Summary
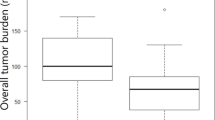
Pembrolizumab treatment is associated with a favorable prognosis in patients with non-small-cell lung cancer (NSCLC). Here, we investigated the associations among pre-treatment clinical factors, baseline overall tumor burden, and development of severe immune-related adverse events (irAEs; grade ≥ 3) after pembrolizumab treatment with or without chemotherapy. We retrospectively examined consecutive patients with advanced NSCLC who received pembrolizumab with or without chemotherapy at Hakodate Goryoukaku Hospital from March 2017 to February 2021. The baseline overall tumor burden was measured as the sum of the unidimensional diameters of up to five target lesions. We defined irAEs as toxicities related to immune checkpoint inhibitors based on the Common Terminology Criteria for Adverse Events, version 5.0. Tumor burden differed significantly between patients with and without severe irAEs (85 vs. 65 mm, p = 0.0367). The cutoff value for overall tumor burden was set to 80 mm. Good performance status (PS = 0) and PD-L1 expression > 80%, but not overall tumor burden, were correlated with severe irAEs, regardless of complementary chemotherapy. The multivariate odds ratios of good PS and high PD-L1 expression for severe irAEs were 3.27 (95% confidence interval [CI]: 1.22–8.77, p = 0.019) and 4.44 (95% CI: 1.59–12.42, p = 0.0044), respectively. Baseline overall tumor burden, good PS, and high PD-L1 expression were associated with severe irAEs in patients with NSCLC treated with first-line pembrolizumab with or without chemotherapy. Patients with these factors should be carefully monitored to prevent irAEs.




Similar content being viewed by others
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Borghaei H, Gettinger S, Vokes EE et al (2021) Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol 39:723–733. https://doi.org/10.1200/JCO.20.01605
Reck M, Rodríguez-Abreu D, Robinson AG et al (2021) Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol 39:2339–2349. https://doi.org/10.1200/JCO.21.00174
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393:1819–1830. https://doi.org/10.1016/S0140-6736(18)32409-7
Rodríguez-Abreu D, Powell SF, Hochmair MJ et al (2021) Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol 32:881–895. https://doi.org/10.1016/j.annonc.2021.04.008
Paz-Ares L, Vicente D, Tafreshi A et al (2020) A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol 15:1657–1669. https://doi.org/10.1016/j.jtho.2020.06.015
Shankar B, Zhang J, Naqash AR et al (2020) Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol 6:1952–1956. https://doi.org/10.1001/jamaoncol.2020.5012
Wang W, Gu X, Wang L et al (2022) The prognostic impact of mild and severe immune-related adverse events in non-small cell lung cancer treated with immune checkpoint inhibitors: a multicenter retrospective study. Cancer Immunol Immunother 71:1693–1703. https://doi.org/10.1007/s00262-021-03115-y
Bruyère CL, Souquet PJ, Dalle S et al (2021) Investigating the impact of immune-related adverse events, glucocorticoid use and immunotherapy interruption on long-term survival outcomes. Cancers (Basel) 13:2365. https://doi.org/10.3390/cancers13102365
Ksienski D, Wai ES, Croteau NS et al (2020) Association of age with differences in immune related adverse events and survival of patients with advanced nonsmall cell lung cancer receiving pembrolizumab or nivolumab. J Geriatr Oncol 11:807–813. https://doi.org/10.1016/j.jgo.2020.01.006
Ksienski D, Wai ES, Alex D et al (2021) Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for advanced non-small cell lung cancer patients with high PD-L1 tumor expression receiving pembrolizumab. Transl Lung Cancer Res 10:355–367. https://doi.org/10.21037/tlcr-20-541
Ksienski D, Wai ES, Croteau N et al (2019) Pembrolizumab for advanced nonsmall cell lung cancer: efficacy and safety in everyday clinical practice. Lung Cancer 133:110–116. https://doi.org/10.1016/j.lungcan.2019.05.005
Noguchi S, Suminaga K, Kaki T et al (2020) Correlation of immune-related adverse events and effects of pembrolizumab monotherapy in patients with non-small cell lung cancer. Lung Cancer (Auckl) 11:53–57. https://doi.org/10.2147/LCTT.S254146
Sugisaka J, Toi Y, Taguri M (2020) Relationship between programmed cell death protein ligand 1 expression and immune-related adverse events in non-small-cell lung cancer patients treated with pembrolizumab. JMA J 3:58–66. https://doi.org/10.31662/jmaj.2019-0005
Shi Y, Fang J, Zhou C et al (2022) Immune checkpoint inhibitor-related adverse events in lung cancer: real-world incidence and management practices of 1905 patients in China. Thorac Cancer 13:412–422. https://doi.org/10.1111/1759-7714.14274
Katsurada M, Nagano T, Tachihara M et al (2019) Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer Res 39:815–825. https://doi.org/10.21873/anticanres.13180
Ishihara H, Kondo T, Nakamura K et al (2021) Association of tumor burden with outcome in first-line therapy with nivolumab plus ipilimumab for previously untreated metastatic renal cell carcinoma. Jpn J Clin Oncol 51:1751–1756. https://doi.org/10.1093/jjco/hyab142
Sakata Y, Kawamura K, Ichikado K et al (2019) The association between tumor burden and severe immune-related adverse events in non-small cell lung cancer patients responding to immune-checkpoint inhibitor treatment. Lung Cancer 130:159–161. https://doi.org/10.1016/j.lungcan.2019.02.011
Dercle L, Ammari S, Champiat S et al (2016) Rapid and objective CT scan prognostic scoring identifies metastatic patients with long-term clinical benefit on anti-PD-1/-L1 therapy. Eur J Cancer 65:33–42. https://doi.org/10.1016/j.ejca.2016.05.031
Roach C, Zhang N, Corigliano E et al (2016) Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol 24:392–397. https://doi.org/10.1097/PAI.0000000000000408
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Fujimoto D, Yoshioka H, Kataoka Y et al (2018) Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: a multicenter retrospective cohort study. Lung Cancer 119:14–20. https://doi.org/10.1016/j.lungcan.2018.02.017
Huang AC, Postow MA, Orlowski RJ et al (2017) T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545:60–65. https://doi.org/10.1038/nature22079
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Paz-Ares L, Luft A, Vicente D, KEYNOTE-407 Investigators et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051. https://doi.org/10.1056/NEJMoa1810865
Kargl J, Busch SE, Yang GH et al (2017) Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun 8:14381. https://doi.org/10.1038/ncomms14381
Kaira K, Imai H, Mouri A et al (2021) Clinical effectiveness of immune checkpoint inhibitors in non-small-cell lung cancer with a poor performance status. Medicina (Kaunas) 57:1273. https://doi.org/10.3390/medicina57111273
Russano M, Cortellini A, Giusti R et al (2022) Clinical outcomes of NSCLC patients experiencing early immune-related adverse events to PD-1/PD-L1 checkpoint inhibitors leading to treatment discontinuation. Cancer Immunol Immunother 71:865–874. https://doi.org/10.1007/s00262-021-03045-9
Fumet JD, Richard C, Ledys F et al (2018) Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer 119:950–960. https://doi.org/10.1038/s41416-018-0220-9
Wang M, Liang H, Wang W et al (2021) Immune-related adverse events of a PD-L1 inhibitor plus chemotherapy versus a PD-L1 inhibitor alone in first-line treatment for advanced non-small cell lung cancer: a meta-analysis of randomized control trials. Cancer 127:777–786. https://doi.org/10.1002/cncr.33270
Riopel ND, Chu Q, Walker J et al (2022) Rapid unmasking of immune-related adverse events after discontinuation of chemotherapy in chemo-immunotherapy regimens. J Immunother 45:207–209. https://doi.org/10.1097/CJI.0000000000000409
Dudnik E, Moskovitz M, Rottenberg Y et al (2021) Pembrolizumab as a monotherapy or in combination with platinum-based chemotherapy in advanced non-small cell lung cancer with PD-L1 tumor proportion score (TPS) ≥50%: real-world data. Oncoimmunology 10:1865653. https://doi.org/10.1080/2162402X.2020.1865653
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Toshiyuki Sumi conceived the idea of the study. Motoki Sekikawa, Yuta Nagahisa, Keigo Matsuura, Naoki Shijubou, Koki Kamada, Hiroki Watanabe, Yuta Koshino, Daiki Nagayama, Haruhiko Michimata, and Yuichi Yamada developed the statistical analysis plan and conducted statistical analyses. Yusuke Tanaka contributed to the interpretation of the results. Toshiyuki Sumi drafted the original manuscript. Hirofumi Chiba supervised the conduct of this study. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Ethics Committee of Hakodate Goryoukaku Hospital (approval no. 2022–019) approved this study.
Consent to participate
Informed consent was obtained using the opt-out method.
Consent to publish
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sumi, T., Koshshino, Y., Sekikawa, M. et al. Risk factors for severe immune-related adverse events after first-line pembrolizumab monotherapy or combination chemotherapy for non-small-cell lung cancer. Invest New Drugs 40, 1298–1305 (2022). https://doi.org/10.1007/s10637-022-01310-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01310-x