Summary
Purpose To date, it is not clear which anticancer agent is useful in combination with trastuzumab and pertuzumab As the first and second selective regimens for advanced or metastatic breast cancer (AMBC), this multicenter, open-label, phase II trial (JBCRG-M03: UMIN000012232) presents a prespecified analysis of eribulin in combination with pertuzumab and trastuzumab. Methods We enrolled 50 patients with no or single prior chemotherapy for HER2-positive AMBC during November 2013–April 2016. All patients received adjuvant or first-line chemotherapy with trastuzumab and a taxane. The treatment comprised eribulin on days 1 and 8 of a 21-day cycle and trastuzumabplus pertuzumab once every 3 weeks, all administered intravenously. While the primary endpoint was the progression-free survival (PFS), secondary endpoints were the response rate and safety. Results Of 50 patients, 49 were eligible for safety analysis, and the full analysis set (FAS) included 46 patients. We treated 8 (16%) and 41 (84%) patients in first- and second-line settings, respectively. While 11 patients (23.9%) had advanced disease, 35 (76.1%) had metastatic disease. The median PFS was 9.2 months for all patients [95% confidence interval (CI): 7.0–11.4]. In the FAS, 44 patients had the measurable lesions and the complete response rate (CR) was 17.4%, and partial response rate (PR) was 43.5%. The grade 3/4 adverse events were neutropenia (5 patients, 10.2%), including febrile neutropenia (2 patients, 4.1%), hypertension (3 patients, 6.1%), and other (1 patient). The average of the left ventricular ejection fraction did not decline markedly. No symptomatic left ventricular systolic dysfunction was observed. Conclusions In patients with HER2-positive AMBC, eribulin, pertuzumab, and trastuzumab combination therapy exhibited substantial antitumor activity with an acceptable safety profile. Hence, we have started a randomized phase III study comparing eribulin and a taxane in combination with pertuzumab and trastuzumab for the treatment of HER2-positive AMBC. Trial registration ID: UMIN-CTR: UMIN000012232.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gene amplification or the protein overexpression of human epidermal growth factor receptor 2 (HER2) is present in 15%–20% of breast cancer tumors [1]. Reportedly, HER2 is overexpressed in several cancer types and contributes to tumor cell proliferation, survival, differentiation, and migration [2,3,4,5]. Trastuzumab, a humanized monoclonal antibody, potently hinders the HER2-mediated signaling pathway and binds to domain IV of HER2. Pertuzumab is also a humanized monoclonal antibody that targets HER2 [6, 7]; however, unlike trastuzumab, it binds to domain II of the receptor and, thus, can disrupt HER2 dimerization and ligand-activated signaling with other growth factor receptors, including other HER family members. HER signaling warrants homo- or heterodimerization. Reportedly, the HER2-HER3 dimer is the most potent to induce cell proliferation [8,9,10].
In the clinical assessment of CLEOPATRA (clinical evaluation of docetaxel, pertuzumab, and trastuzumab), a double-blind randomized phase III trial comparing pertuzumab and trastuzumab+docetaxel with placebo+trastuzumab+docetaxel as the primary treatment for HER2-positive progressive and recurrent breast cancer, the response rates (RR) were 80.2% and 69.3% in the pertuzumab and control groups, respectively. In addition, the progression-free survival (PFS) was significant in the pertuzumab and control groups at 18.7 and 12.4 months, respectively; overall survival [OS; central mean observation period, 50 months; pertuzumab group, 56.5 months; control group, 40.8 months; hazard ratio (HR), 0.68; 95% confidence interval (CI): 0.56–0.84] was markedly prolonged in the pertuzumab group [11, 12]. The addition of pertuzumab did not increase cardiotoxicity or frequent adverse events (AE), except neutropenia (49% vs. 46%), febrile neutropenia (13% vs. 7%), and diarrhea (9% vs. 5%). We have concerns about the safety of the practical implementation of docetaxel for edema, peripheral neuropathy, and other AEs accompanied by a decline in the quality of life. Furthermore, the development of therapeutic methods that could exhibit equivalent effects and decrease side effects is a critical clinical problem regarding patients’ needs.
Reportedly, eribulin, a nontaxane-type microtubule dynamics inhibitor, inhibits tubulin polymerization, stops microtubule function, arrests G2–M phase cell cycle, and induces apoptosis [13,14,15,16]. The EMBRACE study markedly increased the primary endpoint of the OS with eribulin compared with the treatment of physician’s choice (TPC) in MBC patients. In the study, 762 patients with locally recurrent or metastatic breast cancer previously treated with 2–5 chemotherapeutic drugs, including anthracyclines and taxane anticancer drugs, were randomly allocated to the eribulin and TPC treatment groups, and the OS time was extended by 2.7 months [17]. The median PFS in the eribulin and TPC groups was 3.7 and 2.2 months, respectively. The EMBRACE trial included 16% of HER2-positive patients; even within that subset, eribulin exhibited good results over the entire survival period [17].
A phase II trial investigated a combination therapy of eribulin and trastuzumab as a major treatment for HER2-positive, locally advanced or metastatic breast cancer (AMBC) [18]. In the study, 22 (42.3%) of 52 patients had a history of anti-HER2 therapy; median treatment cycles were 10 cycles of eribulin and 11 cycles of trastuzumab, and the RR was 71.2% (n = 37), the median PFS was 11.6 months [18]. In this phase II study, the median PFS was approximately the same as 12 months of the trastuzumab and docetaxel groups in the CLEOPATRA trial. Grade 3/4 AEs were neutropenia (38.5%), peripheral neuropathy (26.9%), and fatigue (7.7%). These findings suggested that the combination of eribulin and trastuzumab is effective, well-tolerated, and could be one treatment option for HER2-positive locally AMBC. Hence, this trial aims to actively investigate the efficacy and safety as a phase II trial of the trastuzumab, pertuzumab, and eribulin combination therapy in HER2-positive breast cancer.
Patients and methods
Study design
We conducted this multicenter, single-arm, phase II study on patients with HER2-positive AMBC and assessed the efficacy and safety of eribulin in combination with trastuzumab and pertuzumab. This study was conducted per the tenets of the Declaration of Helsinki (2008), and the study protocol and informed consent were submitted for approval to the Institutional Review Committee of the participating institution. We obtained written informed consent from all patients before protocol treatment.
Patients
In this study, patients who received first- or second-line therapy for HER2-positive AMBC were eligible. Patients within 12 months of completing perioperative chemotherapy and anti-HER2 therapy were treated as second-line treatment. The HER2-positive tumor was determined by score 3 on gene amplification by immunohistochemical staining or fluorescence in-situ hybridization based on the criteria of the 2013 American Clinical Oncology Association (ASCO/Recommendation of US pathologist (CAP) guideline [19]. In addition, all patients must have received taxanes and trastuzumab as adjuvant or recurrent therapy; previous hormonal therapy was accepted as well. All patients were eligible to assess tumor progression. Other eligibility criteria were as follows: aged 18–70 years with the expected survival time of >6 months, echocardiogram left ventricular ejection fraction (LVEF) at baseline ≥55%, Eastern Cooperative Oncology Group (ECOG) Performance status (PS) 0 or 1 (2 is allowed in case that the cause of decreased PS is bone metastasis), appropriate kidney, and bone marrow function.
Conversely, the exclusion criteria were as follows: symptomatic central nervous system metastasis, active systemic infection, prior use of eribulin, or recurrence in conserved breast or local recurrence, which is an appropriate treatment for reoperation.
Treatment
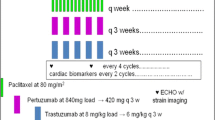
All patients received 1.4-mg/m2 eribulin mesylate intravenously infused over 2–5 min on days 1 and 8 of each 21-day cycle. Pertuzumab and trastuzumab were administered to patients as follows: 840-mg pertuzumab (420 mg every 3 weeks), 8-mg/kg trastuzumab, and a maintenance dose of 6 mg/kg administered every 21 days. Of note, both pertuzumab and trastuzumab were administered intravenously over 90 min on day 1 of cycle 1; after that, it was injected over the course of 30 min on the first day of each cycle. This regimen was continued until the onset of progressive disease (PD) assessed by researchers based on the radiological evidence or until the onset of toxic effects that could not be managed effectively.
Notably, there was a decrease in the dose of eribulin, not pertuzumab and trastuzumab; two reductions (1.1 and 0.7 mg/m2) were allowed before stopping the study treatment or considering postponing the treatment cycle. If eribulin was discontinued owing to toxic effects or patients’ requirement after 6 cycles, pertuzumab and trastuzumab could continue. The use of prophylactic granulocyte colony-stimulating factor (G-CSF) was not allowed. If it is deemed necessary for patients’ welfare and is not expected to interfere with the evaluation of the clinical trial treatment, concomitant medication can be administered at the discretion of the investigator. No other antitumor therapy was permitted during the study treatment was ongoing.
Endpoints
We performed the baseline tumor assessment (computed tomography or magnetic resonance imaging scan) of the chest, abdomen, pelvis, and other areas of known diseases 30 days before the first injection and every 9 weeks during treatment. In this study, the primary endpoint was the PFS, assessed by the investigators using RECIST ver.1.1 [20]. The secondary endpoints were the response rate (RR), safety, OS, efficacy among the patients after pre-use of pertuzumab, eribulin compliance, and efficacy of subsequent treatments. We defined RR as the percentage of patients who attained complete response (CR) and partial response (PR), and PFS as the duration from the date of study registry to the date of first confirming disease progression or death.
In addition, we performed the evaluation of electrocardiogram and echocardiogram at the baseline and every 4 cycles. Besides, laboratory examination of hematology and clinical chemistry was performed for each day of visit in the first cycle; after the second cycle, we managed to omit the laboratory test on the eighth day at the researcher’s discretion. We evaluated the ECOG PS in every visit. Moreover, AEs were rated on a 5-point scale based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0. All AEs were followed until resolution or 30 days after the patients’ last research visit. Notably, patients with SAE were followed until resolution of the event or stabilization of their condition. Nevertheless, AEs for onset peripheral neuropathy and any grade alopecia were followed until resolution or until initiation of another anticancer treatment. Since the Japanese version of PRO-CTCAE was not yet available during this study, side effects were assessed by the CTCAE evaluated by the physician.
Statistical analysis
Based on the median of PFS of 9.2 months with trastuzumab+eribulin, 40% of patients received trastuzumab as preoperative and postoperative treatment, while 50% received a taxane or anthracycline [18]. In this study, we assumed the PFS for first and second line treatment after taxane and trastuzumab treatment as 6.0 months. Considering that the addition of pertuzumab results in 50% prolongation of the PFS based on the CLEOPATRA trial, we assumed the median expected PFS for the trastuzumab, pertuzumab, and eribulin combination therapy to be 9.0 months.
For a one-sided α error of 5%, detection power 80%, and accumulation of 2 and 3 years as a follow-up, 43 cases were necessary. Considering improperness and deviation, we set the enrollment as 48 cases. As the PFS varies depending on the therapeutic line, we made our final decision clinically based on the ratio of primary treatment and secondary treatment.
In this study, all efficacy analyses were primarily based on the full analysis sets (FAS), including all patients who received, at least, one study treatment. We analyzed the PFS using the Kaplan–Meier approach. As subgroup analysis, we evaluated a 95% CI and the median in the PFS for each group. A log-rank test with stratification by the pretreatment status was used to compare the PFS between each subgroup. Moreover, the Cox proportional hazards model was used to estimate HR with 95% CI according to stratification about previous treatment situations. We performed a prespecified subgroup analysis of independently assessed PFS to ascertain the consistency of the treatment effect based on the key baseline characteristics. Furthermore, AEs were assessed descriptively in a safety population (all patients who received, at least, one study drug administration). We used SPSS version 22.0 for windows (IBM Japan, Tokyo, Japan) for statistical analysis.
Results
Patients’ characteristics
Table 1 summarizes demographic and baseline clinical characteristics of the study cohort. We enrolled 50 patients in this trial from November 2013 to February 2016. One patient was dropped before the protocol treatment administration due to ineligibility, and 49 patients (median age: 56 years) were evaluated for safety. The FAS contained 46 patients. Of note, we excluded 3 patients from the FAS for the following reasons: (i) trastuzumab was not preceded; (ii) taxane was not preceded; and (iii) the interval from adjuvant therapy was too short for eligibility. The data blocking date for the effectiveness analysis was October 31, 2016, and the end date of safety analysis was at the completion of eight cycles for each patient.
Of all 49 patients, 23 (47%) patients were estrogen receptor–positive, while 15 (31%) were progesterone receptor–positive. All patients with progesterone receptor–positive were estrogen receptor–positive. In addition, visceral metastasis was reported in 25 patients (51%). Previous treatment of trastuzumab was administered to all patients; 25 for (neo) adjuvant and 25 in the metastatic setting. Prior pertuzumab therapy was used in 12 patients (24%). Pretreatment T-DM1 was not an exclusion criterion. But there were no patients who used T-DM1 as perioperative or post-recurrence treatment. The median relative median dose of eribulin was 93.3% (range: 77%–100%).
Efficacy
Overall, the median PFS was 9.2 months for all patients (95% CI: 7.0–11.4; Fig. 1). Notably, the median PFS was 20.5 (95% CI: 2.8–38.2) months in the first-line treatment and 8.3 (95% CI: 6.8–9.8) months in the second-line treatment.
As the second-line treatment, the median PFS was 10.2 (95% CI: 7.5–12.8) months and 3.9 (95% CI: 2.7–5.1) months in patients treated without pertuzumab and with pertuzumab respectively. 46 patients in the full analysis set were evaluable for RR (60.9%; Table 2). Based on investigator assessment, the CR was 8 (17.4%), and the PR was 20 (43.5%). The RR of first-line treatment patients was 87.5% (Table 2), whereas it was 55.3% in second-line treatment patients. In particular, the RR of patients who received prior pertuzumab treatment was low; only 2 out of 10 achieved PR. The Swimmer plot revealed that 40 patients continued eribulin with trastuzumab and pertuzumab until disease progression or the blocking data point. By the time of drafting this manuscript, 10 patients were still ongoing treatment at this analysis (Fig. 2).
Safety
Table 3 lists the overall safety profile. All patients in this study reported treatment-related AEs. The hematological AEs with incidence >10% were leukopenia (14.3%), neutropenia (14.3%), and anemia (10.2%). The leading non-hematological AEs were peripheral neuropathy (34.7%), malaise (18.4%), alopecia (18.4%), nausea (12.2%), and appetite loss/diarrhea/mucositis/dysgeusia (10.2%). Furthermore, the leading grade 3/4 AEs were neutropenia/leukopenia (4.1%), febrile neutropenia (4.1%), peripheral neuropathy (2.0%), and appetite loss (2.0%,).
In this study, we encountered 7 cases of serious AEs; 1 case was of interstitial pneumonia with recovery noted after 16 days. In addition, all cases of infusion-related reaction, febrile neutropenia, and vomiting recovered. Owing to the breast cancer progression, we observed one patient declined consciousness level, one patient bleeding from the liver, and one patient vomiting respectively. We did not observe asymptomatic left ventricular systolic dysfunction (includes asymptomatic LVEF drop of >10 percentage points below the baseline and value, 50%; symptomatic LVEF drop that required treatment or that led to treatment discontinuation) (Fig. 3).
Discussion
From the CLEOPATRA study (n = 808), pertuzumab and trastuzumab+docetaxel is the standard therapy for patients with HER2-positive AMBC. In the phase II trial of pertuzumab and trastuzumab+paclitaxel as first- (n = 51) or second-line treatment (n = 69), the overall PFS was 19.5 (95% CI: 14–26) months, PFS was 24.2 (95% CI: 14 months–not reached) months and 16.4 months (95% CI, 8.5 months–not reached) for those without and with prior treatment, respectively [21]. In the study, grade ≥ 3 AEs were fatigue (6%), diarrhea, peripheral neuropathy, AST/ALT elevation, and limb syndrome (3%), skin dryness, and nausea (1.5%); no case of febrile neutropenia was noted. Overall, pertuzumab and trastuzumab+paclitaxel exhibited good efficacy and high tolerability. In preclinical data, vinca alkaloid vinorelbine demonstrated synergistic activity with trastuzumab against HER2-overexpressing breast cancer cells [22]. In HERNATA study, TTP (15.3 months vs. 12.4 months; HR: 094), OS (35.7 months vs. 38.8 months; HR: 1.01) was analogous to the study assessing trastuzumab+vinorelbine compared with trastuzumab+docetaxel as the primary treatment of HER2-positive advanced and recurrent breast cancer [23]. In this study, Median time to treatment failure for study chemotherapy was 5.6 months in the vinorelbine versus 7.7 months in the docetaxel, suggesting that vinorelbine may have achieved longer TTP. It is difficult to cure patients with recurrent breast cancer, and the purpose of treatment is to alleviate symptoms and prolong survival. Prolonged chemotherapy tends to have a longer prognosis [24], but docetaxel is difficult to continue after 8 cycles [11, 23]. Thus, vinorelbine was considered a candidate for combination with pertuzumab and trastuzumab. Reportedly, the median PFS was 14.3 months in the VELVET trial assessing vinorelbine+pertuzumab and trastuzumab treatment as the first-line treatment of HER2-positive AMBC [25].
In the EMBRACE trial, the OS time of eribulin-treated patients is longer compared with the TPC group [17]. Some studies have reported tumor blood vessel normalization and epithelial-to-mesenchymal transition (EMT) suppression as a new mechanism of action of eribulin [26, 27]. Reportedly, eribulin decreases TGF-β in tumors and plays a vital role in not only EMT but also the immune response in the tumor microenvironment [28,29,30,31]. In fact, in vivo experiments have demonstrated that the penetration of NK cells is induced by using eribulin [32]. Arguably, eribulin might prolong the OS by these mechanisms of action. Furthermore, a phase II trial investigated a combination therapy of eribulin and trastuzumab as a major treatment for HER2-positive, locally AMBC. All these findings indicate that the combination of eribulin and trastuzumab is effective, well-tolerated, and could be one treatment option for HER2-positive locally AMBC. Araki et al. [33] reported the safety and efficacy of eribulin, trastuzumab, and pertuzumab combination on 23 subjects as the third-line or later treatment; the combination therapy exhibited an acceptable safety profile, and the RR was 34.8%, which is favorable as a late-line therapy. To validate the efficacy of the eribulin, trastuzumab, and pertuzumab combination therapy on the front line treatment, our study was limited to primary and secondary treatments.
In our study, the eribulin, trastuzumab, and pertuzumab combination therapy exhibited many AEs, but most of them were not severe and their management was easy. The eribulin, trastuzumab, and pertuzumab combination therapy might be an altanative treatment of HER2-positive AMBC that has not previously used pertuzumab. In addition, this regimen could be an option if the avoidance of severe side effects is desirable in patients who have received taxanes pre- and postoperatively, Although the number of primary treatments was as small as 8 people, the median PFS exceeded 20 months in this study.
The limitation is that this trial is a single arm Phase 2 trial and we didn’t compare the eribulin, trastuzumab, and pertuzumab combination therapy with the standard therapy. Hence, we started a phase III trial (JBCRG M 06 study: NCT03264547) comparing eribulin and a taxane in combination with pertuzumab and trastuzumab for the treatment of HER2-positive AMBC [34].
Conclusions
This study establishes that the first- and second-line treatment with the eribulin, pertuzumab, and trastuzumab combination therapy exhibits substantial antitumor activity with an acceptable safety profile in patients with HER2-positive AMBC. Hence, this study might provide evidence of the combined use of eribulin, trastuzumab, and pertuzumab as a new first- or second-line treatment for HER2-positive AMBC patients.
References
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology, College of American Pathologists (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
English DP, Roque DM, Santin AD (2013) HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol Diagn Ther 17:85–99
Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R (2015) HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev 34:157–164
Hudis CA (2007) Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med 357:39–51
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr, Leahy DJ (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin fab. Nature 421:756–760
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5:317–328
Nahta R, Hung MC, Esteva FJ (2004) The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 64:2343–2346
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 69:9330–9336
Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX (2002) Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2:127–137
Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM (2012) CLEOPATRA study group. Pertuzumab and trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109–119
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortés J (2015) CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372:724–734
Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, Littlefield BA, Wilson L (2005) The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther 4:1086–1095
Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA (2008) Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther 7:2003–2011
Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, Jordan MA (2010) Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 49:1331–1337
Jordan MA, Kamath K (2007) How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets 7:730–742
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C (2011) EMBRACE (Eisai metastatic breast Cancer study assessing Physician’s choice versus E7389) investigators. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377:914–923
Wilks S, Puhalla S, O’Shaughnessy J, Schwartzberg L, Berrak E, Song J, Cox D, Vahdat L (2014) Phase 2, multicenter, single-arm study of eribulin mesylate with trastuzumab as first-line therapy for locally recurrent or metastatic HER2-positive breast cancer. Clin Breast Cancer 14:405–412
Wolff AC, Hammond ME, Hicks DG, Dowsett M, LM MS, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Dang C, Iyengar N, Datko F, D’Andrea G, Theodoulou M, Dickler M, Goldfarb S, Lake D, Fasano J, Fornier M (2015) Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 33:442–447
Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ (2004) Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 96:739–749
Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, Karlsson P, Tange UB, Sørensen PG, Møller S, Bergh J, Langkjer ST (2011) Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 29:264–271
Gennari A, Stockler M, Puntoni M, Sormani M, Nanni O, Amadori D, Wilcken N, D'Amico M, DeCensi A, Bruzzi P (2011) Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 29(16):2144–2149
Perez EA, López-Vega JM, Petit T, Zamagni C, Easton V, Kamber J, Restuccia E, Andersson M (2016) Safety and efficacy of vinorelbine in combination with pertuzumab and trastuzumab for first-line treatment of patients with HER2-positive locally advanced or metastatic breast cancer: VELVET cohort 1 final results. Breast Cancer Res 18:126
Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, Uesugi M, Agoulnik S, Taylor N, Funahashi Y, Matsui J (2014) Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer 110:1497–1505
Twelves C, Cortes J, Kaufman PA, Yelle L, Awada A, Binder TA, Olivo M, Song J, O’Shaughnessy JA, Jove M, Perez EA (2015) “New” metastases are associated with a poorer prognosis than growth of pre-existing metastases in patients with metastatic breast cancer treated with chemotherapy. Breast Cancer Res 17:150
Ueda S, Saeki T, Takeuchi H, Shigekawa T, Yamane T, Kuji I, Osaki A (2016) In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br J Cancer 114:1212–1218
Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E (2018) TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554:538–543
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T (2018) TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554:544–548
Budhu S, Schaer DA, Li Y, Toledo-Crow R, Panageas K, Yang X, Zhong H, Houghton AN, Silverstein SC, Merghoub T, Wolchok JD (2017) Blockade of surface-bound TGF-β on regulatory T cells abrogates suppression of effector T cell function in the tumor microenvironment. Sci Signal 10:eaak9702
Ito K, Hamamichi S, Abe T, Akagi T, Shirota H, Kawano S, Asano M, Asano O, Yokoi A, Matsui J, Umeda IO, Fujii H (2017) Antitumor effects of eribulin depend on modulation of the tumor microenvironment by vascular remodeling in mouse models. Cancer Sci 108:2273–2280
Araki K, Fukada I, Yanagi H, Kobayashi K, Shibayama T, Horii R, Takahashi S, Akiyama F, Ohno S, Ito Y (2017) First report of eribulin in combination with pertuzumab and trastuzumab for advanced HER2-positive breast cancer. Breast 35:78–84
Yamashita T, Masuda N, Saji S, Araki K, Ito Y, Takano T, Takahashi M, Tsurutani J, Koizumi K, Kitada M, Kojima Y, Sagara Y, Tada H, Iwasa T, Kadoya T, Iwatani T, Hasegawa H, Morita S, Ohno S (2020) Trastuzumab, pertuzumab, and eribulin mesylate versus trastuzumab, pertuzumab, and a taxane as a first-line or second-line treatment for HER2-positive, locally advanced or metastatic breast cancer: study protocol for a randomized controlled, non-inferiority, phase III trial in Japan (JBCRG-M06/EMERALD). Trials 21(1):391
Acknowledgments
We appreciate the JBCRG Office and Data Center members, especially Shina Kurai for supporting this study.
Funding
This study was sponsored by the Japanese Breast Cancer Research Group (JBCRG), part of a public health research foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Yamashita has received honoraria from Chugai, Eisai, Novartis, Nippon Kayaku, Taiho, AstraZeneca, Kyowa Kirin, Pfizer, and Eli Lilly; funding from Chugai, Taiho, Kyowa Kirin, and Nippon Kayaku. H. Kawaguchi has received honoraria from Chugai, AstraZeneca. Eisai, Kvowa Kirin. Novartis, Taiho, Pfizer, Eli Lilly, and Takeda; and consulting fee from AstraZeneca, Chugai, Eisai, and Pfizer. Norikazu Masuda has received honoraria from Chugai, AstraZeneca, Pfizer, Eli Lilly, Eisai, and Takeda; funding from Chugai, AstraZeneca, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Eli Lilly, Eisai, and Daiichi Sankyo. Tetsuhiro Yoshinami and M. Kashiwaba has received honoraria from Chugai. S. Morita has received honoraria from AstraZeneca, Bristol-Myers Squibb Company, Chugai, Eisai, Eli Lilly, MSD, Pfizer, Taiho. S Ohno received honoraria from Chugai, AstraZeneca, Taiho, Pfizer, Novartis, and Kyowa Kirin; funding from Taiho and Eisai. M Toi received honoraria for lecture from Eisai, Chugai, Daiichi Sankyo, Kyowa Kirin, Konica-Minolta, AstraZeneca, MSD, Pfizer, Eli Lilly, Novartis, Taiho, and Takeda; consulting fee from Kyowa Kirin, Daiichi Sankyo; and funding from Eisai, AstraZeneca, Taiho, Shimadzu, Astellas, AFI, Chugai, GL Science, Nippon Kayaku, Kyowa Kirin, and Shionogi. MK, KN, M.H, NM, KY, TK, TN, HB, HY, YH, and RN do not have any conflicts of interest to declare.
Ethical approval
The experiments in this study comply with the current laws of the country in which they were performed.
Informed consent
We obtained informed consent from all participants in this study.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was reviewed and approved by each institution’s review board and/or ethics committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamashita, T., Kawaguchi, H., Masuda, N. et al. Efficacy of the eribulin, pertuzumab, and trastuzumab combination therapy for human epidermal growth factor receptor 2–positive advanced or metastatic breast cancer: a multicenter, single arm, phase II study (JBCRG-M03 study). Invest New Drugs 39, 217–225 (2021). https://doi.org/10.1007/s10637-020-00991-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00991-6