Summary
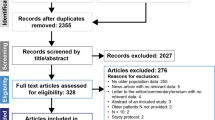
Introduction The number of cancer cases among the elderly continue to increase as the worldwide population ages. This patient subset is underrepresented in clinical trials, partly because of unresolved uncertainties about age-associated tolerabilities and antitumor activities. We reviewed phase 1 trial data to study tolerance and efficacy of novel agents used for treatment of elderly patients with cancer. Methods Data from 773 consecutive evaluable patients in 85 phase 1 clinical trials (2008–2016) at START Madrid-CIOCC were analyzed according to age, with respect to objective response, survival, and toxicity. Results The mean age was 58.7 (range: 18–87) years; 260 (33.6%) patients were >65 y (elderly group). One hundred thirty-seven (17.8%) patients received immunotherapy drugs, 308 (39.8%) received targeted agents, and 328 (42.4%) received chemotherapy. No statistically significant differences in overall survival, objective response, or severe toxicity rates were found according to treatment type. Similar toxicities and clinical activities were found between the two age subgroups; 18.8% of the elderly and 20.7% of the younger patients experienced severe hematological toxicity (p=0.5), and 30.2% and 32.7%, respectively, experienced severe non-hematological toxicity (p=0.4). Regarding antitumor activity, 12.4% of the elderly and 15% of the younger patients achieved objective responses (p=0.41). There were no significant between-group differences in overall survival (9.7 versus 11.5 months, respectively, p=0.1) or progression-free survival (2.3 versus 2.2 months, respectively, p=0.7). Conclusions This retrospective study found that elderly and younger populations had comparable antitumor activities and toxicity profiles. These results support including elderly patients with cancer in early-phase trials.

Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in the published article.
References
Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W et al (2009) SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Bethesda, MD, https://www.seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010
White MC, Holman DM, Boehm JE et al (2014) Age and cancer risk: A potentially modifiable relationship. Am J Prev Med 46:1–16. https://doi.org/10.1016/j.amepre.2013.10.029
Henley SJ, Ward EM, Scott S et al (2020) Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 126:2225–2249. https://doi.org/10.1002/cncr.32802
Finkel T, Serrano M, Blasco MA (2007) The common biology of cancer and ageing. Nature 448:767–774. https://doi.org/10.1038/nature05985
Arciero VS, Cheng S, Mason R et al (2018) Do older and younger patients derive similar survival benefits from novel oncology drugs? A systematic review and meta-analysis. Age Ageing 47:654–660. https://doi.org/10.1093/ageing/afy079
Shenoy P, Harugeri A (2015) Elderly patients’ participation in clinical trials. Perspect Clin Res 6:184–189. https://doi.org/10.4103/2229-3485.167099
Quoix E, Zalcman G, Oster JP et al (2011) Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet (London, England) 378:1079–1088. https://doi.org/10.1016/S0140-6736(11)60780-0
LoConte NK, Smith M, Alberti D et al (2010) Amongst eligible patients, age and comorbidity do not predict for dose-limiting toxicity from phase I chemotherapy. Cancer Chemother Pharmacol 65:775–780. https://doi.org/10.1007/s00280-009-1084-8
Javid SH, Unger JM, Gralow JR et al (2012) A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist 17:1180–1190. https://doi.org/10.1634/theoncologist.2011-0384
Fang P, He W, Gomez DR et al (2017) Influence of Age on Guideline-Concordant Cancer Care for Elderly Patients in the United States. Int J Radiat Oncol Biol Phys 98:748–757. https://doi.org/10.1016/j.ijrobp.2017.01.228
Townsley CA, Chan KK, Pond GR et al (2006) Understanding the attitudes of the elderly towards enrolment into cancer clinical trials. BMC Cancer 6:1–9. https://doi.org/10.1186/1471-2407-6-34
Townsley CA, Naidoo K, Pond GR et al (2003) Are older cancer patients being referred to oncologists? A mail questionnaire of Ontario primary care practitioners to evaluate their referral patterns. J Clin Oncol Off J Am Soc Clin Oncol 21:4627–4635. https://doi.org/10.1200/JCO.2003.06.073
Kemeny MM, Peterson BL, Kornblith AB et al (2003) Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 21:2268–2275. https://doi.org/10.1200/JCO.2003.09.124
Siu LL, Shepherd FA, Murray N et al (1996) Influence of age on the treatment of limited-stage small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 14:821–828. https://doi.org/10.1200/JCO.1996.14.3.821
Takuwa H, Tsuji W, Yotsumoto F (2018) Overall survival of elderly patients with breast cancer is not related to breast-cancer specific survival: A single institution experience in Japan. Breast Dis 37:177–183. https://doi.org/10.3233/BD-170280
Olver I (2000) Chemotherapy for elderly patients with advanced cancer: is it worth it? Aust Prescr 23:80–82. https://doi.org/10.18773/austprescr.2000.090
Greil R (1998) Prognosis and management strategies of lymphatic neoplasias in the elderly. I. Aggressive non-Hodgkin’s lymphomas. Oncology 55:189–217. https://doi.org/10.1159/000011855
Kanesvaran R, Cordoba R, Maggiore R (2018) Immunotherapy in Older Adults With Advanced Cancers: Implications for Clinical Decision-Making and Future Research. Am Soc Clin Oncol Educ B 400–414 https://doi.org/10.1200/edbk_201435
Johnstone J, Parsons R, Botelho F et al (2017) T-Cell Phenotypes Predictive of Frailty and Mortality in Elderly Nursing Home Residents. J Am Geriatr Soc 65:153–159. https://doi.org/10.1111/jgs.14507
Daste A, Chakiba C, Domblides C et al (2016) Targeted therapy and elderly people: A review. Eur J Cancer 69:199–215. https://doi.org/10.1016/j.ejca.2016.10.005
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Cancer Therapy Evaluation Program (CTEP) (2017) Common Terminology Criteria for Adverse Events (CTCAE).v.5.0 [5x7]. Cancer Ther Eval Progr 155
Garrido-Laguna I, Janku F, Vaklavas C et al (2012) Validation of the Royal Marsden Hospital prognostic score in patients treated in the Phase I Clinical Trials Program at the MD Anderson Cancer Center. Cancer 118:1422–1428. https://doi.org/10.1002/cncr.26413
Templeton AJ, McNamara MG, Šeruga B et al (2014) Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106:dju124. https://doi.org/10.1093/jnci/dju124
Xue P, Kanai M, Mori Y et al (2014) Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. J Natl Cancer Inst 106:406–415. https://doi.org/10.1093/jnci/dju124
Ferrucci PF, Ascierto PA, Pigozzo J et al (2016) Baseline neutrophils and derived neutrophilto-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 27:732–738. https://doi.org/10.1093/annonc/mdw016
Sacdalan DB, Lucero JA, Sacdalan DL (2018) Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: A review and meta-analysis. Onco Targets Ther 11:955–965. https://doi.org/10.2147/OTT.S153290
Li B, Zhou P, Liu Y et al (2018) Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin Chim Acta 483:48–56. https://doi.org/10.1016/j.cca.2018.04.023
Baldini C, Le Saux O, Helissey C et al (2018) Are phase I trials safe for older patients? J Geriatr Oncol 9:87–92. https://doi.org/10.1016/j.jgo.2017.08.012
Schwandt A, Harris PJ, Hunsberger S et al (2014) The role of age on dose-limiting toxicities in Phase i dose-escalation trials. Clin Cancer Res 20:4768–4775. https://doi.org/10.1158/1078-0432.CCR-14-0866
Extermann M, Overcash J, Lyman GH et al (1998) Comorbidity and functional status are independent in older cancer patients. J Clin Oncol Off J Am Soc Clin Oncol 16:1582–1587. https://doi.org/10.1200/JCO.1998.16.4.1582
Caillet P, Canoui-Poitrine F, Vouriot J et al (2011) Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol Off J Am Soc Clin Oncol 29:3636–3642. https://doi.org/10.1200/JCO.2010.31.0664
Liu R, Gomes A, Ao G et al (2020) 592P A predictive score of antitumour activity of novel agents in cancer patients treated in early phase studies. Ann Oncol 31:S497. https://doi.org/10.1016/j.annonc.2020.08.706
Denson AC, Mahipal A (2014) Participation of the elderly population in clinical trials: Barriers and solutions. Cancer Control 21:209–214. https://doi.org/10.1177/107327481402100305
Belgioia L, Desideri I, Errico A et al (2019) Safety and efficacy of combined radiotherapy, immunotherapy and targeted agents in elderly patients: A literature review. Crit Rev Oncol Hematol 133:163–170. https://doi.org/10.1016/j.critrevonc.2018.11.009
Hurria A, Browner IS, Cohen HJ et al (2012) Senior adult oncology: Clinical practice guidelines in oncology. JNCCN J Natl Compr Cancer Netw 10:162–209. https://doi.org/10.6004/jnccn.2012.0019
Acknowledgments
None to report.
Funding
This work was supported by START Madrid at CIOCC as a Ph.D. studentship.
Author information
Authors and Affiliations
Contributions
Conception and design: G.A., L.U., M.M., E.C. Writing, review, and/or revision of manuscript: G. A., M.M., A.G., L.U., A.C., R.L, V.B., I.M., E.C. Acquisition of data: G.A., L.U. Analysis and interpretation of data: G.A., L.U., M.M., J.M.C., E.C.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study complies with the guidelines for human studies and was performed ethically in accordance with the World Medical Association Declaration of Helsinki. The study was exempt from ethical committee approval because the work was a review of different Pharma-sponsored phase 1 clinical trials that were formally approved by the respective ethics committees and the Spanish regulatory agency. The clinical studies data that we used to perform our analyses were properly anonymized and complied with the laws of Spain relevant to protection of personal data. Informed consent was obtained from each patient at the time of original data collection.
Informed consent
Not applicable. This study was a pooled retrospective analysis of associations of characteristics of patients. Per standard requirements, no informed consent was required.
Research involving human and animals participants
Not applicable. No specific research involving human participants and/or animals is applicable because this study was a retrospective analysis of patients treated during previously published phase 1 clinical trials.
Disclosures of potential conflicts of interest
-
• Geriletu Ao declares no potential conflicts of interest
-
• Maria de Miguel reports research funding from MSD, Pharmamar, Roche, Novartis, Abbvie, Array, Eisai, and Sanofi. Speaker´s Bureau: MSD, Janssen, Roche.
-
• Ana Gomes declares no potential conflicts of interest
-
• Runhan Liu declares no potential conflicts of interest
-
• Valentina Boni reports employment at START Madrid-CIOCC, Hm Hospitales Sanchinarro. Consulting or advisory roles at Puma Biotechnology, Ideaya Biosciences, Loxo Therapeutics, CytomX Therapeutics, Guidepoint, Oncoart. Institutional financial support for clinical trials from Abbvie, ACEO, Adaptaimmune, Amcure, AMGEN, AstraZeneca, BMS, Cytomx, GSK, Genentech/Roche, H3, Incyte, Janssen, Kura, Lilly, Loxo, Nektar, Macrogenics, Menarini, Merck, Merus, Nanobiotix, Novartis, Pfizer, PharmaMar, Principia, PUMA, Sanofi, Taiho, Tesaro, BeiGene, Transgene, Takeda, Incyte, Innovio, MSD, PsiOxus, Seattle Genetics, Mersana, GSK, Daiichi, Nektar, Astellas, ORCA, Boston Therapeutics, Dynavax, DebioPharm, Boehringen Ingelheim, Regeneron, Millenium, Synthon, Spectrum, Rigontec, Zenith. Memberships at SEOM, ESMO, ASCO, SOLTI (scientific committee member), GETHI.
-
• Irene Moreno reports research funding from Abbvie, Astellas, BMS, Pharmamar, Kyowa and Symphogen. Speaker´s Bureau: MSD.
-
• José Miguel Cárdenas declares no potential conflicts of interest.
-
• Lisardo Ugidos reports advisory role for Astra Zeneca.
-
• Antonio Cubillo reports no potential conflicts of interest.
-
• Emiliano Calvo reports honoraria or consultation fees from Astellas, Novartis, Nanobiotix, Pfizer, Janssen-Cilag, GLG, PsiOxus Therapeutics, Merck, Medscape, BMS, Gilead, Seattle Genetics, Pierre Fabre, Boehringer Ingelheim, Cerulean Pharma, EUSA, Gehrmann Consulting, AstraZeneca, Roche, Guidepoint, Servier, Celgene, Abbvie, Amcure, OncoDNA, Alkermes. Director of Clinical Research, START Madrid and Director of Clinical Research at HM Hospitals Group, Madrid. Stocks or ownership in START, Oncoart Associated, International Cancer Consultants. No licensing fees or royalties. Direct research funding as project lead from Novartis, AstraZeneca, Beigene. Institutional financial support for clinical trials from Abbvie, ACEO, Adaptaimmune, Amcure, AMGEN, AstraZeneca, BMS, Cytomx, GSK, Genentech/Roche, H3, Incyte, Janssen, Kura, Lilly, Loxo, Nektar, Macrogenics, Menarini, Merck, Merus, Nanobiotix, Novartis, Pfizer, PharmaMar, Principia, PUMA, Sanofi, Taiho, Tesaro, BeiGene, Transgene, Takeda, Incyte, Innovio, MSD, PsiOxus, Seattle Genetics, Mersana, GSK, Daiichi, Nektar, Astellas, ORCA, Boston Therapeutics, Dynavax, DebioPharm, Boehringer Ingelheim, Regeneron, Millenium, Synthon, Spectrum, Rigontec. Member of scientific board at PsiOxus, non-financial interest. Founder and president of non-profit foundation INTHEOS (Investigational Therapeutics in Oncological Sciences), medical society. Memberships in SEOM, EORTC, ESMO, ASCO. Other relationships: HM Hospitals Group and START, Program of Early Phase Clinical Drug Development in Oncology; Employed as Medical Oncologist, Director, Clinical Research. Methods in Clinical Cancer Research (MCCR) Workshop, Zeist, Netherlands (Joint ECCO-AACR-EORTC-ESMO Workshop on Methods in Clinical Cancer, Research), Co-director.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ao, G., de Miguel, M., Gomes, A. et al. Toxicity and antitumor activity of novel agents in elderly patients with cancer included in phase 1 studies. Invest New Drugs 39, 1694–1701 (2021). https://doi.org/10.1007/s10637-021-01150-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01150-1