Rao SS, Yimer H, Chaudhry A, Gandhi MD, Guthrie TH, Kingsley E, Tumula PK, Manda S, Chen D-Y, Cohen A, By K, Xu L, Liu Y, Sharman JP (2021) Phase 2 study of Zanubrutinib in BTK inhibitor-intolerant patients (pts) with Relapsed/Refractory B-Cell malignancies. Blood 138:1410–1410. https://doi.org/10.1182/blood-2021-148544
Signorovitch JE, Wu EQ, Yu AP, Gerrits CM, Kantor E, Bao Y, Gupta SR, Mulani PM (2010) Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. PharmacoEconomics 28:935–945. https://doi.org/10.2165/11538370-000000000-00000
Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, Gao S, Zhou D, Hu J, Feng R, Huang H, Ji M, Guo H, Huang J, Novotny W, Feng S, Li J (2020) Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study. J Hematol Oncol 13:48. https://doi.org/10.1186/s13045-020-00884-4
Xu W, Yang S, Zhou K, Pan L, Li Z, Zhou J, Gao S, Zhou D, Hu J (2021) Feng R Zanubrutinib monotherapy in patients with relapsed or refractory chronic lymphocytic leukemia: 34-month follow-up results. In: European Hematology Association 2021 Virtual Congress
Xu W, Song Y, Wang T, Yang S, Liu L, Hu Y, Zhang W, Zhou J, Gao S, Ding K, Zhang H, Zhu Z, Wang S, Xu B, Hu J, Liu T, Ji C, Xia Z, Li Y, Wang X, Zhao R, Zhang B, Li J (2021) Updated efficacy and safety results of orelabrutinib in the treatment of relapsed or refractory chronic lymphocytic leukemia/small cell leukemia. Hematol Oncol. https://doi.org/10.1002/hon.43_2880
Xu W, Song Y, Wang T, Yang S, Liu L, Hu Y, Zhang W, Zhou J, Gao S, Ding K, Zhang H, Zhu Z, Wang S, Xu B, Hu J, Liu T, Ji C, Xia Z, Li Y, Wang X, Zhang B, Zhao R, Li J (2021) Orelabrutinib monotherapy in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma: updated long term results of phase II study. Blood 138:2638. https://doi.org/10.1182/blood-2021-146491
Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, Ji J, Xu W, Jin J, Lv F, Feng R, Gao S, Guo H, Zhou L, Elstrom R, Huang J, Novotny W, Wei R, Zhu J (2020) Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton’s tyrosine kinase. Clin Cancer Res 26:4216–4224. https://doi.org/10.1158/1078-0432.ccr-19-3703
Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, Ji J, Xu W, Jin J, Lv F, Feng R, Gao S, Guo H, Zhou L, Huang J, Novotny W, Kim P, Yu Y, Wu B, Zhu J (2022) Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study. Blood 139:3148–3158. https://doi.org/10.1182/blood.2021014162
Song Y, Song Y, Liu L, Zhang M, Li Z, Ji C, Xu W, Liu T, Xu B, Wang X, Gao S, Zhang H, Hu Y, Li Y, Cheng Y, Yang H, Cao J, Zhu Z, Hu J, Zhang W, Jing H, Ding K, Lu Z, Zhang B, Zhao R, Xu Z, Zhu J (2019) Safety and Efficacy of Orelabrutinib Monotherapy in chinese patients with relapsed or refractory mantle cell lymphoma: a multicenter, open-label, phase II study. Blood 134:755–755. https://doi.org/10.1182/blood-2019-126305
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, Hillmen P, Keating MJ, Montserrat E, Rai KR, Kipps TJ, International Workshop on Chronic Lymphocytic L (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111:5446–5456. https://doi.org/10.1182/blood-2007-06-093906
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance AL, Lymphoma G, Eastern Cooperative Oncology G, European Mantle Cell Lymphoma C, Italian Lymphoma F, European Organisation for R, Treatment of Cancer/Dutch Hemato-Oncology G, Grupo Espanol de Medula O, German High-Grade Lymphoma Study G, German Hodgkin’s Study G, Japanese Lymphorra Study G, Lymphoma Study A, Group NCT, Nordic Lymphoma Study G, Southwest Oncology G, United Kingdom National Cancer Research I (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068. https://doi.org/10.1200/JCO.2013.54.8800
Phillippo D, Ades T, Dias S, Palmer S, Abrams KR, Welton N (2016) NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE
Liu N, Zhou Y, Lee JJ (2021) IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol 21:111. https://doi.org/10.1186/s12874-021-01308-8
Owen C, Peters A, Puckrin R, Shafey M, Tilley D, Cancer Care Alberta, Alberta Health Services (2022) Clinical practice guideline on chronic lymphocytic leukemia, version 8
Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, Kipps TJ, Moreno C, Montillo M, Burger JA, Byrd JC, Hillmen P, Dai S, Szoke A, Dean JP, Woyach JA (2019) Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol 94:1353–1363. https://doi.org/10.1002/ajh.25638
Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, Kaplan P, Kraychok I, Illes A, de la Serna J, Dolan S, Campbell P, Musuraca G, Jacob A, Avery E, Lee JH, Liang W, Patel P, Quah C, Jurczak W (2020) ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 38:2849–2861. https://doi.org/10.1200/jco.19.03355
Tam CS, Brown JR, Kahl BS, Ghia P, Giannopoulos K, Jurczak W, Šimkovič M, Shadman M, Österborg A, Laurenti L, Walker P, Opat S, Chan H, Ciepluch H, Greil R, Tani M, Trněný M, Brander DM, Flinn IW, Grosicki S, Verner E, Tedeschi A, Li J, Tian T, Zhou L, Marimpietri C, Paik JC, Cohen A, Huang J, Robak T, Hillmen P (2022) Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol 23:1031–1043. https://doi.org/10.1016/s1470-2045(22)00293-5
Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, Jelinek DF, Braggio E, Leis JF, Zhang CC, Coutre SE, Barr PM, Cashen AF, Mato AR, Singh AK, Mullane MP, Little RF, Erba H, Stone RM, Litzow M, Tallman M (2019) Ibrutinib-Rituximab or Chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 381:432–443. https://doi.org/10.1056/NEJMoa1817073
Chanan-Khan A, Liu T, Yang K, Cohen A, Fahrbach K, Campbell J, Tang B (2022) PB1887: Network meta-analysis of progression free survival in the treatment of relapsed of refractory chronic lymphocytic leukemia. HemaSphere. https://doi.org/10.1097/01.HS9.0000850400.19760.77
Schieber M, Gordon LI, Karmali R (2018) Current overview and treatment of mantle cell lymphoma. F1000Res. https://doi.org/10.12688/f1000research.14122.1
Rule S, Dreyling M, Goy A, Hess G, Auer R, Kahl B, Hernández-Rivas J, Qi K, Deshpande S, Parisi L, Wang M (2019) Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica 104:e211–e214. https://doi.org/10.3324/haematol.2018.205229
Song Y, Zhou K, Zou D (2021) Zanubrutinib (zanu) in patients (pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL): long-term efficacy and safety results from a phase 2 study. In: European Hematology Association 2021 Virtual Congress
Acknowledgements
The authors thank the patients, investigators and research staff who participated in the study. Medical writing and editorial assistance were supported by Beigene.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors were involved in the study concept and design, data analysis and interpretation, drafting or substantively revising the manuscript, and reviewing and approving the final manuscript for submission.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, Y., Zhou, K., Yang, S. et al. Indirect comparisons of efficacy of zanubrutinib versus orelabrutinib in patients with relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma or relapsed or refractory mantle cell lymphoma. Invest New Drugs 41, 606–616 (2023). https://doi.org/10.1007/s10637-023-01376-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01376-1