Summary
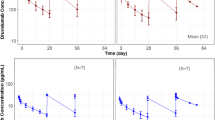
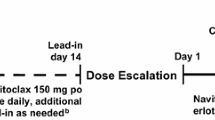
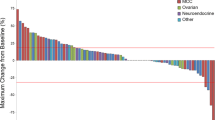
Background LY3022855 is a recombinant, immunoglobulin, human monoclonal antibody targeting the colony-stimulating factor-1 receptor. This phase 1 trial determined the safety, pharmacokinetics, and antitumor activity of LY3022855 in combination with durvalumab or tremelimumab in patients with advanced solid cancers who had received standard anti-cancer treatments. Methods In Part A (dose-escalation), patients received intravenous (IV) LY3022855 25/50/75/100 mg once weekly (QW) combined with durvalumab 750 mg once every two weeks (Q2W) IV or LY3022855 50 or 100 mg QW IV with tremelimumab 75/225/750 mg once every four weeks. In Part B (dose-expansion), patients with non-small cell lung cancer (NSCLC) or ovarian cancer (OC) received recommended phase 2 dose (RP2D) of LY3022855 from Part A and durvalumab 750 mg Q2W. Results Seventy-two patients were enrolled (median age 61 years): Part A = 33, Part B = 39. In Part A, maximum tolerated dose was not reached, and LY3022855 100 mg QW and durvalumab 750 mg Q2W was the RP2D. Four dose-limiting equivalent toxicities occurred in two patients from OC cohort. In Part A, maximum concentration, area under the concentration-time curve, and serum concentration showed dose-dependent increase over two cycles of therapy. Overall rates of complete response, partial response, and disease control were 1.4%, 2.8%, and 33.3%. Treatment-emergent anti-drug antibodies were observed in 21.2% of patients. Conclusions LY3022855 combined with durvalumab or tremelimumab in patients with advanced NSCLC or OC had limited clinical activity, was well tolerated. The RP2D was LY3022855 100 mg QW with durvalumab 750 mg Q2W. ClinicalTrials.gov ID: NCT02718911 (Registration Date: May 3, 2011).


Similar content being viewed by others
Data availability
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request six months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment for up to two years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
Code availability
Not applicable.
References
Yin Z, Bai L, Li W, Zeng T, Tian H, Cui J (2019) Targeting T cell metabolism in the tumor microenvironment: an anti-cancer therapeutic strategy. J Exp Clin Cancer Res 38(1):403. https://doi.org/10.1186/s13046-019-1409-3
Thommen DS, Schumacher TN (2018) T cell dysfunction in Cancer. Cancer Cell 33(4):547–562. https://doi.org/10.1016/j.ccell.2018.03.012
Buchbinder EI, Desai A (2016) CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 39(1):98–106. https://doi.org/10.1097/COC.0000000000000239
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Marquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schoffski P, Carlino MS, Lebbe C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD (2019) Five-year survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med 381(16):1535–1546. https://doi.org/10.1056/NEJMoa1910836
Gonzalez-Martin A, Sanchez-Lorenzo L (2019) Immunotherapy with checkpoint inhibitors in patients with ovarian cancer: still promising? Cancer 125(Suppl 24):4616–4622. https://doi.org/10.1002/cncr.32520
Papadopoulos KP, Gluck L, Martin LP, Olszanski AJ, Tolcher AW, Ngarmchamnanrith G, Rasmussen E, Amore BM, Nagorsen D, Hill JS, Stephenson J Jr (2017) First-in-human study of AMG 820, a monoclonal anti-Colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin Cancer Res 23(19):5703–5710. https://doi.org/10.1158/1078-0432.CCR-16-3261
Cassetta L, Kitamura T (2018) Targeting tumor-associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front Cell Dev Biol 6:38. https://doi.org/10.3389/fcell.2018.00038
Saung MT, Muth S, Ding D, Thomas DL 2nd, Blair AB, Tsujikawa T, Coussens L, Jaffee EM, Zheng L (2018) Targeting myeloid-inflamed tumor with anti-CSF-1R antibody expands CD137+ effector T-cells in the murine model of pancreatic cancer. J Immunother Cancer 6(1):118. https://doi.org/10.1186/s40425-018-0435-6
Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, Wolchok JD (2016) Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine 6:50–58. https://doi.org/10.1016/j.ebiom.2016.02.024
Li M, Li M, Yang Y, Liu Y, Xie H, Yu Q, Tian L, Tang X, Ren K, Li J, Zhang Z, He Q (2020) Remodeling tumor immune microenvironment via targeted blockade of PI3K-gamma and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J Control Release 321:23–35. https://doi.org/10.1016/j.jconrel.2020.02.011
Peng X, Hou P, Chen Y, Dai Y, Ji Y, Shen Y, Su Y, Liu B, Wang Y, Sun D, Jiang Y, Zha C, Xie Z, Ding J, Geng M, Ai J (2019) Preclinical evaluation of 3D185, a novel potent inhibitor of FGFR1/2/3 and CSF-1R, in FGFR-dependent and macrophage-dominant cancer models. J Exp Clin Cancer Res 38(1):372. https://doi.org/10.1186/s13046-019-1357-y
Boulakirba S, Pfeifer A, Mhaidly R, Obba S, Goulard M, Schmitt T, Chaintreuil P, Calleja A, Furstoss N, Orange F, Lacas-Gervais S, Boyer L, Marchetti S, Verhoeyen E, Luciano F, Robert G, Auberger P, Jacquel A (2018) IL-34 and CSF-1 display an equivalent macrophage differentiation ability but a different polarization potential. Sci Rep 8(1):256. https://doi.org/10.1038/s41598-017-18433-4
IMC CS4. Adis Insight. 2011. Available on https://adisinsight.springer.com/drugs/800034411. Accesssed 16 March 2020
Autio KA, Klebanoff CK, Schaer D, Kauh JS, Slovin SF, Blinder VS, Comen EA, Danila DC, Hoffman DMJ, Kang S, McAndrew P, Modi S, Morris MJ, Rathkopf DE, Sanford RA, Tate SC, Yu D, McArthur HL (2019) Phase 1 study of LY3022855, a colony-stimulating factor-1 receptor (CSF-1R) inhibitor, in patients with metastatic breast cancer (MBC) or metastatic castration-resistant prostate cancer (MCRPC). J Clin Oncol 37(15_suppl):2548–2548. https://doi.org/10.1200/JCO.2019.37.15_suppl.2548
Holmgaard RB, Brachfeld A, Gasmi B, Jones DR, Mattar M, Doman T, Murphy M, Schaer D, Wolchok JD, Merghoub T (2016) Timing of CSF-1/CSF-1R signaling blockade is critical to improving responses to CTLA-4 based immunotherapy. Oncoimmunology 5(7):e1151595. https://doi.org/10.1080/2162402X.2016.1151595
Hamanishi J, Mandai M, Konishi I (2016) Immune checkpoint inhibition in ovarian cancer. Int Immunol 28(7):339–348. https://doi.org/10.1093/intimm/dxw020
Santini FC, Hellmann MD (2018) PD-1/PD-L1 Axis in lung Cancer. Cancer J 24(1):15–19. https://doi.org/10.1097/PPO.0000000000000300
Peters S, Kerr KM, Stahel R (2018) PD-1 blockade in advanced NSCLC: a focus on pembrolizumab. Cancer Treat Rev 62:39–49. https://doi.org/10.1016/j.ctrv.2017.10.002
Doo DW, Norian LA, Arend RC (2019) Checkpoint inhibitors in ovarian cancer: a review of preclinical data. Gynecol Oncol Rep 29:48–54. https://doi.org/10.1016/j.gore.2019.06.003
U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available on https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accesssed 16 March 2020
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D (2017) Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 5(1):53. https://doi.org/10.1186/s40425-017-0257-y
von Tresckow B, Morschhauser F, Ribrag V, Topp MS, Chien C, Seetharam S, Aquino R, Kotoulek S, de Boer CJ, Engert A (2015) An open-label, multicenter, phase I/II study of JNJ-40346527, a CSF-1R inhibitor, in patients with relapsed or refractory Hodgkin lymphoma. Clin Cancer Res 21(8):1843–1850. https://doi.org/10.1158/1078-0432.CCR-14-1845
Gomez-Roca CA, Italiano A, Le Tourneau C, Cassier PA, Toulmonde M, D'Angelo SP, Campone M, Weber KL, Loirat D, Cannarile MA, Jegg AM, Ries C, Christen R, Meneses-Lorente G, Jacob W, Klaman I, Ooi CH, Watson C, Wonde K, Reis B, Michielin F, Ruttinger D, Delord JP, Blay JY (2019) Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol 30(8):1381–1392. https://doi.org/10.1093/annonc/mdz163
Wesolowski R, Sharma N, Reebel L, Rodal MB, Peck A, West BL, Marimuthu A, Severson P, Karlin DA, Dowlati A, Le MH, Coussens LM, Rugo HS (2019) Phase Ib study of the combination of pexidartinib (PLX3397), a CSF-1R inhibitor, and paclitaxel in patients with advanced solid tumors. Ther Adv Med Oncol 11:1758835919854238. https://doi.org/10.1177/1758835919854238
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, Gupta M, Tang M, Allison DE, Lu D, Zhang Y, Joshi A, Dresser MJ (2012) A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet 51(2):119–135. https://doi.org/10.2165/11596370-000000000-00000
Pradel LP, Ooi CH, Romagnoli S, Cannarile MA, Sade H, Ruttinger D, Ries CH (2016) Macrophage susceptibility to Emactuzumab (RG7155) treatment. Mol Cancer Ther 15(12):3077–3086. https://doi.org/10.1158/1535-7163.MCT-16-0157
Cassier PA, Garin G, Eberst L, Delord J-P, Chabaud S, Terret C, Montane L, Bidaux A-S, Laurent S, Jaubert L, Ferlay C, Bernardin M, Tabone-Eglinger S, Gilles-Afchain L, Menetrier-Caux C, Caux C, Treilleux I, Pérol D, Gomez-Roca CA (2019) MEDIPLEX: A phase 1 study of durvalumab (D) combined with pexidartinib (P) in patients (pts) with advanced pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC). J Clin Oncol 37(15_suppl):2579–2579. https://doi.org/10.1200/JCO.2019.37.15_suppl.2579
Dowlati A, Rugo HS, Harvey RD, Kudchadkar RR, Carvajal RD, Manji GA, Hamid O, Klempner SJ, Tang S, Yu D, Kauh JS, Schaer DA, Tate SC, Wesolowski R (2017) A phase I study of LY3022855, a colony stimulating factor-1 receptor (CSF-1R) inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol 35(15_suppl):2523–2523. https://doi.org/10.1200/JCO.2017.35.15_suppl.2523
Baverel PG, Dubois VFS, Jin CY, Zheng Y, Song X, Jin X, Mukhopadhyay P, Gupta A, Dennis PA, Ben Y, Vicini P, Roskos L, Narwal R (2018) Population pharmacokinetics of Durvalumab in Cancer patients and association with longitudinal biomarkers of disease status. Clin Pharmacol Ther 103(4):631–642. https://doi.org/10.1002/cpt.982
Ryman JT, Meibohm B (2017) Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol 6(9):576–588. https://doi.org/10.1002/psp4.12224
Pognan F, Couttet P, Demin I, Jaitner B, Pang Y, Roubenoff R, Sutter E, Timsit Y, Valentin MA, Vogel B, Woerly G, Wolf A, Schramm U (2019) Colony-stimulating Factor-1 antibody Lacnotuzumab in a phase 1 healthy volunteer study and mechanistic investigation of safety outcomes. J Pharmacol Exp Ther 369(3):428–442. https://doi.org/10.1124/jpet.118.254128
Acknowledgements
We extend our gratitude to the patients and their families and caregivers for participating in this trial. Medical writing support was provided by Karan Sharma from Eli Lilly Services India Pvt. Ltd.
Funding
This study was funded by Eli Lilly and Company.
Author information
Authors and Affiliations
Contributions
S. Chapman contributed to study conception and design. W. Zhang and S. Chapman contributed to development of methodology. GS. Falchook, JE. Cohen, M. Peeters, RD. Carvajal, TM. Bauer, R. Obermannova, LY. Dirix contributed to acquisition of data. S. Chapman, W. Zhang, DA. Peterson, GS. Falchook, M. Peeters, RD. Carvajal, LY. Dirix, R. Perets, RS. Frommer contributed to analysis and interpretation of data. All authors contributed to writing, review, and/or critical revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was performed in accordance with the International Conference on Harmonization guidelines and Declaration of Helsinki 1964 and its amendments. The Institutional Review Board of each study site approved the study protocol, information brochure, and the informed consent form before study initiation.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients provided informed consent to regarding publishing their data.
Conflict of interest
GS. Falchook reports receiving grants from Eli Lilly and Company during the conduct of the study. He also received grants from 3-V Biosciences, Abbisko, ADC Therapeutics, Aileron, American Society of Clinical Oncology, Amgen, ARMO, Beigene, Bioatla, Bioinvent, Biothera, Bicycle, Celldex, Celgene, Ciclomed, Curegenix, Curis, Cyteir, Daiichi, DelMar, eFFECTOR, grants and other from EMD Serono, Epizyme, Exelixis, grants and other from Fujifilm, Genmab, GSK, Hutchison MediPharma, from Igynta, Incyte, Jacobio, Jouce, Kolltan, Loxo, MedImmune, grants and other from Millenium, Merck, miRNA Therapeutics, NIH, Novartis, Oncomed, Oncorus, Oncothyreon, from Poseida, Precision Oncology, Prelude, Regeneron, Rgenix, Ribon, Sapience, Strategia, Syndax, Synthorx, Taiho, Takeda, Tesaro, Tocagen, Turning Point Therapeutics, U.T. MD Anderson Cancer Center, Vegenics, Xencor, other from Wolters-Kluver, other from Sarah Cannon Research Institute, other from Total Health Conferencing, other from Rocky Mountain Oncology Society. He also received Royalties (self): Wolters Kluwer (2014-present) Advisory role (to institution): Fujifilm (2018) Údvisory role (self): EMD Serono (2010, 2011) Travel (self, for work or research related to institution): Bristol-Myers Squibb (2015), EMD Serono (2011, 2012, 2013), Fujifilm (2018), Millennium (2013), Sarah Cannon Research Institute Speakers honorarium for CME: Total Health Conferencing (2019), Rocky Mountain Oncology Society (2020) Research funding (to institution) from Ó-V Biosciences, Abbisko, Abbvie, ADC Therapeutics, Aileron, American Society of Clinical Oncology, Amgen, ARMO, AstraZeneca, BeiGene, Bioatla, Bioinvent, Biothera, Bicycle, Celldex, Celgene, Ciclomed, Curegenix, Curis, Cyteir, Daiichi, DelMar, eFFECTOR, Eli Lilly and Company, EMD Serono, Epizyme, Exelixis, Fujifilm, Genmab, GlaxoSmithKline, Hutchison MediPharma, Ignyta, Incyte, Jacobio, Jounce, Kolltan, Loxo, MedImmune, Millennium, Merck, miRNA Therapeutics, National Institutes of Health, Novartis, OncoMed, Oncorus, Oncothyreon, Poseida, Precision Oncology, Prelude, Regeneron, Rgenix, Ribon, Sapience, Strategia, Syndax, Synthorx, Taiho, Takeda, Tarveda, Tesaro, Tocagen, Turning Point Therapeutics, U.T. MD Anderson Cancer Center, Vegenics, and Xencor.
M. Peeters reports receiving advisory fee from Eli Lilly and Company.
S. Rottey, LY. Dirix, R. Obermannova, and JE. Cohen have no conflicts to disclose.
R. Perets reports receiving personal fees from BiolineRx and Karyopharm Therapeutics.
RD. Carvajal reports receiving personal fees and other from BMS, Incyte, Immunocore, and Merck Roche/Genentech; personal fees from Castle Biosciences, Compugen, Foundation Medicine, I-Mab, PureTech, Sanofi Genzyme, Sorrento Therapeutics, Aura Biosciences, Chimeron, and Rgenix; other from Amgen, Novartis, Pfizer, AstraZeneca, Bellicum, Plexxikon, Mirati, Macrogenics, Corvus, Bayer, Eli Lilly and Company, and Astellis.
J. Sabari reports receiving personal fees from AstraZeneca, Janssen, and Pfizer.
DA. Peterson, S. Chapman, W. Zhang, and B. Calderon are full-time employees of Eli Lilly and Company.
RS. Frommer reports receiving personal fees from MSD, BMS, Roche, Novartis, Clovis oncology, and Astra Zeneca.
TM. Bauer reports receiving grants from Eli Lilly and Company during the conduct of the study; grants from Daiichi Sankyo, Medpacto, Incyte, Mirati Therapeutics, MedImmune, Abbvie, AstraZeneca, MabVax, Stemline Therapeutics, Merck, Eli Lilly and Company, GlaxoSmithKline, Novartis, Genentech, Deciphera, Merrimack, Immunogen, Millennium, Phosplatin Therapeutics, Calithera Biosciences, Kolltan Pharmaceuticals, Principa Biopharma, Peleton, Immunocore, Roche, Aileron Therapeutics, Bristol-Myers Squibb, Amgen, Onyx, Sanofi, Boehringer-Ingelheim, Astellas Pharma, Five Prime Therapeutics, Jacobio, Top Alliance BioScience, Janssen, Clovis Oncology, Takeda, Karyopharm Therapeutics, Foundation Medicine, and ARMO Biosciences; grants and other from Leap Therapeutics; grants and non-financial support and other from Ignyta and Moderna Therapeutics; grants, personal fees and other from Pfizer; grants, personal fees and non-financial support from Loxo and Bayer; personal fees and non-financial support from Guardant Health; personal fees from Exelesis.
JS. Wang reports receiving research funding to institution from Genentech, Vedanta Biosciences, Relay Therapeutics, Synthorx, Ribon Therapeutics, Clovis Pharma, Xencor, Black Diamond Therapeutics, Vigeo Therapeutics, Qilu Puget Sound Biotherapeutics, Daiichi Sankyo Pharma, Cyteir Therapeutics, Bicycle Therapeutics, Kymab, Prelude Therapeutics, ADC Therapeutics, Revolution Medicines, Klus Pharma, Janssen R&D, Birdie Biopharmaceuticals, Merck, Jacobio Pharmaceuticals, MacroGenics, TopAlliance Biosciences, Moderna, Agenus, Boehringer Ingelheim, Eli Lilly and Company, Tesaro, GlaxoSmithKline, Stemline Therapeutics, BioNTech, Hutchinson MediPharma, Evelo Biosciences, Ignyta, EMD Serono, Novartis Pharmaceuticals, Loxo Oncology, Acerta Pharma, Portola Pharmaceuticals, Celgene Corporation, Taiho Oncology, LSK BioPartners, Olema Pharmaceuticals, and Phoenix Molecular Designs; personal fees and AstraZeneca, H3 Biomedicine, and Syndax.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 324 kb)
Rights and permissions
About this article
Cite this article
Falchook, G.S., Peeters, M., Rottey, S. et al. A phase 1a/1b trial of CSF-1R inhibitor LY3022855 in combination with durvalumab or tremelimumab in patients with advanced solid tumors. Invest New Drugs 39, 1284–1297 (2021). https://doi.org/10.1007/s10637-021-01088-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01088-4