Summary
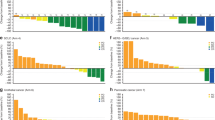
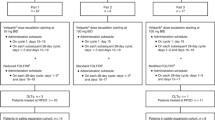
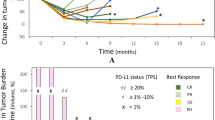
Background The IND.226 study was a phase Ib study to determine the recommended phase II dose of durvalumab + tremelimumab in combination with standard platinum-doublet chemotherapy. Sequential administration of multiple agents increases total chair time adding costs overall and inconvenience for patients. This cohort of the IND.226 study evaluated the safety and tolerability of durvalumab + tremelimumab given either sequentially (SEQ) or concurrently (CON). Methods Patients with advanced solid tumours were enrolled and randomised to either SEQ tremelimumab 75 mg IV over 1 h followed by durvalumab 1500 mg IV over 1 h q4wks on the same day, or CON administration over 1 h. The serum pharmacokinetic profile of SEQ versus CON of durvalumab and tremelimumab administration was also evaluated. Results 14 patients either received SEQ (n = 7pts) or CON (n = 7 pts). There were no infusion related reactions. Drug related adverse events (AEs) were mainly low grade and manageable, and comparable in frequency between SEQ/CON- fatigue (43%/57%), rash (43%/43%), pruritus (43%/29%) and nausea (14%/29%). One patient in each cohort discontinued treatment due to toxicity. The PK profiles of durvalumab and tremelimumab were similar between CON and SEQ, and to historical reference data. Conclusions Concurrent administration of durvalumab and tremelimumab over 1 h is safe with a comparable PK profile to sequential administration.

Similar content being viewed by others
References
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723. https://doi.org/10.1056/NEJMoa1003466
Ferris RL, Blumenschein G, Fayette J et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856–1867. https://doi.org/10.1056/NEJMoa1602252
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus Docetaxel in advanced nonsquamous non–small-cell lung Cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung Cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813. https://doi.org/10.1056/NEJMoa1510665
Wolchok JD, Chiarion-Sileni V, Gonzalez R et al (2017) Overall survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med 377:1345–1356. https://doi.org/10.1056/NEJMoa1709684
Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus Ipilimumab versus Sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277–1290. https://doi.org/10.1056/NEJMoa1712126
Hellmann MD, Paz-Ares L, Bernabe Caro R et al (2019) Nivolumab plus Ipilimumab in advanced non–small-cell lung Cancer. N Engl J Med 381:2020–2031. https://doi.org/10.1056/NEJMoa1910231
Antonia S, Goldberg SB, Balmanoukian A et al (2016) Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 17:299–308. https://doi.org/10.1016/S1470-2045(15)00544-6
Siu LL, Even C, Mesía R et al (2019) Safety and efficacy of Durvalumab with or without Tremelimumab in patients with PD-L1–low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical TrialDurvalumab Monotherapy and combination therapy in PD-L1–low/negative HNSCCDurvalu. JAMA Oncol 5:195–203. https://doi.org/10.1001/jamaoncol.2018.4628
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050
Pfirschke C, Engblom C, Rickelt S et al (2016) Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 44:343–354. https://doi.org/10.1016/j.immuni.2015.11.024
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non–small-cell lung Cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Burtness B, Harrington KJ, Greil R et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394:1915–1928. https://doi.org/10.1016/S0140-6736(19)32591-7
Ismael G, Hegg R, Muehlbauer S et al (2012) Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol 13:869–878. https://doi.org/10.1016/S1470-2045(12)70329-7
Launay-Vacher V (2013) An appraisal of subcutaneous trastuzumab: a new formulation meeting clinical needs. Cancer Chemother Pharmacol 72:1361–1367. https://doi.org/10.1007/s00280-013-2289-4
Pivot X, Gligorov J, Müller V et al (2013) Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol 14:962–970. https://doi.org/10.1016/S1470-2045(13)70383-8
Davies A, Merli F, Mihaljević B et al (2017) Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial. Lancet Haematol 4:e272–e282. https://doi.org/10.1016/S2352-3026(17)30078-9
De Cock E, Kritikou P, Sandoval M et al (2016) Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS One 11:e0157957. https://doi.org/10.1371/journal.pone.0157957
Rule S, Briones J, Smith R et al (2014) PSY89 - preference for rituximab subcutaneous (Sc) and intravenous (iv) among patients with Cd20+ non-Hodgkin’s lymphoma (Nhl) completing the Rasq measure in randomized phase iii studies Prefmab and Mabcute. Value Health 17:A537. https://doi.org/10.1016/j.jval.2014.08.1719
Radhakrishnan V, Banavali S, Gupta S et al (2019) 1209PExcellent CBR and prolonged PFS in non-squamous NSCLC with oral CA-170, an inhibitor of VISTA and PD-L1. Ann Oncol 30. https://doi.org/10.1093/annonc/mdz253.035
Tang J, Shalabi A, Hubbard-Lucey VM (2017) Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 29:84–91. https://doi.org/10.1093/annonc/mdx755
Andersson M, López-Vega JM, Petit T et al (2017) Efficacy and safety of Pertuzumab and Trastuzumab administered in a single infusion bag, followed by Vinorelbine: VELVET cohort 2 final results. Oncologist 22:1160–1168. https://doi.org/10.1634/theoncologist.2017-0079
Funding
This trial was sponsored and supported by the Canadian Clinical Trials Group. AstraZeneca Pharmaceuticals provided drug and partial funding support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. J. Nehra: No conflicts of interest.
Dr. P.A. Bradbury – Honoraria: Abbvie, Pfizer, Lilly, Merck. Advisory boards- Abbvie, BI.
Dr. P.M. Ellis- Advisory Boards: AstraZeneca, Abbvie, Takeda, Pfizer. Honoraria for speaking for: AstraZeneca, Abbvie, Pfizer, Bristol-Myers Squibb.
Dr. J. Laskin: honoraria/ad boards from Roche, BI, Takeda and AstraZeneca. My institution has received research funding from Roche, and AstraZeneca.
Dr. C. Kollmannsberger – No conflicts of interest.
Dr. D. Hao – Advisory Boards: AstraZeneca, Roche, Merck, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly.
Dr. R.A. Juergens – Advisory board, honoraria and grants: AstraZeneca, Bristol-Myers Squibb, Merck. Advisory board and honoraria: Amgen, Boehringer Ingelheim, Novartis, Roche, Pfizer, and Takeda.
Dr. G. D. Goss reports Honoraria: AstraZeneca, BMS, IO Biotech. Travel reimbursement: Boehringer Ingelheim.
Dr. P. Wheatley-Price – Advisory Boards: Takeda, Bristol-Myers Squibb, AstraZeneca, Merck, Abbvie, Roche.
Dr. S. Hotte: Astra Zeneca: Research Funding (institution); honoraria/Advisory boards.
Dr. K. Gelmon: Pharmaceutical Company Associations. Advisory Boards – AstraZeneca, Roche, Novartis, Pfizer, Oncotheryon, Nanostring, Merck, Mylan, Lilly, Genomic Health, Nanostring, BMS. Research Funding – Pfizer, Roche, AstraZeneca, Novartis, BMS. Expert Testimony - Genentech.
Dr. A.V. Tinker: No conflicts of interest.
Dr. P. Brown-Walker – No conflicts of interest.
I. Gauthier: No conflicts of interest.
Dr. D. Tu – No conflicts of interest.
Dr. X. Song – Employee of AstraZeneca and owns stock in AstraZeneca.
A. Khan: Employee of AstraZeneca and owns stock in AstraZeneca.
Dr. L. Seymour – Received funds from AstraZeneca Pharmaceuticals on behalf of CCTG to support the conduct of the trial. Owns stock in AstraZeneca.
Dr. M. Smoragiewicz – No conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nehra, J., Bradbury, P.A., Ellis, P.M. et al. A Canadian cancer trials group phase IB study of durvalumab (anti-PD-L1) plus tremelimumab (anti-CTLA-4) given concurrently or sequentially in patients with advanced, incurable solid malignancies. Invest New Drugs 38, 1442–1447 (2020). https://doi.org/10.1007/s10637-020-00904-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00904-7