Summary
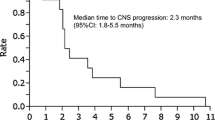
Objectives In EGFR-mutated non-small cell lung cancer (NSCLC) patients, approximately 80–90% of leptomeningeal metastasis (LM) develops after failed initial treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (EGFR-TKI). However, the efficacy of rechallenging with previously administered EGFR-TKIs in patients with EGFR-mutated NSCLC and the LM that develops following EGFR-TKI treatment failure remains unknown. Materials and methods We retrospectively reviewed medical records of patients with EGFR-mutated NSCLC and LM, from November 2011 to August 2019. The patients were classified according to the LM treatment type: switched to previously unadministered EGFR-TKIs (Switch-TKI) or rechallenge with previously administered EGFR-TKIs (Rechallenge-TKI). Results In total, 50 patients treated with EGFR-TKI after LM diagnosis were included; 35 were treated with Switch-TKI and 15 with Rechallenge-TKI. The median overall survival (OS) from the time of LM diagnosis was 6.2 months in all study patients. According to the treatment type, the median OS from the time of LM diagnosis was 6.9 months in Switch-TKI patients and 4.9 months in Rechallenge-TKI patients. There was no significant difference in the OS between the Switch-TKI and Rechallenge-TKI groups (P = 0.864). Thirty-five patients were treated with erlotinib and 15 with osimertinib; Regardless of the type for EGFR-TKI, there was no significant difference in OS between patients treated with Switch-TKI and those treated with Rechallenge-TKI. Conclusion Rechallenge of previously administered EGFR-TKIs may be a therapeutic option for LM development after EGFR-TKI treatment failure in patients with EGFR-mutated NSCLC, not only switching to previously unadministered EGFR-TKIs.




Similar content being viewed by others
Data availability
All data and material are available on reasonable request.
References
Cheng H, Perez-Soler R (2018) Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol 19:e43–e55. https://doi.org/10.1016/S1470-2045(17)30689-7
Le Rhun E, Taillibert S, Chamberlain MC (2013) Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int 4:S265–S288. https://doi.org/10.4103/2152-7806.111304
Alexander M, Lin E, Cheng H (2020) Leptomeningeal metastases in non-small cell lung cancer: optimal systemic management in NSCLC with and without driver mutations. Curr Treat Options Oncol 21:72. https://doi.org/10.1007/s11864-020-00759-3
Li YS, Jiang BY, Yang JJ et al (2016) Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol 11:1962–1969. https://doi.org/10.1016/j.jtho.2016.06.029
Wu YL, Zhao Q, Deng L et al (2019) Leptomeningeal metastasis after effective first-generation EGFR TKI treatment of advanced non-small cell lung cancer. Lung Cancer 127:1–5. https://doi.org/10.1016/j.lungcan.2018.11.022
Lee SJ, Lee JI, Nam DH et al (2013) Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol 8:185–191. https://doi.org/10.1097/JTO.0b013e3182773f21
Lee E, Keam B, Kim DW et al (2013) Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 8:1069–1074. https://doi.org/10.1097/JTO.0b013e318294c8e8
Ahn MJ, Chiu CH, Cheng Y et al (2020) Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol 15:637–648. https://doi.org/10.1016/j.jtho.2019.12.113
Lee J, Choi Y, Han J et al (2020) Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol 15:1758–1766. https://doi.org/10.1016/j.jtho.2020.06.018
Yang JCH, Kim SW, Kim DW et al (2020) Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol 38:538–547. https://doi.org/10.1200/JCO.19.00457
Flippot R, Biondani P, Auclin E et al (2019) Activity of EGFR tyrosine kinase inhibitors in NSCLC with refractory leptomeningeal metastases. J Thorac Oncol 14:1400–1407. https://doi.org/10.1016/j.jtho.2019.05.007
Nosaki K, Yamanaka T, Hamada A et al (2020) Erlotinib for non-small cell lung cancer with leptomeningeal metastases: a phase II study (LOGIK1101). Oncologist 25:e1869–e1878. https://doi.org/10.1634/theoncologist.2020-0640
Togashi Y, Masago K, Masuda S et al (2012) Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 70:399–405. https://doi.org/10.1007/s00280-012-1929-4
Yan W, Liu Y, Li J et al (2019) Whole brain radiation therapy does not improve the overall survival of EGFR-mutant NSCLC patients with leptomeningeal metastasis. Radiat Oncol 14:168. https://doi.org/10.1186/s13014-019-1376-z
Ramalingam SS, Vansteenkiste J, Planchard D et al (2020) Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 382:41–50. https://doi.org/10.1056/NEJMoa1913662
Mitsuya K, Nakasu Y, Hayashi N et al (2019) Palliative cerebrospinal fluid shunting for leptomeningeal metastasis-related hydrocephalus in patients with lung adenocarcinoma: a single-center retrospective study. PLoS ONE 14:e0210074. https://doi.org/10.1371/journal.pone.0210074
Tomizawa Y, Fujita Y, Tamura A et al (2010) Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer 68:269–272. https://doi.org/10.1016/j.lungcan.2009.06.025
Koizumi T, Agatsuma T, Ikegami K et al (2012) Prospective study of gefitinib readministration after chemotherapy in patients with advanced non–small-cell lung cancer who previously responded to gefitinib. Clin Lung Cancer 13:458–463. https://doi.org/10.1016/j.cllc.2012.01.006
Song T, Yu W, Wu SX (2014) Subsequent treatment choices for patients with acquired resistance to EGFR-TKIs in non-small cell lung cancer: restore after a drug holiday or switch to another EGFR-TKI? Asian Pac J Cancer Prev 15:205–213. https://doi.org/10.7314/apjcp.2014.15.1.205
Li D, Ambrogio L, Shimamura T et al (2008) BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27:4702–4711. https://doi.org/10.1038/onc.2008.109
Bozzetti C, Tiseo M, Lagrasta C et al (2008) Comparison between epidermal growth factor receptor (EGFR) gene expression in primary non-small cell lung cancer (NSCLC) and in fine-needle aspirates from distant metastatic sites. J Thorac Oncol 3:18–22. https://doi.org/10.1097/JTO.0b013e31815e8ba2
Suda K, Murakami I, Yu H et al (2016) Heterogeneity of EGFR aberrations and correlation with histological structures: analyses of therapy-naive isogenic lung cancer lesions with EGFR mutation. J Thorac Oncol 11:1711–1717. https://doi.org/10.1016/j.jtho.2016.05.017
Morris PG, Reiner AS, Szenberg OR et al (2012) Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol 7:382–385. https://doi.org/10.1097/JTO.0b013e3182398e4f
Hoffknecht P, Tufman A, Wehler T et al (2015) Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)–pretreated Non–small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 10:156–163. https://doi.org/10.1097/JTO.0000000000000380
Togashi Y, Masago K, Fukudo M et al (2010) Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol 5:950–955. https://doi.org/10.1097/JTO.0b013e3181e2138b
Nanjo S, Hata A, Okuda C et al (2018) Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer 118:32–37. https://doi.org/10.1038/bjc.2017.394
Xing L, Pan Y, Shi Y et al (2018) P1.13–25 efficacy and safety of osimertinib in EGFR T790M-positive advanced NSCLC patients with brain metastases (Apollo study). J Thorac Oncol 13:S592. https://doi.org/10.1016/j.jtho.2018.08.882
Soria JC, Ohe Y, Vansteenkiste J et al (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113–125. https://doi.org/10.1056/NEJMoa1713137
Acknowledgements
We thank Editage (https://www.editage.jp) for editing this manuscript.
Author information
Authors and Affiliations
Contributions
TM and HK wrote the manuscript and researched data. HK reviewed and edited the manuscript. KM is a professional biostatistician and responsible for statistical analysis. All authors reviewed, approved the final version of the manuscript.
Corresponding author
Ethics declarations
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with 1964 Helsinki declaration and its later amendments or with comparable ethical standards. The institutional ethics review board of Shizuoka Cancer Center approved this study (approval no. J2020-177–2020-1).
Informed consent
For this type of study, formal consent is not required. We have also applied an opt-out method to obtain consent for this study by posting a document about this study. The document has been approved by the institutional ethics review board of Shizuoka Cancer Center.
Conflicts of interest
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Dr. Kenmotsu reports grants and personal fees from Chugai Pharmaceutical Co, Ltd., personal fees from Ono Pharmaceutical Co, Ltd., personal fees from Boeringer Ingelheim, personal fees from Eli Lilly K.K, personal fees from Kyowa Hakko Kirin Co., Ltd., personal fees from Bristol-Myers Squibb, personal fees from MSD, grants and personal fees from Novartis Pharma K.K., grants and personal fees from Daiichi-Sankyo Co., Ltd., grants and personal fees from AstraZeneca K.K., personal fees from Pfizer, personal fees from Taiho Pharma, outside the submitted work. Dr. Mamesaya reports personal fees from AstraZeneca KK, Pfizer Japan, Inc., personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Boehringer Ingelheim, personal fees from MSD K.K., personal fees from TAIHO PHARMACEUTICAL CO., LTD., personal fees from ONO PHARMACEUTICAL CO., LTD., outside the submitted work. Dr. Kobayashi reports personal fees from Eli Lilly K.K, personal fees from Taiho Pharmaceutical, personal fees from AstraZeneca, outside the submitted work. Dr. Omori reports personal fees from Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical, AstraZeneca K.K., Boehringer Ingelheim, Taiho Pharmaceutical, and MSD, which are unrelated to the submitted work. Dr. Wakuda reports grants and personal fees from Chugai Pharmaceutical Co., Ltd., personal fees from Taiho Pharmaceutical, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly K.K., personal fees from Ono Pharmaceutical, personal fees from MSD, grants and personal fees from Astrazeneca, grants from Novartis, grants from Abbvie, outside the submitted work. Dr. Ono reports grants from Taiho Pharmaceutical, grants from Ono Pharmaceutical, grants from Chugai Pharmaceutical Co., Ltd., grants from Novartis Pharma K.K., outside the submitted work. Dr. Murakami reports personal fees from AstraZeneca K.K., Ono Pharmaceutical, Bristol-Myers Squibb Japan, Chugai Pharmaceutical Co., Ltd., Pfizer Inc., Novartis Pharma K.K., Boehringer Ingelheim, Taiho Pharmaceutical, Eli Lilly K.K., and MSD, which are unrelated to the submitted work. Dr. Harada reports personal fees from Daiichi Sankyo Pharmaceutical Co. during the conduct of the study as well as personal fees from Daiichi Sankyo Pharmaceutical Co., AstraZeneca K.K., Brain Labo Co., and Chugai Pharmaceutical Co. and grants from the Japan Agency for Medical Research and Development and the National Cancer Center Research and Development Fund, which are unrelated to the submitted work. Dr. Kazuhisa Takahashi reports grants and personal fees from AstraZeneca K.K., Pfizer Japan, Inc., Eli Lilly K.K., MSD, and Boehringer Ingelheim as well as grants from Takeda Pharmaceutical Company Ltd., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., KYORIN Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., GlaxoSmithKline Consumer Healthcare Japan K.K., SHIONOGI & CO., LTD., and Novartis Pharma K.K., which are unrelated to the submitted work. Dr. Toshiaki Takahashi reports grants and personal fees from AstraZeneca KK, Pfizer Japan, Inc., grants and personal fees from Eli Lilly Japan K.K., grants and personal fees from Chugai Pharmaceutical Co., Ltd., grants and personal fees from Ono Pharmaceutical Co., Ltd., grants and personal fees from MSD K.K., grants and personal fees from Boehringer Ingelheim Japan, Inc., grants and personal fees from Pfizer Japan, Inc., personal fees from Roche Diagnostics K.K., outside the submitted work. Others—Declarations of interest: none.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyawaki, T., Kenmotsu, H., Yabe, M. et al. Rechallenge with previously administered epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR-mutated non-small cell lung cancer with leptomeningeal metastasis. Invest New Drugs 39, 1732–1741 (2021). https://doi.org/10.1007/s10637-021-01140-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01140-3