Summary
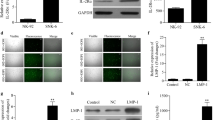
Background Chemotherapy resistance is a main reason for treatment failure in extranodal NK/T-cell lymphoma (ENKTL). Interleukin-10 (IL-10) is closely related to the occurrence and prognosis of ENKTL. We intended to study the role and molecular mechanism of IL-10 in ENKTL resistance. Methods Fifty serum samples were collected from patients with a histologically proven diagnosis of ENKTL. Fifty healthy volunteers were enrolled as a control group. The level of serum IL-10 was detected by ELISA. The NK/T-cell lymphoma cell lines YT and NK-92 were divided into the control group (untreated), IL-10 group (treated with IL-10), IL-10 + GEM group (treated with IL-10 and gemcitabine simultaneously) and GEM group (treated with gemcitabine). A CCK8 assay and flow cytometry were employed to detect the effects of IL-10 on each group. Western blotting was applied to detect the expression of ABC membrane transporter family proteins and signaling pathway proteins in each group. Results Serum IL-10 levels were higher in ENKTL patients as well asin patients with ineffective treatment. The IC50 value for gemcitabine in YT and NK-92 cells increased significantly in the presence of IL-10. The effects of gemcitabine resulting in cell killing, cell cycle arrest, and apoptosis promotion were also weakened by IL-10. The expression of ABCC4, STAT1, p-STAT1, Tyk2 and p-Tyk2 was significantly increased by IL-10. Conclusion Our results indicate that IL-10 contributes to the resistance of ENKTL cells via ABCC4 and that IL-10 regulates the JAK/STAT signaling pathway in YT and NK-92 cells.




Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Xiong J, Zhao WL (2018) Advances in multiple omics of natural-killer/T cell lymphoma. J Hematol Oncol 11(1):134
Au WY et al (2009) Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 113(17):3931–3937
Li YX et al (2009) Variable Clinical Presentations of Nasal and Waldeyer Ring Natural Killer/T-Cell Lymphoma. Clin Cancer Res 15(8):2905–2912
Fox CP et al (2020) Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell Project. Lancet Haematol 7(4):E284–E294
Zhang XD et al (2018) Conserved cell populations in doxorubicin-resistant human nasal natural killer/T cell lymphoma cell line: super multidrug resistant cells? Cancer Cell Int 18:150–154
Wang L et al (2014) Combination of gemcitabine, L-asparaginase, and oxaliplatin (GELOX) is superior to EPOCH or CHOP in the treatment of patients with stage IE/IIE extranodal natural killer/T cell lymphoma: a retrospective study in a cohort of 227 patients with long-term follow-up. Med Oncol 31(3):860–865
Zhang L et al (2016) Efficacy and safety of cisplatin, dexamethasone, gemcitabine and pegaspargase (DDGP) regimen in newly diagnosed, advanced-stage extranodal natural killer/T-cell lymphoma: interim analysis of a phase 4 study NCT01501149. Oncotarget 7(34):55721–55731
Tse E, Kwong YL (2017) The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol 10(1):85–91
Yang Y et al (2015) Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood 126(12):1424–1432
Zhu LA et al (2019) c-Myc mediated upregulation of long noncoding RNA SNHG12 regulates proliferation and drug sensitivity in natural killer/T-cell lymphoma. J Cell Biochem 120(8):12628–12637
Fowler NH et al (2016) Role of the tumor microenvironment in mature B-cell lymphoid malignancies. Haematologica 101(5):531–540
Kim S et al (2015) Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 121(13):2129–2136
Diakos CI et al (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15(11):E493–E503
Elinav E et al (2013) Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 13(11):759–771
Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F (2009) The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood 114(16):3367–3375
Nolen BM et al (2014) Circulating Mediators of Inflammation and Immune Activation in AIDS-Related Non-Hodgkin Lymphoma. PLoS One 9(6):99144–99153
Kawakami K, Ito R, Tono Y (2012) Orbital inflammatory lesion as an initial manifestation of systemic nasal type NK/T-cell lymphoma. J Clin Exp Hematop 52(2):137–139
Mannino MH et al (2015) The paradoxical role of IL-10 in immunity and cancer. Cancer Lett 367(2):103–107
Wu C et al (2017) Co-delivery of multiple drug resistance inhibitors by polymer/inorganic hybrid nanoparticles to effectively reverse cancer drug resistance. Colloids Surf B Biointerfaces 149:250–259
Wu XS et al (2019) IL-10 is overexpressed in human cutaneous T-cell lymphoma and is required for maximal tumor growth in a mouse model. Leuk Lymphoma 60(5):1244–1252
Boyano MD et al (2000) Soluble interleukin-2 receptor, intercellular adhesion molecule-1 and interleukin-10 serum levels in patients with melanoma. Br J Cancer 83(7):847–852
Nemunaitis J et al (2001) Comparison of serum interleukin-10 (IL-10) levels between normal volunteers and patients with advanced melanoma. Cancer Invest 19(3):239–247
Hassuneh MR, Nagarkatti M, Nagarkatti PS (2013) Role of interleukin-10 in the regulation of tumorigenicity of a T cell lymphoma. Leuk Lymphoma 54(4):827–834
Danoch H et al (2015) Sensitizing B- and T- cell Lymphoma Cells to Paclitaxel/Abraxane-Induced Death by AS101 via Inhibition of the VLA-4-IL10-Survivin Axis. Mol Cancer Res 13(3):411–422
Vahl JM et al (2017) Interleukin-10-regulated tumour tolerance in non-small cell lung cancer. Br J Cancer 117(11):1644–1655
Kaio E, Tanaka S, Oka S (2003) Clinical significance of thrombospondin-1 expression in relation to vascular endothelial growth factor and interleukin-10 expression at the deepest invasive tumor site of advanced colorectal carcinoma. Int J Oncol 23(4):901–911
Soria JC et al (2003) Lack of interleukin-10 expression could predict poor outcome in patients with stage I non-small cell lung cancer’. Clin Cancer Res 9(5):1785–1791
Conroy SM et al (2013) Non-Hodgkin Lymphoma and Circulating Markers of Inflammation and Adiposity in a Nested Case-Control Study: The Multiethnic Cohort. Cancer Epidemiol Biomark Prev 22(3):337–347
McKay K et al (2011) Association between natural killer cells and regression in melanocytic lesions. Hum Pathol 42(12):1960–1964
Adedipe F et al (2019) Structural and functional insights into the Diabrotica virgifera virgifera ATP-binding cassette transporter gene family. BMC Genomics 20(1):899–901
Liu Z, Fu S, Ma X (2020) Resistance to Bacillus thuringiensis Cry1Ac toxin requires mutations in two Plutella xylostella ATP-binding cassette transporter paralogs. PLoS Pathog 16(8):1008697–1008702
Carlson ML, Bao H, Duong F (2016) Formation of a Chloride-conducting State in the Maltose ATP-binding Cassette (ABC) Transporter. J Biol Chem 291(23):12119–12125
Hirabayashi K et al (2015) Functional Dynamics Revealed by the Structure of the SufBCD Complex, a Novel ATP-binding Cassette (ABC) Protein That Serves as a Scaffold for Iron-Sulfur Cluster Biogenesis. J Biol Chem 290(50):29717–29731
Li X et al (2017) Identification of drug-resistance related genes in natural killer/T cell lymphoma through digltal gene expression and RNA-sequencing. Chin J Exp Surg 34:1754–1756
Changkija B, Konwar R (2012) Role of interleukin-10 in breast cancer. Breast Cancer Res Treat 133:11–21
Hamed Anber N, El-Sebaie AH, Darwish NH (2019) Prognostic value of some inflammatory markers in patients with lymphoma. Biosci Rep 39(3):20182174–20182174
Wang H et al (2015) Increased serum levels of interleukin-10 predict poor prognosis in extranodal natural killer/T-cell lymphoma patients receiving asparaginase-based chemotherapy. Onco Targets Ther 8:2589–2599
Gemelli C et al (2014) MafB is a downstream target of the IL-10/STAT3 signaling pathway, involved in the regulation of macrophage de-activation. BBA-Mol Cell Res 1843(5):955–964
Funding
This research was supported by the National Natural Science Foundation of China (8157010926).
Author information
Authors and Affiliations
Contributions
J. H. and L. F. were involved in the conception and design of this study. J. H., L. F., and M. J. performed the data analysis and interpreted the results. J. H. and M. J. prepared the first draft of the manuscript. Z. L. and M. Z. critically revised the manuscript. M. Z. supervised the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients and healthy volunteers signed an informed consent form, and the research was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Informed consent
All patients and healthy volunteers signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The author reports no conflicts of interest in this work.
Research involving human participants and/or animals
All of the procedures performed in the study involving human participants were in accordance with the ethical standards of the relevant institutional and national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huo, J., Fu, L., Jin, M. et al. IL-10 contributes to gemcitabine resistance in extranodal NK/T-cell lymphoma cells via ABCC4. Invest New Drugs 40, 537–545 (2022). https://doi.org/10.1007/s10637-022-01224-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01224-8